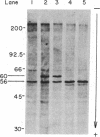
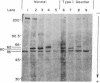
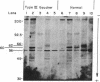
Abstract
Fibroblasts from normal subjects and patients with the three types of Gaucher disease were labeled with [3H]leucine. Glucocerebrosidase antigen was immunoprecipitated using affinity-purified Sepharose-bound antibody. Normal cells initially formed a 60-kDa polypeptide antigen that was gradually replaced by a broad band of antigen averaging 63 kDa. This position corresponds with that of mature fibroblast and placental enzyme. Processing of glucocerebrosidase in six unrelated patients with type I Gaucher disease and one patient with type III Gaucher disease was exactly the same as normal. In contrast, three patients with the severe infantile (type II) form of the disease manifested a very unstable enzyme; the 60-kDa band appeared transiently and the mature 63-kDa band was never seen. These results indicate that type II Gaucher disease may well be distinguishable from type I disease by virtue of the very unstable enzyme precursor. Contrary to some earlier reports, processing of glucocerebrosidase in type I disease appears to be entirely normal.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler E., Kuhl W., Sorge J. Cross-reacting material in Gaucher disease fibroblasts. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6506–6510. doi: 10.1073/pnas.81.20.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E., Kuhl W., Sorge J. Glucocerebrosidase "processing" and gene expression in various forms of Gaucher disease. Am J Hum Genet. 1985 Nov;37(6):1062–1070. [PMC free article] [PubMed] [Google Scholar]
- Erickson A. H., Ginns E. I., Barranger J. A. Biosynthesis of the lysosomal enzyme glucocerebrosidase. J Biol Chem. 1985 Nov 15;260(26):14319–14324. [PubMed] [Google Scholar]
- Ginns E. I., Brady R. O., Pirruccello S., Moore C., Sorrell S., Furbish F. S., Murray G. J., Tager J., Barranger J. A. Mutations of glucocerebrosidase: discrimination of neurologic and non-neurologic phenotypes of Gaucher disease. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5607–5610. doi: 10.1073/pnas.79.18.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginns E. I., Choudary P. V., Tsuji S., Martin B., Stubblefield B., Sawyer J., Hozier J., Barranger J. A. Gene mapping and leader polypeptide sequence of human glucocerebrosidase: implications for Gaucher disease. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7101–7105. doi: 10.1073/pnas.82.20.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski G. A., Dinur T., Osiecki K. M., Kruse J. R., Legler G., Gatt S. Gaucher disease types 1, 2, and 3: differential mutations of the acid beta-glucosidase active site identified with conduritol B epoxide derivatives and sphingosine. Am J Hum Genet. 1985 May;37(3):499–510. [PMC free article] [PubMed] [Google Scholar]
- Gravel R. A., Leung A. Complementation analysis in Gaucher disease using single cell microassay techniques. Evidence for a single "Gaucher gene". Hum Genet. 1983;65(2):112–116. doi: 10.1007/BF00286645. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Sorge J., Gelbart T., West C., Westwood B., Beutler E. Heterogeneity in type I Gaucher disease demonstrated by restriction mapping of the gene. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5442–5445. doi: 10.1073/pnas.82.16.5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge J., West C., Westwood B., Beutler E. Molecular cloning and nucleotide sequence of human glucocerebrosidase cDNA. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7289–7293. doi: 10.1073/pnas.82.21.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]