Abstract
High photosynthetic benthic primary production (P) represents a key ecosystem service provided by tropical coral reef systems. However, benthic P budgets of specific ecosystem compartments such as macrophyte-dominated reef lagoons are still scarce. To address this, we quantified individual and lagoon-wide net (Pn) and gross (Pg) primary production by all dominant functional groups of benthic primary producers in a typical macrophyte-dominated Caribbean reef lagoon near Puerto Morelos (Mexico) via measurement of O2 fluxes in incubation experiments. The photosynthetically active 3D lagoon surface area was quantified using conversion factors to allow extrapolation to lagoon-wide P budgets. Findings revealed that lagoon 2D benthic cover was primarily composed of sand-associated microphytobenthos (40%), seagrasses (29%) and macroalgae (27%), while seagrasses dominated the lagoon 3D surface area (84%). Individual Pg was highest for macroalgae and scleractinian corals (87 and 86 mmol O2 m−2 specimen area d−1, respectively), however seagrasses contributed highest (59%) to the lagoon-wide Pg. Macroalgae exhibited highest individual Pn rates, but seagrasses generated the largest fraction (51%) of lagoon-wide Pn. Individual R was highest for scleractinian corals and macroalgae, whereas seagrasses again provided the major lagoon-wide share (68%). These findings characterise the investigated lagoon as a net autotrophic coral reef ecosystem compartment revealing similar P compared to other macrophyte-dominated coastal environments such as seagrass meadows and macroalgae beds. Further, high lagoon-wide P (Pg: 488 and Pn: 181 mmol O2 m−2 lagoon area d−1) and overall Pg:R (1.6) indicate substantial benthic excess production within the Puerto Morelos reef lagoon and suggest the export of newly synthesised organic matter to surrounding ecosystems.
Introduction
Tropical coral reefs are among the most productive global ecosystems on account of a diverse community of benthic photoautotrophs sustaining reef community biomass and ecosystem productivity [1]. For an entire coral reef ecosystem, composed of various physiographic zones (e.g., reef lagoon, reef flat and outer reef slope), gross primary production (Pg) and respiration (R) appear largely balanced (Pg:R 1.07) [2], [3]. Consequently, net primary production (Pn) is low, and hardly any excess production or export of organic matter occurs on the ecosystem scale [4]–[6]. This balanced energetic budget in oligotrophic reef-surrounding waters is driven by the efficient utilization and regeneration of organic and inorganic nutrients via tightly coupled biogeochemical element cycles [7]–[10]. From a more detailed perspective, particular reef ecosystem compartments (e.g., lagoon or reef flat) show considerable differences in benthic primary production (P) and R, which in turn underlie considerable variability [8]. This results from variable contributions of the main functional groups of photoautotrophic primary producers (e.g., seagrasses, macroalgae and scleractinian corals) to benthic community composition, but also from their individual metabolic activity [2], [3].
In contrast to entire coral reef ecosystems and reef compartments dominated by scleractinian corals, there is still very few information available on benthic metabolism and P budgets of macrophyte-dominated reef compartments such as sandy lagoons, seagrass meadows or macroalgae beds [11]–[14]. In addition, the majority of fundamental key studies on the metabolism of tropical coral reefs and their associated coastal habitats is relatively old [1], [15], and thus may not generally suit to address the present state of shifting and/or degraded benthic reef communities observed world-wide over the past decades [16]–[18], calling for a contemporary reassessment.
Most previous studies assessing ecosystem metabolism to generate benthic P budgets in tropical coastal environments have quantified bulk metabolic fluxes on the ecosystem scale using flow respirometry [5], [19], [20]. Other studies have focussed on the physiology of specific benthic primary producers [11], [14], [21], [22] without taking into account the full range of individual contributions to ecosystem productivity within the diverse community of benthic photoautotrophs. However, for reliable benthic P budget calculations, individual P and R of all dominant benthic primary producers and their specific contributions to ecosystem P need to be quantified. In addition, reliable 3D surface area estimates of all investigated organisms, as ecological interface with the surrounding environment, are of paramount importance for the quantification of individual metabolic rates and the calculation of benthic P budgets on ecosystem scale [23], [24].
The goals of this study therefore were to (1) characterise the benthic community composition in a Caribbean fringing reef lagoon and identify the dominant benthic primary producers, (2) quantify individual P and R for all dominant primary producers and their respective functional groups based on estimates of actual 3D surface area, and hence (3) generate a benthic P budget for a macrophyte-dominated tropical coral reef lagoon.
Materials and Methods
Study site
This study was carried out from July 15th to August 2nd 2008 (Caribbean summer) in a semi-enclosed Caribbean coral reef lagoon near Puerto Morelos, Mexico (20°52. 058 N, 86°52.030 W) bordering the north–south orientated Mesoamerican barrier reef system (Figure 1). Field work was conducted under permits issued by the Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentacion to the National Autonomous University of Mexico (UNAM). All measurements were conducted on site in the aquarium and laboratory facilities of the Institute of Marine Sciences and Limnology (ICML) belonging to UNAM.
Figure 1. Map of the study site displaying the Puerto Morelos reef lagoon (Mexico) and the total lagoon area considered for primary production budget calculations.
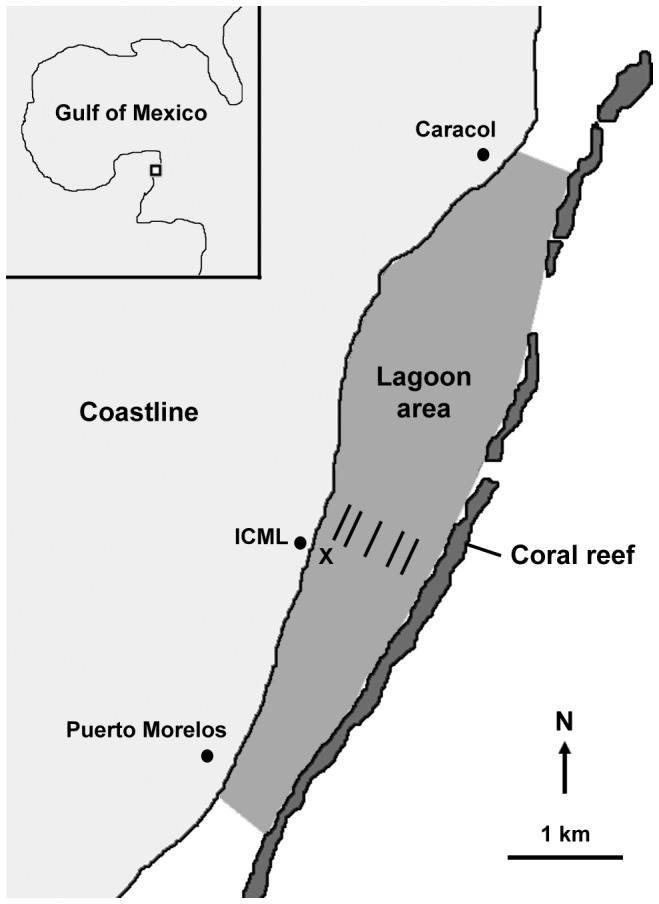
The monitoring station for data logger deployments is indicated by X. Parallel lines represent locations of benthic transect surveys. Abbreviation: ICML = Institute of Marine Sciences and Limnology.
The coral reef ecosystem of Puerto Morelos features an extensive reef lagoon framed by the coastline and the barrier reef at widths ranging from 0.2 to 1.5 km [25]. Central parts of the lagoon reach maximum water depths of ca. 4 m. Mean residence time for the entire lagoon water body is ca. 3 h and current speeds average 0.1 m s−1 under calm conditions [26]. The reef lagoon is affected by seasonal variability in water temperature (up to 5 °C) and light availability due to differences in daytime duration (ca. 2.5 h) between summer maxima and winter minima [27].
Line point intercept surveys (LPI) were conducted to quantify benthic lagoon community composition and identify dominant benthic primary producers. In total, five LPI of 100 m length each were carried out for various distances from land (100, 200, 400, 800 and 1000 m) along a transect perpendicular to the shore-line in front of the ICML (Figure 1). The transect location was selected to include all major lagoon zones (i.e., coastal, mid-lagoon and back reef) to be representative of the lagoon-wide mean seafloor coverage by all dominant benthic functional groups and substrate types. The representative quality of the transect location was confirmed by previous studies on lagoon-wide spatial macroalgae biomass distribution and mean seagrass shoot density by dominant benthic macrophytes (i.e., Halimeda incrassata, Thalassia testudinum) [28]–[30]. Intervals of 0.5 m between intercepts resulted in 201 data points per LPI. Results obtained from all LPI were used to calculate the lagoon-wide mean percentage coverage for each recorded benthic taxon or substrate type. The 2D area of the entire reef lagoon (ca. 8.1423 km2) extending between shore-line locations at the level of the town centres of Puerto Morelos and Caracol (located northerly, Figure 1) was determined from an aerial digital photograph using image processing software (ImageJ, V. 1.37 m, National Institutes of Health).
Sample collection and maintenance
Specimens representing the major functional groups of primary producers (i.e., seagrasses, macroalgae, sand-associated microphytobenthos and scleractinian corals) were collected from similar intermediate water depths (2.0–2.5 m) using SCUBA. From each functional group, the main representative taxa were collected: 1) seagrasses: Thalassia and Syringodium, 2) macroalgae: green (Halimeda and Avrainvillea), brown (mainly Lobophora) and several unidentified red algae taxa, 3) scleractinian corals: Porites and Manicina, 4) microphytobenthic mixed sand community: not identified taxonomically. This selection of organisms together accounted for 95% of the 2D lagoon seafloor coverage and for 98% of all benthic primary producers. Collection techniques and post-sampling treatment for the respective organisms are described below.
A flow-through cultivation tank (volume: 200 L) supplied with freshly pumped lagoon water (exchange rate: 150–200 L h−1) was set up for the collected seagrass, macroalgae and coral specimens to heal under in situ temperature (study period range: 29–32°C) and light conditions (mid-day photosynthetically active radiation ca. 600 µmol quanta m−2 s−1) before experimentation. Both, temperature and light availability were continuously recorded during the study period by data loggers (Onset HOBO Pendant UA-002-64, n = 3) in the cultivation and experimental tanks and in the lagoon at a reference site (water depth: 2.5 m, 1 min resolution, Figure 1). To ensure that conditions were congruent throughout the entire study period, data loggers were initially deployed for 2 d prior to the first transfer of organisms to the cultivation tank, and thereafter analysed and redeployed every second day. Light availability in the cultivation and experimental tanks (daytime mean±SD: 17,161±16,270 lux) was adjusted to in situ lagoon conditions using net cloth, while water temperature was controlled by regulating flow-through rates.
Branching colonies of Porites (diameter 7–9 cm, n = 12) and solitary Manicina polyps (diameter: 3–9 cm, n = 12) were sampled from the lagoon seafloor 1 wk prior to measurements. All coral specimens were collected and transferred using individual zip lock bags to avoid mechanical damage during transport. In the lab, corals were fixed onto ceramic tiles using epoxy glue (Reef Construct, Aqua Medic) to avoid direct tissue contact during handling. During the recovery period, ceramic tiles were regularly cleaned from overgrowth. Seagrasses (height: 12–26 cm, n = 12 for both taxa) and benthic macroalgae specimens (height: 5–12 cm, n = 12 for each of 4 taxa) were collected 48 h prior to physiological measurements and left to heal. Lagoon sand samples (n = 12) were obtained using a custom-made “mini-corer” apparatus with a defined 2D surface area (5.73 cm2), volume (3.4 ml) and sediment core depth (1.5 cm). These samples were immediately transported to the laboratory for subsequent quantitative net primary production (Pn) and respiration (R) measurements of the associated microphytobenthos. During handling, special care was taken to transfer all specimens without air exposure and to exclude seagrasses, macroalgae and corals showing extensive epibiont or endolith infestations potentially affecting O2 fluxes during incubation experiments.
Surface area quantification
Digital photographs of spread out macroalgae and seagrasses were used to quantify the 2D leaf surface area by image processing software (ImageJ, V. 1.37 m, National Institutes of Health). The planar area of each specimen was subsequently multiplied by the factor 2 to obtain the 3D surface area representing both sides of the outspread macrophytes. The 3D surface area of each macroalgal and seagrass specimen was related to the respective 2D lagoon area at the sand-water interface determined during LPI surveys to generate the 2D to 3D area conversion factor for each taxon. Skeletal 3D surface area of scleractinian corals was quantified using the Advanced Geometry technique with subsequent application of coral growth form specific approximation factors [31]. Estimates for skeletal 3D surface area and planar projected surface areas of scleractinian coral specimens, likewise quantified by digital image analysis, were used to generate 2D to 3D surface area conversion factors for both coral taxa. The specific resulting 2D to 3D conversion factors are summarised in Table 1. For samples of the microphytobenthic sand community, specimen surface area was represented by the sampled 2D area defined by the “mini-corer” apparatus (cf. Sample collection and maintenance).
Table 1. Individual primary production and respiration rates measured for all dominant benthic primary producers in a Caribbean reef lagoon (Puerto Morelos, Mexico).
| Functional group | Genus | Pg | R | Pn | 2D:3D | n |
| (mmol O2 m−2 specimen area d−1) | ||||||
| Seagrasses | Syringodium | 24±9 | 15±9 | 11±8 | 80.5±7.3 | 6 |
| Thalassia | 13±3 | 11±5 | 4±2 | 46.3±3.5 | 6 | |
| Mean | 18±9 | 13±7 | 8±7 | |||
| Macroalgae | ||||||
| Green algae | Halimeda | 75±9 | 32±9 | 42±9 | 13.3±3.7 | 6 |
| Avrainvillea | 52±5 | 25±5 | 27±5 | 14.0±2.2 | 6 | |
| Mean | 63±14 | 29±8 | 35±11 | |||
| Brown algae | Lobophora | 40±7 | 17±3 | 23±7 | 2.5±0.1 | 6 |
| Red algae | unidentified | 182±31 | 89±25 | 93±31 | 1.3±0.1 | 6 |
| Mean | 87±60 | 41±32 | 46±33 | |||
| Microphytobenthos | unidentified | 46±10 | 34±8 | 16±10 | 1.0 | 6 |
| Scleractinian corals | Manicina | 91±10 | 79±29 | 12±10 | 1.6±0.1 | 6 |
| Porites | 81±13 | 72±10 | 16±9 | 2.3±0.1 | 6 | |
| Mean | 86±12 | 74±19 | 14±9 | |||
g, R and Pn are given as mean±SD, 2D:3D factors as mean±SE. Abbreviations: Pg = gross primary production, Pn = net primary production, R = respiration, n = number of replicates. Values for P
Physiological measurements
To quantify individual Pn and R rates via O2 fluxes, specimens were incubated with fresh lagoon seawater inside gas-tight glass chambers (500 mL), which were submerged into a 200 L flow-through experimental tank serving as a water bath. The temperature and light conditions in the experimental tank were adjusted to be identical to those of the cultivation tank and congruent to in situ. For each taxon-specific incubation experiment, one set of specimens (n = 6) was incubated in the light (Pn), another set (n = 6) in the dark (R), while 8 additional chambers containing only lagoon seawater served as seawater controls for Pn (n = 4) and R (n = 4) incubations. R treatment and seawater control incubation chambers were covered using an opaque plastic foil preventing ambient light penetration. All chamber incubations were started at ca. 10∶00 to minimise potential effects of potential circadian rhythms and, except for scleractinian corals, lasted for 24 h to obtain representative diel rates for Pn. For scleractinian corals, the incubation period was reduced to 6 h to prevent physiological damage by hypoxic or hyperoxic conditions. Water flow velocities inside the incubation chambers could not be accurately adjusted to flow conditions comparably low as in situ (mean: 0.1 m s−1), without risking substantial overestimation of physiological rates by elevated flow velocities [32]–[36]. Thus, all chamber incubations were conducted under no-flow conditions to generate conservative estimates for benthic primary producer Pn and R rates. No-flow conditions ensured higher measurement accuracy of Pn and R rates ruling out flow-induced effects on O2 transfer velocities across the surface boundary of the incubation chambers [37], while also allowing for comparison with previous chamber incubation studies [24], [38]. At the start and end of all chamber incubation, the concentration of dissolved O2 (DO) was measured in the incubation medium using a DO optode sensor (Hach Lange, HQ10) after gently stirring for 5 s before every reading to ensure homogeneous DO distribution.
Data analyses
Pn and R for each incubated specimen were derived from DO concentration differences calculated by subtracting start from end concentrations. These results were corrected for DO concentration differences measured in seawater controls and normalised to incubation volume, specimen surface area and incubation period. Diel rates of Pn and R for both scleractinian coral taxa were calculated by extrapolation of 6 h incubation periods to an approximate 12∶12 h light/dark cycle (07:00–19:00) measured in situ during the study period. Individual gross primary production (Pg) rates were calculated by adding mean R rates for each specific taxon to the individual Pn of each incubated specimen. This resulted in individual Pg, R and Pn rates for all incubated taxa (given as: mmol O2 m−2 specimen surface area d−1).
Individual Pg, R and Pn rates were used to calculate the contribution of each investigated taxon and their respective functional groups to lagoon-wide benthic metabolism taking into account the specific percentage cover related to the entire lagoon area (ca. 8.1423 km2, Figure 1) and respective mean 2D to 3D conversion factors. To account for the notable contribution (11% of green algae cover) by unidentified taxa of the diverse benthic green algae community [39], mean metabolic rates and conversion factors derived from Halimeda and Avrainvillea were applied. Resulting taxon- and functional group-specific contributions to lagoon-wide Pg, R and Pn were normalised to seafloor area by the total 2D lagoon area and expressed as mmol O2 m−2 lagoon area d−1. Individual and lagoon area-related taxon- and functional group-specific Pg, R and Pn data sets were analysed statistically using one-way ANOVA with Tukey or Games-Howell post-hoc tests (SPSS software packages, v. 14.0, IBM), if not mentioned differently, after testing for equal variances (Levene test) and normal distribution (Kolmogorov–Smirnov test).
Results
Benthic community composition
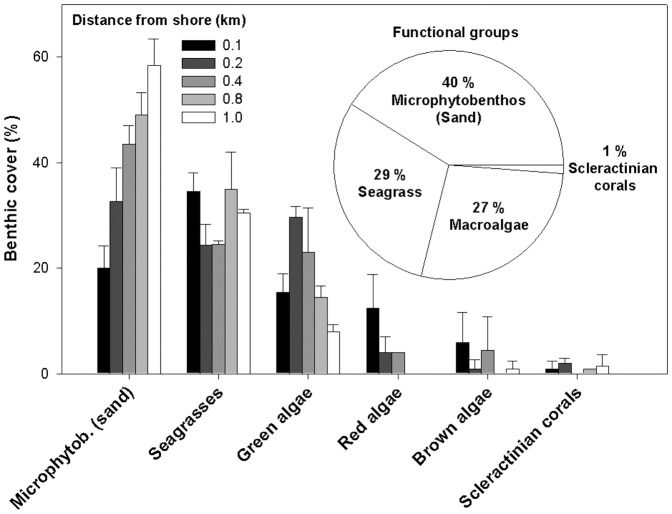
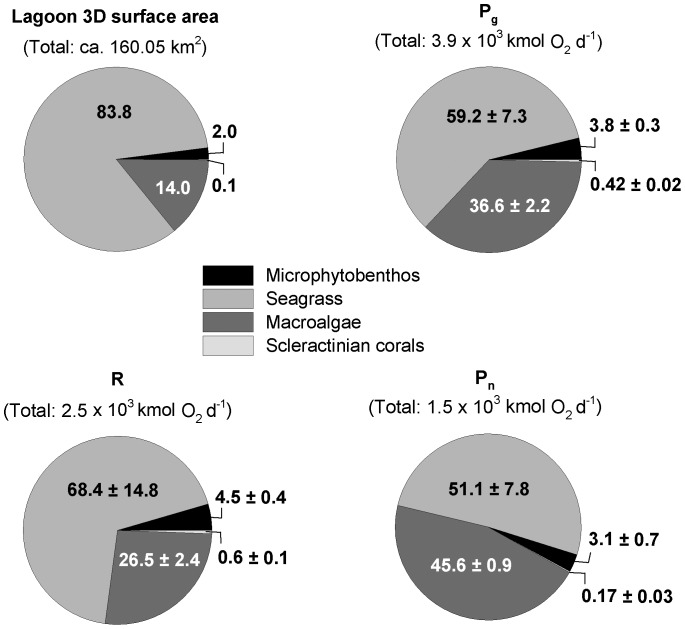
In terms of 2D lagoon areal coverage, calcareous sand accounted for most of the seafloor area (mean: 40%), and thus sand-associated microphytobenthos represented the spatially dominant functional group (Figure 2). However, after 2D to 3D surface area conversion including all functional groups and substrate types (total 3D lagoon surface area: 160.05 km2), the percentage contribution by microphytobenthos was minor (Figure 3). Seagrasses (29.2%) and macroalgae (26.8%) occupied similar considerable fractions of the 2D lagoon area, which converted into the major (83.8%) and second (14.0%) largest share of 3D lagoon surface area, respectively. In contrast, scleractinian coral cover was minimal for 2D (1.2%) and 3D (0.1%) lagoon surface area estimates.
Figure 2. Benthic coverage by dominant primary producers in the Puerto Morelos reef lagoon (Mexico).
Bar chart shows percentage cover of the lagoon 2D area by the monitored categories, sand (microphytobenthos), seagrasses (Thalassia and Syringodium), green algae (Avrainvillea, Halimeda, Rhipocephalus, Penicillus and other unidentified taxa), red algae (unidentified), brown algae (mainly Lobophora) and scleractinian hard corals (Porites and Manicina) by increasing distance to shore. Values are given as mean±SD. Pie chart indicates the lagoon-wide mean percentage cover by the respective functional groups.
Figure 3. Benthic primary production budget for a Caribbean reef lagoon (Puerto Morelos, Mexico).
Values in pie-chart slices are given as percentage mean±SE of percentage contribution to 3D lagoon surface area, primary production or respiration. Abbreviations: Pg = gross primary production, Pn = net primary production, R = respiration.
Metabolic rates of benthic primary producers
Scleractinian corals and macroalgae showed the highest (F3,50 = 8.87, p<0.018), yet similar (p = 1.00), individual gross primary production (Pg) of all functional groups, while scleractinian corals exhibited the highest individual respiration (R) (F3,50 = 12.33, p = 0.01) and seagrasses the lowest individual R (p<0.002) (Table 1). Net primary production (Pn) was highest for macroalgae (F3,50 = 8.38, p<0.04) and similar for seagrasses, microphytobenthos and scleractinian corals (p>0.90).
Within functional groups, Pg was similar for the scleractinian coral genera Manicina and Porites (t-test, t = 1.54, df = 10, p = 0.155), while amongst macroalgae Pg rates were highest for red and lowest for brown algae (p<0.001, p<0.029; Table 1). Pg of the seagrass Syringodium was elevated compared to Thalassia (Mann-Whitney U-test, z = -2.24, p = 0.03). The overall highest individual R rates were measured for red algae (F3,50 = 28.76, p<0.025), which were however similar to R of both coral taxa (p>0.77). Individual R rates of both seagrasses were similar (t-test, t = 4.94, df = 10, p = 0.31). Pn was similar for both investigated scleractinian corals (t-test, t = −0.56, df = 10, p = 0.59) and among seagrasses (Mann-Whitney U-test, z = −2.24, p = 0.06), while red algae showed the highest Pn of all investigated macroalgae taxa (p<0.028).
Benthic lagoon primary production budget
Seagrasses exhibited the highest lagoon area-related Pg compared to all other functional groups (F3,50 = 42.48, p<0.001), while contributing the largest share (59.2%) to lagoon-wide Pg (Table 2, Figure 3). Pg of seagrasses was substantially elevated compared to R (Pg:R = 1.4±0.2, mean±SE), resulting in the likewise highest contribution (51.1%) to lagoon-wide Pn (Figure 3). Macroalgae Pg related to lagoon area was higher compared to microphytobenthos (p = 0.036) and scleractinian corals (p<0.001), and contributed the second largest share to lagoon-wide Pg (Figure 3). Macroalgae R was only less than 50% of macroalgae Pg, resulting in the second highest area-related Pn of all investigated functional groups (Table 2). High individual macroalgae Pn rates were reflected in the second largest contribution (45.6%) to lagoon-wide Pn (Figure 3). In contrast, microphytobenthos contributed only minor fractions to lagoon-wide Pg and R, eventually accounting for only 3.1% of lagoon-wide Pn, as a result of low 3D area coverage. Area-related Pg and R rates for both scleractinian corals were nearly balanced and lower than for all other functional groups (F3,50 = 42.48, p<0.001; F3,50 = 33.72, p<0.007, respectively), likewise showing extremely low Pn rates, and consequently negligible contributions to lagoon-wide metabolism (Figure 3).
Table 2. Primary production and respiration rates of all dominant benthic primary producers normalised to lagoon seafloor area.
| Functional group | Genus | Pg | R | Pn | Pg:R |
| (mmol O2 m−2 lagoon area d−1) | |||||
| Seagrasses | Syringodium | 164±62 | 106±61 | 74±54 | 1.6±0.3 |
| Thalassia | 125±25 | 104±51 | 34±18 | 1.2±0.3 | |
| Mean | 144±50 | 105±53 | 57±45 | 1.4±0.2 | |
| Sum | 289±88 | 210±111 | 109±72 | ||
| Macroalgae | |||||
| Green algae | Halimeda | 85±10 | 37±11 | 48±10 | 2.3±0.3 |
| Avrainvillea | 62±6 | 30±6 | 32±6 | 2.1±0.1 | |
| Mean | 69±20 | 32±11 | 38±14 | 2.2±0.1 | |
| Brown algae | Lobophora | 4±1 | 1.6±0.3 | 2±1 | 2.3±0.1 |
| Red algae | unidentified | 10±2 | 5±1 | 5±2 | 2.0±0.1 |
| Mean | 39±35 | 18±17 | 21±20 | 2.2±0.2 | |
| Sum | 178±18 | 81±18 | 97±18 | ||
| Microphytobenthos | unidentified | 19±4 | 14±3 | 7±4 | 1.3±0.1 |
| Scleractinian corals | Manicina | 0.5±0.1 | 0.5±0.2 | 0.07±0.06 | 1.2±0.2 |
| Porites | 1.5±0.2 | 1.3±0.2 | 0.3±0.2 | 1.1±0.1 | |
| Mean | 1.0±0.5 | 1.0±0.5 | 0.162±0.159 | 1.0±0.1 | |
| Sum | 2.0±0.3 | 1.8±0.4 | 0.4±0.2 | ||
| Total | 488±59 | 307±94 | 181±28 | ||
| Mean | 1.6±0.4 | ||||
g, R and Pn are given as mean±SD. Pg:R ratios are presented as mean±SE. Abbreviations: Pg = gross primary production, Pn = net primary production, R = respiration. Values for P
Discussion
Budget of benthic lagoon primary production
This study represents one of the very few field investigations succeeding early fundamental works [1] that generates a benthic primary production (P) budget for a coral reef-associated habitat (i.e., reef lagoon) based on individual metabolic rates of all dominant benthic primary producers. To our knowledge, it is the first to calculate a benthic P budget by combining taxon-specific metabolic rates with the respective estimates of photosynthetic 3D surface areas. Our findings characterise the investigated Caribbean reef lagoon as a net autotrophic and macrophyte-dominated benthic environment, in which seagrasses and macroalgae account for the main fractions of 2D (i.e., seagrasses + macroalgae) and 3D (seagrasses) benthic lagoon area. P budget calculations based on individual gross primary production (Pg), net primary production (Pn) and respiration (R) rates identify seagrasses and benthic macroalgae as the major contributors to lagoon-wide high photosynthetic Pn. Considerable excess P by benthic photoautotrophs suggests rapid turnover by microbial degradation in lagoon overlying waters or the potential export from this macrophyte-dominated coral reef ecosystem compartment.
As the present study was conducted exclusively during Caribbean summer season, our findings for individual metabolic rates may be elevated compared to other seasons and to annual average. P of benthic primary producers may be influenced by seasonal variations in key environmental parameters, such as water temperature and light availability [27], [29], [30]. However, all investigated seagrass and macroalgae taxa, the major contributors to lagoon area coverage, show no obvious seasonal dynamics in biomass and seafloor coverage [27]. This indicates the annual validity of our summer lagoon area coverage data and further suggests a similar percentage contribution by the respective primary producers to the lagoon P budget over a yearly cycle. However, as all chamber incubations were conducted under no-flow conditions, individual metabolic rates are expected to be higher under in situ flow conditions [32]–[36], and may eventually cause a potential increase of the calculated lagoon-wide P.
With respect to the high 2D benthic cover by macrophytes and the substantial contribution by seagrasses to lagoon-wide photosynthetic 3D surface area, our present findings for metabolic rates can most adequately be compared to tropical coastal habitats featuring seagrass meadows. This is supported by our findings for area-related lagoon-wide Pg, Pn and R, which fall into the upper range of metabolic rates recently reviewed and summarised for tropical seagrass meadows [13] that likewise show identical mean Pg:R ratios (Table 3). Further, our area-related Pg, Pn and R for both investigated seagrass taxa are similar to mean metabolic rates listed for monospecific meadows likely dominated by the same seagrass species, or at least same genus, investigated here (i.e., Thalassia testudinum and Syringodium filiforme) [13]. Consistent with our findings for the Puerto Morelos reef lagoon, most seagrass ecosystems tend to be net autotrophic [13], [40], [41] showing pronounced Pn similar to other macrophyte-dominated ecosystems, such as mangroves forests or macroalgae beds (Table 3). Previous work in the study site has shown that this net productive state is even largely sustained during and after severe disturbances such as tropical storm events with substantially reduced ambient seawater temperature and light availability [42]. Further, our results for lagoon-wide Pg related to seafloor area are in the range of established literature values for entire reef ecosystems, while lagoon-wide Pn rates are even approximately one order of magnitude higher (Table 3). This in turn suggests a substantial input by the lagoon compartment to ecosystem-wide P in tropical coral reefs, which eventually may dependent on the areal contribution of the lagoon to the entire reef ecosystem area.
Table 3. Overview of benthic primary production in coral reef ecosystem compartments and reef-associated tropical coastal habitats.
| Benthic ecosystem | Region | Pg | Pn | Pg:R | Source |
| (mmol O2 m−2 seafloor area d−1) | |||||
| Coral-dominated | |||||
| Reef flats | various Pacific | 583±50 | 1.0±0.1 | [4] | |
| Entire reefs | various Pacific | 395±58 | 27±20 | 1.07±0.1 | [3] |
| Macrophyte-dominated | |||||
| Reef lagoon | Caribbean | 488±59 | 181±28 | 1.6±0.4 | This study |
| Seagrass meadows | Tropical | 252±14 | 24±8 | 1.6 | [13] |
| Macroalgae beds | Central Pacific | 520 | 1.04 | [14] | |
| Caribbean | 877 | 582 | 3.0 | [11] | |
| Sediments | |||||
| Lagoon sand | Caribbean | 19±4 | 7±4 | 1.3±0.1 | This study |
| Northern Red Sea | 19±4 | −1±2 | 0.99 | [12] | |
| Lagoon soft sediments | SW Pacific | 33 | 0.88 | [45] | |
| Reef sand | SC Pacific | 91 | 7 | 1.07 | [21] |
| Mangroves | mainly Caribbean | 633±117 | 400±83 | 1.4±0.4 | [3] |
| Estuaries | various Atlantic and Pacific | 67±8 | −17±8 | 0.8±0.1 | [3] |
Seagrasses and macroalgae contribute the major fractions to Pg, Pn and R in the Puerto Morelos reef lagoon. Notably, seagrasses account for the largest lagoon-wide shares, although their individual metabolic rates are lower than of the local benthic macroalgae community covering comparable fractions of 2D lagoon area. This apparent inconsistency clearly results from the dominance of seagrasses regarding lagoon-wide photosynthetic 3D surface area, quantified here for the first time by the generation of taxon-specific 2D to 3D conversion factors. However, high seagrass R likewise affects the lagoon-wide Pn shares of all other functional groups, thus revealing the substantial contribution of benthic macroalgae to the production of newly photosynthesised organic matter. This finding supports a recent study describing the dynamic growth of the benthic macroalgae community in this reef lagoon, thereby rapidly spreading over newly available benthic substrate generated by environmental disturbances [27].
Microphytobenthos and scleractinian corals represent the functional groups with low to insignificant contributions to lagoon-wide Pg, Pn and R. The very low 2D and 3D areal cover by scleractinian corals determines their insignificant contribution to lagoon-wide metabolism, despite the fact that individual coral Pg and R rates are among the highest of all functional groups, and comparable to other Caribbean coral taxa investigated at the study site [43]. Likewise, sand-associated microphytobenthos shows the highest individual Pn rates and covers the major fraction of 2D lagoon area, resulting in the third largest share of lagoon-wide Pn. However, this share appears low due to the disproportionally minor contribution by microphytobenthos to the lagoon-wide 3D surface area. In fact, microphytobenthos Pn rates measured at this Caribbean site are substantially higher than described for other shallow coral reef lagoons (Table 3), as in sandy lagoons of the Northern Red Sea [12]. There, microphytobenthos Pg and R are balanced (Pg:R = 0.99), characterising these benthic substrates as largely independent of allochthonous organic matter input, while exhibiting low to no photosynthetic Pn. Interestingly, Pg rates of these Red Sea lagoon sands seem identical to those of the present study site (Table 3), suggesting substantially higher R in Red Sea lagoons, likely sustained by abundant and specialised heterotrophic microbes [46]. Low R of calcareous lagoon sands may indicate a decreased, or regionally variable, intensity of their biocatalytical filter function with potential implications for biogeochemical cycling and the fate of lagoon-wide Pn.
Benthic Pn and Pg:R of this coral reef-associated lagoon are in the range of previous findings for other macrophyte-dominated coastal habitats, such as mangrove forests or seagrass meadows (Table 3), known for their substantial export of photosynthetic excess production. Substantial amounts of Pn (24.3–43.5%) may be exported from macrophyte-dominated benthic communities as detritus, undergo burial processes and microbial decomposition, e.g., in neighbouring beach ecosystems, or enter export to the open ocean [6], [47]–[49]. However, previous work at the study site also indicates that the majority of benthic primary producers, in particular seagrasses and macroalgae, show a constant release of newly photosynthesised Pn in the form of dissolved (DOM) and particulate (POM) organic matter, and that this organic matter stimulates microbial O2 consumption in local lagoon overlying waters [50]. DOM release rates by benthic macroalgae (3.8 mmol C m−2 d−1) account for ca. 26% of mean benthic macroalgae Pn quantified by the present study, thus ranging within margins of current Pn export estimates for macrophyte-dominated benthic communities [6]. Release of DOM and POM with labile biochemical properties and its subsequent decomposition by planktonic microbes may thus initiate short linked benthic-pelagic element cycling between the lagoon compartments similar to that described for sandy Red Sea lagoons [51], [52]. Further integrated laboratory and in situ studies may elucidate the role of this potential trophic pathway and increase our understanding of the biogeochemical processes determining the fate of benthic photosynthetic Pn in reef-associated lagoon environments.
Acknowledgments
We want to thank the staff of the ICML (UNAM), in particular F. Colombo and C. Morera Román, for welcoming us and for logistical support.
Funding Statement
This work was funded by German Research Foundation (DFG) grant Wi 2677/2-1 and Wi 2677/6-1 to C.W., and Bleaching Work Group funding within the CRTR project. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Odum HT, Odum EP (1955) Trophic structure and productivity of a windward coral reef community on Eniwetok Atoll. Ecol Monogr 25: 1415–1444. [Google Scholar]
- 2. Hatcher BG (1988) Coral reef primary productivity: a beggar's banquet. Tree 5: 106–111. [DOI] [PubMed] [Google Scholar]
- 3. Gattuso JP, Frankignoulle M, Wollast R (1998) Carbon and carbonate metabolism in coastal aquatic ecosystems. Annu Rev Ecol Syst 29: 405–434. [Google Scholar]
- 4.Kinsey DW (1983) Standards of performance in coral reef primary production and carbon turnover. In: Barnes DJ, editor. Perspectives on Coral Reefs. Brian Clouston, A.C.T., Australia. pp. 209–220.
- 5. Kinsey DW (1985) Metabolism, calcification, and carbon production: I. Systems level studies. Proc 5th Int Coral Reef Symp 4: 505–526. [Google Scholar]
- 6. Duarte CM, Cebrian J (1996) The fate of marine autotrophic production. Limnol Oceanogr 41: 1758–1766. [Google Scholar]
- 7. Muscatine L, Porter JW (1977) Reef corals-mutualistic symbioses adapted to nutrient-poor environments. Bioscience 27: 454–460. [Google Scholar]
- 8. Hatcher BG (1990) Coral reef primary productivity: a hierarchy of pattern and process. Tree 5: 149–155. [DOI] [PubMed] [Google Scholar]
- 9. Richter C, Wunsch M (1999) Cavity-dwelling suspension feeders in coral reefs a new link in reef trophodynamics. Mar Ecol Prog Ser 188: 105–116. [Google Scholar]
- 10. Bythell JC, Wild C (2011) Biology and ecology of coral mucus release. J Exp Mar Biol Ecol 408: 88–93. [Google Scholar]
- 11. Wanders JWB (1976) The role of benthic algae in the shallow reef of Curacao (Netherlands Antilles) II: Primary productivity of the Sargassum beds on the North-East coast submarine plateau. Aquat Bot 2: 327–335. [Google Scholar]
- 12. Wild C, Naumann MS, Haas A, Struck U, Mayer F, et al. (2009) Coral sand O2 uptake and pelagic-benthic coupling in a subtropical fringing reef, Aqaba, Red Sea. Aquat Biol 6: 133–142. [Google Scholar]
- 13. Duarte CM, Marbà N, Gacia E, Fourqurean JW, Beggins J, et al. (2010) Seagrass community metabolism: Assessing the carbon sink capacity of seagrass meadows. Global Biogeochem Cycles 24: 1–8 DOI:10.1029/2010GB003793 [Google Scholar]
- 14. Falter JL, Atkinson MJ, Schar DW, Lowe RJ, Monismith SG (2011) Short-term coherency between gross primary production and community respiration in an algal-dominated reef flat. Coral Reefs 30: 53–58. [Google Scholar]
- 15. Kinsey DW (1977) Seasonality and zonation in coral reef productivity and calcification. Proc 3rd Int Coral Reef Symp 2: 383–388. [Google Scholar]
- 16. McClanahan T, Muthiga NA (1998) An ecological shift in a remote coralatoll of Belize over 25 years. Environ Conserv 25: 122–130. [Google Scholar]
- 17. Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, et al. (2003) Climate change, human impacts, and the resilience of coral reefs. Science 301: 929–933. [DOI] [PubMed] [Google Scholar]
- 18.Wilkinson C (2008) Status of coral reefs of the world: 2008. Global Coral Reef Monitoring Network and Reef and Rainforest Research Center, Townsville, Australia.
- 19. Odum HT (1957) Primary production measurements in eleven Florida Springs and a marine turtle-grass community. Limnol Oceanogr 2: 85–97. [Google Scholar]
- 20. Cuet P, Atkinson MJ, Blanchot J, Casareto BE, Cordier E, et al. (2011) CNP budgets of a coral-dominated fringing reef at La Reunion, France: coupling of oceanic phosphate and groundwater nitrate. Coral Reefs 30: 45–55. [Google Scholar]
- 21. Boucher G, Clavier J, Hily C, Gattuso JP (1990) Contribution of benthic biomass to overall metabolism in New Caledonia lagoon sediments. Mar Ecol Prog Ser 64: 271–280. [Google Scholar]
- 22. Fourqurean JW, Zieman JC (1991) Photosynthesis, respiration and whole plant carbon budget of the seagrass Thalassia testudinum . Mar Ecol Prog Ser 69: 161–169. [Google Scholar]
- 23. Dahl AL (1973) Surface area in ecological analysis: quantification of benthic coral-reef algae. Mar Biol 23: 239–249. [Google Scholar]
- 24. Naumann MS, Richter C, Mott C, el-Zibdah M, Manasrah R, Wild C (2012) Budget of coral-derived organic carbon in a fringing coral reef of the Gulf of Aqaba, Red Sea. J Mar Syst 105–108: 20–29. [Google Scholar]
- 25. Jordan TP, Merino M, Moreno O, Martin E (1981) Community structure of coral reefs in the Mexican Caribbean. Proc 4th Int Coral Reef Symp 2: 303–308. [Google Scholar]
- 26. Coronado C, Candela J, Iglesias-Prieto R, Sheinbaum J, Lopez M, et al. (2007) On the circulation in the Puerto Morelos fringing reef lagoon. Coral Reefs 26: 149–163. [Google Scholar]
- 27. Van Tussenbroek BI (2011) Dynamics of seagrasses and associated algae in coral reef lagoons. Hidrobiológica 21: 293–310. [Google Scholar]
- 28. Enríquez S, Pantoja-Reyes NI (2005) Form-function analysis of the effect of canopy morphology on leaf self-shading in the seagrass Thalassia testudinum . Oecologia 145: 235–243. [DOI] [PubMed] [Google Scholar]
- 29. Van Tussenbroek BI (1995) Thalassia testudinum leaf dynamics in a Mexican Caribbean reef lagoon. Mar Biol 122: 33–40. [Google Scholar]
- 30. Van Tussenbroek BI, van Dijk JK (2007) Spatial and temporal variability in biomass and production of psammophytic Halimeda incrassata (Bryopsidales, Chlorophyta) in a Caribbean reef lagoon. J Phycol 43: 69–77. [Google Scholar]
- 31. Naumann MS, Niggl W, Laforsch C, Glaser C, Wild C (2009) Coral surface area quantification – evaluation of established methods by comparison with computer tomography. Coral Reefs 28: 109–117. [Google Scholar]
- 32. Carpenter RC, Hackney JM, Adey WH (1991) Measurements of primary productivity and nitrogenase activity of coral reef algae in a chamber incorporating oscillatory flow. Limnol Oceanogr 36: 40–49. [Google Scholar]
- 33. Lesser MP, Weis VM, Patterson MR, Jokiel PL (1994) Effects of morphology and water motion on carbon delivery and productivity in the reef coral, Pocillopora damicornis: diffusion barriers, inorganic carbon limitation, and biochemical plasticity. J Exp Mar Biol Ecol 178: 153–179. [Google Scholar]
- 34. Bruno JF, Edmunds PJ (1998) Metabolic consequences of phenotypic plasticity in the coral Madracis mirabilis: the effect of morphology and water flow on aggregate respiration. J Exp Mar Biol Ecol 229: 187–195. [Google Scholar]
- 35. Mass T, Genin A, Shavit U, Grinstein M, Tchernov D (2010) Flow enhances photosynthesis in marine benthic autotrophs by increasing the efflux of oxygen from the organism to the water. Proc Natl Acad Sci USA 107: 2527–2531 doi:10.1073/pna [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wild C, Naumann MS (2013) Effect of active water movement on energy and nutrient acquisition in coral reef-associated benthic organisms. Proc Natl Acad Sci USA 100: 8767–8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu EY, Barazanji KW, Johnson RLJ (1997) Sources of error in A-aD-O2 calculated from blood stored in plastic and glass syringes. J Appl Physiol 82: 196–202. [DOI] [PubMed] [Google Scholar]
- 38. Naumann MS, Haas A, Struck U, Mayr C, el-Zibdah M, et al. (2010) Organic matter release by the dominant hermatypic corals of the Northern Red Sea. Coral Reefs 29: 649–660. [Google Scholar]
- 39. Collado-Vides L, Ortegon-Aznar I, Comba-Barrera L, Senties Granados A, Gonzalez-Gonzalez J (1998) Macroalgae of Puerto Morelos Reef System, Mexican Caribeean. Hidrobiologia 8: 133–143. [Google Scholar]
- 40.Fourqurean JW, Duarte CM, Kennedy H, Marba N, Holmer M, et al.. (2012) Seagrass ecosystems as a globally significant carbon stock. Nat Geosci 1–5. DOI:10.1038/NGEO1477
- 41.Marba N, Holmer M, Gacia E, Barron C (2006) Seagrass beds and coastal biogeochemistry. In: Larkum AWD, Orth RJ, Duarte C, editors. Seagrasses: biology, ecology and conservation. Springer. pp. 135–57.
- 42.Naumann MS, Haas AF, Jantzen C, Iglesias-Prieto R, Wild C (2012) Benthic-pelagic coupling in a Caribbean reef lagoon affected by hurricane "Dolly". Proc 12th Int Coral Reef Symp Cairns, Australia http://www.icrs2012.com/proceedings/manuscripts/ICRS2012_4C_4.pdf
- 43. Colombo-Pallotta MF, Rodriguez-Roman A, Iglesias-Prieto R (2010) Calcification in bleached and unbleached Montastraea faveolata: evaluating the role of oxygen and glycerol. Coral Reefs 29: 899–907. [Google Scholar]
- 44.Kirk JTO (1983) Light and Photosynthesis in Aquatic Ecosystems. CambridgeUK: Cambridge University Press. 401 p. [Google Scholar]
- 45. Clavier J, Garrigue C (1999) Annual sediment primary production and respiration in a large coral reef lagoon (SW New Caledonia). Mar Eco1 Prog Ser 191: 79–89. [Google Scholar]
- 46. Wild C, Laforsch C, Huettel M (2006) Detection and enumeration of microbial cells within highly porous calcareous reef sands. Mar Freshw Res 57: 415–420. [Google Scholar]
- 47. Duarte CM, Middelburg JJ, Caraco N (2005) Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences 2: 1–8. [Google Scholar]
- 48. Heck KL Jr, Carruthers TJB, Duarte CM, Randall Hughes A, Kendrick G, et al. (2008) Trophic transfers from seagrass meadows subsidize diverse marine and terrestrial consumers. Ecosystems 11: 1198–1210. [Google Scholar]
- 49. Pollard PC, Kogure K (1993) Bacterial decomposition of detritus in a tropical seagrass (Syringodium isoetifolium) ecosystem, measured with (Methyl-super-3H) thymidine. Aust J Mar Freshwat Res 44: 155–172. [Google Scholar]
- 50. Haas A, Jantzen C, Naumann MS, Iglesias-Prieto R, Wild C (2010) Organic matter release by the dominant primary producers in a Caribbean reef lagoon: implication for in situ O2 availability. Mar Ecol Prog Ser 409: 53–60. [Google Scholar]
- 51. Mayer FW, Wild C (2010) Coral mucus release and following particle trapping contribute to rapid nutrient recycling in a Northern Red Sea fringing reef. Mar Freshw Res 61: 1006–1014. [Google Scholar]
- 52. Wild C, Niggl W, Naumann MS, Haas AF (2010) Organic matter release by Red Sea coral reef organisms – potential effects on microbial activity and in-situ O2 availability. Mar Ecol Prog Ser 411: 61–71. [Google Scholar]