Abstract
Background
Thymic stromal lymphopoietin (TSLP) has been reported to activate myeloid dendritic cells (mDCs) to induce Th2 T lymphocyte responses. Its effect on plasmacytoid dendritic cells (pDCs) with TLR ligands has not yet been studied. We investigated the effects of TSLP and TLR ligands on mDCs and pDCs subsets.
Material/Methods
Myeloid dendritic cells (mDC) and plasmacytoid dendritic cells (pDC) were stimulated by TLR ligands (mDC with TLR1/2 LTA, TLR2 PGN, TLR3 poly I: C, TLR4 LPS, TLR5 Flagellin) (pDC with TLR9 CpG2006, CpG 2216, TLR7 loxoribine) in the presence or absence of TSLP. Supernatants from mDCs and pDCs were analyzed for cytokine production. mDCs and pDCs were collected and cultured with allogeneic naïve T cells and after 7 days of co-culture. DC-primed CD4+ T cells were washed and restimulated with PMA and ionomycin. Cytokine production in supernatants from restimulated cells - IL-4, IL-5, IL-10, IL-13, TNF-α was analyzed by Luminex.
Results
TSLP alone induced the expression of maturation markers on mDCs and increased their ability to polarize lymphocytes into the Th2 phenotype. We demonstrated that pDCs also have the capacity to become even more potent inducers of Th2 immune responses, but only after combined treatment with TSLP and TLR ligands, particularly with TLR9 ligand CpG 2006.
Conclusions
TSLP plays a major role in Th2 polarization of immune response mediated by myeloid DCs. Here, we demonstrate that plasmacytoid DCs, exposed to TSLP together with TLR ligands, acquire significant potential towards Th2 polarization.
Keywords: dendritic cell, T cell, allergy, TLR ligand
Background
Thymic stromal lymphopoietin (TSLP) is one of a number of molecules involved in the regulation and polarization of immune reactions [1,2]. TSLP was originally isolated from mouse thymus and characterized as a lymphocyte growth factor [3]. A TSLP homolog was subsequently found in humans, together with its receptor (TSLPR) [4]. Its originally described thymic location and its role in lymphocyte development was confirmed in human studies [5], including its effect on the development of FoxP3+ regulatory T cells of thymic origin [6,7]. Thymic epithelial cells were found to be a major source of TSLP in thymus [6]. Over the last decade, TSLP has attracted major attention, particularly due to its additional and crucial role in Th2 direction of immune response [8]. Due to its importance in the pathogenesis of allergic diseases, the mode of action of TSLP has been studied in detail. It was repeatedly shown that TSLP activity is mediated via its binding to the TSLP receptor preferentially expressed on dendritic cells [9]. Myeloid DCs (mDC) were described as a primary target of TSLP binding [10,11]. TSLP-activated DCs subsequently promote the proliferation and activation of Th2 CD4+ T cells.
While the effect of TSLP on myeloid DCs is well studied [10], much less is know about its influence on other DCs subsets, such as plasmacytoid DCs (pDC), which is of special importance with regard to the proven role of TSLP in allergy. Accumulation of pDCs at the site of allergic reaction, such as allergic inflammation in nasal mucosa, has been described [12]. TSLP is an epithelial-derived molecule and its presence at the site of allergic inflammation such as asthmatic lungs or atopic skin is well documented. Its increased amount directly in nasal mucosa of allergic patients has also recently been described [13]. In the allergic environment, the interaction between pDCs and TSLP is thus inevitable.
Therefore, all DCs populations, including pDCs, participate in a DCs network orchestrating allergic reactions. Given the importance of both TSLP and mDCs/pDCs in regulation the immune response and direction of allergic responses, the mutual interaction of these molecules is of special importance.
For these reasons we studied the effect of TSLP treatment on blood-derived myeloid and plasmacytoid DCs. We analyzed the phenotypic and functional changes after treatment with TSLP. We further studied the T cell stimulatory capacity of TSLP-treated DCs.
To closer mimic the natural microenvironment of allergic reactions, we followed the additional influence of TLR ligands on DCs subpopulations and on subsequent T cell activation.
Material and Methods
Material
Peripheral mononuclear cells were obtained by Ficoll centrifugation of the buffy coats of healthy volunteer donors provided by the Institute of Hematology and Blood Transfusion in Prague. All donors provided signed informed consent.
Separation of blood DC populations
(BDCA-1)+ myeloid DCs were isolated using the CD1c (BDCA-1)+ Dendritic Cell Isolation Kit (Miltenyi). Plasmacytoid DCs were separated using the Plasmacytoid Dendritic Cell Isolation Kit (Miltenyi).
Stimulation of DCs
All cell populations were cultured in RPMI containing 10% fetal bovine serum (FBS), 1% L-glutamine, and 1% penicillin-streptomycin (complete medium).
mDCs were seeded at 0.5×106/ml in flat-bottomed plates in the presence or absence of TSLP (30 ng/ml, R&D Systems), lipopolysaccharide (LPS 1 μg/ml, SIGMA-ALDRICH), POLY (I: C) (POLY (I: C), 50 μg/ml, SIGMA-ALDRICH), lipoteichoic acid (LTA, 2 μg/ml, Invivogen), peptidoglycan (PGN, 5 μg/ml, Invivogen), and Flagellin (1 μg/ml, Invivogen).
pDCs were seeded at 0.5×106/ml in flat-bottomed plates in the presence or absence of TSLP (30 ng/ml, R&D Systems), CpG ODN 2216 (5 μg/ml, Invivogen), CpG ODN 2006 (5 μg/ml, Invivogen), and loxoribine (500 μM, Invivogen) in the presence or absence of IL-3 (10 ng/ml, PEPROTECH).
Flow cytometry
After stimulation, mDCs were collected and stained with CD83 (Beckman), CD86 (BD), OX40-L (Biolegend), TSLPR (Biolegend), and IL-7Rα (Biolegend). pDCs were collected and stained with CD80 (Immunotech), CD86 (BD), ICOS-L (eBioscience), TSLPR (Biolegend), and IL-7Rα (Biolegend). DCs were stained for 30 min at 4°C, washed in PBS and analyzed on FACS Aria (BD Biosciences) using Diva software. Samples were gated according to their FSC and SSC properties, and dead cells were excluded from the analysis. For each experiment, 5000 viable DCs were acquired.
DCs cytokine production
Myeloid DCs and pDCs culture supernatants were collected after 36 h and frozen at −20°C. Supernatants from mDCs were analyzed with ELISA kits for the production of IL-6 (BIOSOURCE) and IL-12p70 (Eli-pair). Supernatants from pDCs were analyzed by Luminex for the production of IL-6 and TNF-α (Millipore) and by ELISA for IFN-α production (BenderMed).
Stimulation of T cells
mDCs and pDCs were collected after 36 h of culture under corresponding conditions, washed twice and cultured with 5×104 CD4+ allogeneic naïve T cells isolated using the Human CD4+ T Cell Enrichment kit (StemCell) in round-bottomed 96-well culture plates. After 7 days of co-culture, DC-primed CD4+ T cells were washed and restimulated with PMA and ionomycin in flat-bottomed 96-well plates at a concentration of 1×106/ml for 24 h. Cytokine production was analyzed by Luminex for intracellular cytokine staining.
Luminex – IL-4, IL-5, IL-10, IL-13, TNF-α (Millipore) was used to analyze T cell cytokine production in supernatants from restimulated cells. For intracellular cytokine analysis, brefeldin A (10 μg/ml) was added at the beginning of the restimulation. The cells were stained with IFN-γ (BD) and CD3 (Exbio) using the FIX and PERM kit (eBioscience).
Statistics
A nonparametric paired t-test was used to compare the phenotype changes and cytokine production of DCs treated or untreated with TSLP. P<0.05 was considered to be significant.
Results
Expression of TSLPR on dendritic cells
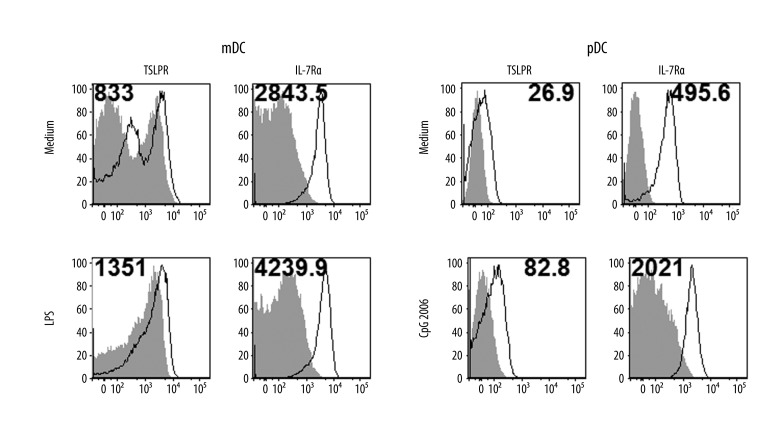
The TSLP receptor is a heterodimer that consists of the common γ-like receptor chain called the TSLP receptor (TSLPR) and IL-7 receptor α chain (IL-7Rα). TSLPR was expressed very weakly, whereas IL-7R was expressed more strongly in unstimulated mDCs, and both were slightly upregulated after LPS stimulation (Figure 1). We detected significant expression of TSLPR and IL-7R in unstimulated pDCs. Stimulation with CpG 2006 further upregulated surface expression of both TSLPR subunits in pDCs
Figure 1.
Expression of TSLPR receptor in dendritic cells. Surface TSLPR and IL-7R expression in myeloid DCs and plasmacytoid DCs was measured by flow cytometry. Cell populations were magnetically separated and in vitro stimulated 24 hours with selected TLR agonists (LPS – lipopolysaccharide, CpG 2006). Open histograms represent staining of TSLPR or IL-7R, shaded histograms represent isotype controls. Mean fluorescence intensity (MFI) of samples substrated with MFI of isotype controls is indicated. Data represent 1 of 3 independent experiments.
Phenotype of dendritic cells stimulated with TSLP and TSLP-TLR agonists
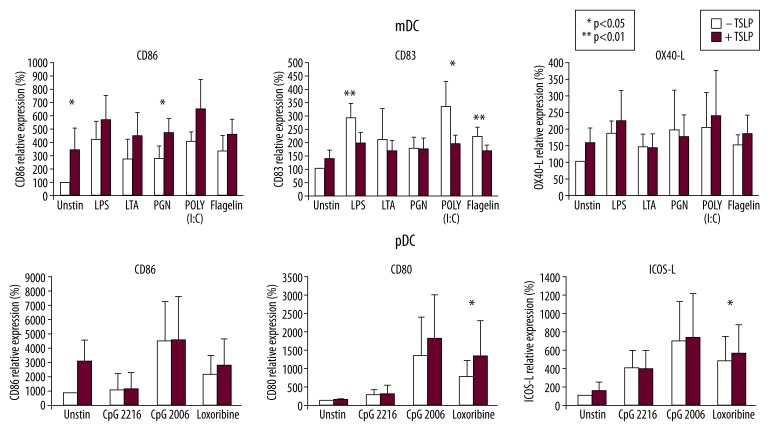
We studied the effect of simultaneous TSLP and Toll-like receptor agonist treatment on all tested DCs subsets. Myeloid and plasmacytoid DCs were stimulated with TSLP alone or TSLP together with TLR agonists. Myeloid DCs were exposed to TSLP or TSLP plus LPS, LTA, PGN, POLY (I: C), and flagellin. As expected, TSLP itself and all TLR agonists upregulated the surface expression of CD86, CD83, and OX40-L in mDCs when compared with medium alone (Figure 2). Additional effects of TSLP to TLR agonists were observed as an increase of CD86 expression in the majority of donors. However, when mDCs were stimulated simultaneously with TLR activators (LPS, POLY (I: C), and flagellin) and TSLP, expression of CD83 was significantly lower when compared to TLR ligands alone (Figure 2).
Figure 2.
TSLP and TSLP with TLR agonists induce phenotype changes in dendritic cells. DC populations were stimulated with TSLP with or without TLR agonists and expression of maturation markers CD86, CD80, CD83, and OX40-L was measured by flow cytometry. Relative expression of analyzed molecules compared to the unstimulated sample is shown. Data were analyzed by paired t test. Statistical significance is indicated as 1 (p<0.05) or 2 asterisks (p<0.01). Myeloid DCs – mean levels from 5 donors, Plasmacytoid DCs – mean levels from 13 donors.
Plasmacytoid DCs were stimulated with TSLP or TSLP plus appropriate TLR ligands, CpG 2216, CpG 2006, and loxoribine. CpG 2006 and loxoribine induced marked upregulation of surface markers. In contrast to mDCs, pDCs did not upregulate surface markers such as CD86, CD80, or ICOS-L after TSLP stimulation alone. However, when combined with TLR agonists, TSLP further upregulated (mainly) CD80 expression.
Cytokine production by DCs activated by TSLP and TSLP plus TLR agonists
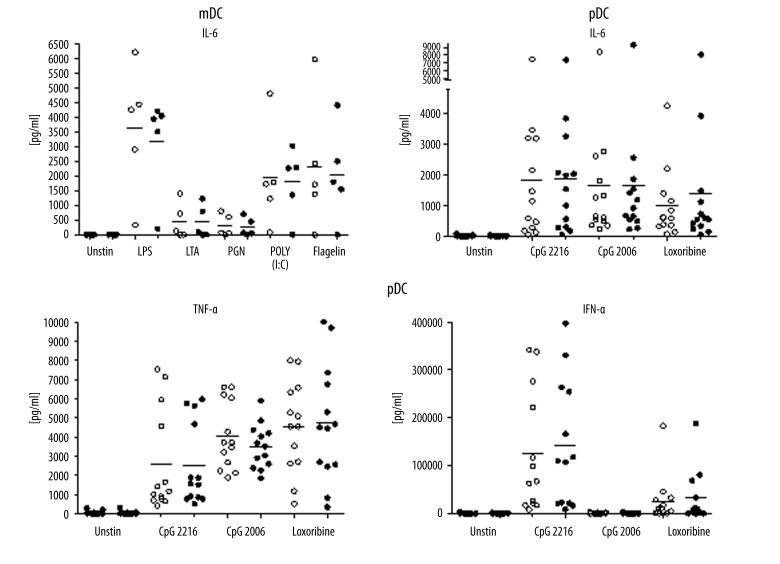
To further characterize the effect of TSLP on DCs, we measured their cytokine production after stimulation with TSLP and TSLP plus TLR agonists. Generally, we observed high inter-individual differences in cytokine production.
Cytokine production was observed only after TLR stimulation. TSLP alone did not induce any measurable cytokine secretion by DCs, and any additional effect of TSLP on TLR induced cytokine production was negligible.
IL-6 was produced after stimulation with all tested TLR agonists. The highest levels of IL-6 were observed after LPS, POLY (I: C), and flagellin stimulation of mDCs (Figure 3). In pDCs, all TLR agonists were able to induce TNF-α and IL-6. Strong production of IFN-α was observed specifically after CpG 2216 activation, without any significant effect of TSLP (Figure 3).
Figure 3.
Cytokine production by DCs activated with TSLP and TSLP with TLR agonists. DC populations were stimulated with TSLP with or without TLR agonists. Concentrations of cytokines were measured in the cell culture supernatants. For statistical analysis, paired t test was used. Horizontal lines represent median values. IL-6 was produced by myeloid DCs in 5 donors. IL-6, TNF-α, and IFN-α were produced by plasmacytoid DCs in 13 donors.
Polarization potential of TSLP stimulated DC
To evaluate how TSLP affects the function of DCs with regard to their Th polarization potential, we performed polarization experiments with both DC populations. Myeloid DCs were tested with TSLP alone or in combination with TLR ligands LPS and POLY (I: C). pDCs were stimulated with TSLP and/or appropriate TLR ligands CpG 2216, CpG 2006, and loxoribine.
mDCs
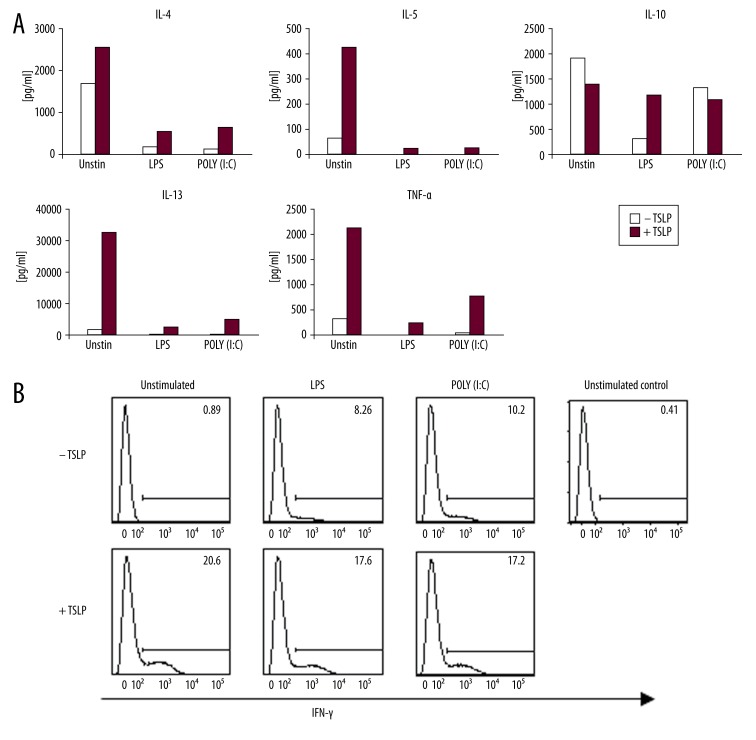
Lymphocytes activated by TSLP-treated mDCs produced more Th2 cytokines IL-4, IL-5, IL-13, TNF-α and slightly less IL-10 than lymphocytes activated by untreated mDCs. Although the effect of TSLP treated mDCs on T lymphocyte polarization was prominent, the effect of TLR ligands in the same situation was only marginal (Figure 4A).
Figure 4.
Cytokine production by allogeneic naïve CD4+ T cells primed for 7 days with TSLP or TSLP+TLR-stimulated myeloid DCs. (A) Production of IL-4, IL-5, IL-10, IL-13, and TNF-α by CD4+ T cells after 24-h restimulation with PMA and ionomycin measured in cell culture supernatant. Data represent 1 of 3 independent experiments. (B) Production of IFN-γ was measured by intracellular staining of T cells after 6-h restimulation with PMA and ionomycin. Data represent 1 of 3independent experiments.
TSLP-stimulated mDCs also induced naïve CD4 T cells to produce IFN-γ irrespective of the TLR agonist’s presence (Figure 4B).
pDCs
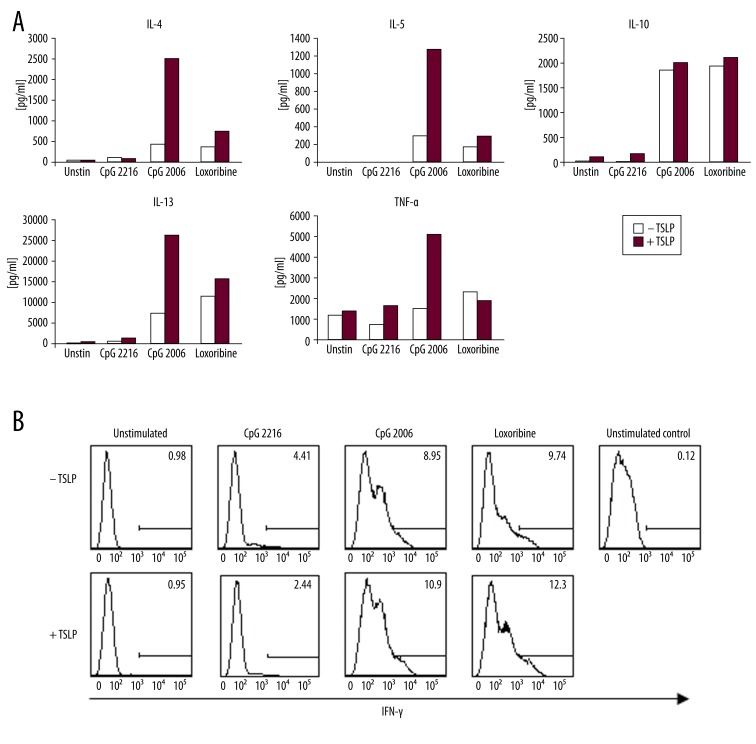
The polarization potential of TSLP and/or TLR ligand-treated pDCs towards T lymphocytes is in stark contrast to the effect of mDCs. Unstimulated or TSLP-stimulated pDCs induced negligible production of cytokines in lymphocytes. The situation dramatically changes when pDCs are exposed to TLR ligands. Plasmacytoid DCs stimulated with CpG 2006 and loxoribine, but not CpG 2216, acquire significant ability to activate lymphocyte production of IL-4, IL-5, IL-13, TNF-α and IL-10. This capacity is markedly augmented by TSLP (Figure 5A). We thus demonstrate strong potential of TSLP-influenced pDCs towards Th2 polarization, which occurs only in synergy with TLR ligands.
Figure 5.
Cytokine production by allogeneic naïve CD4+ T cells primed for 7 days with TSLP or TSLP+TLR-stimulated plasmacytoid DCs. (A) Production of IL-4, IL-5, IL-10, IL-13, and TNF-α by CD4+ T cells after 24-h restimulation with PMA and ionomycin measured in cell culture supernatant. Data represent 1 of 3 independent experiments. (B) Production of IFN-γ was measured by intracellular staining of T cells after 6-h restimulation with PMA and ionomycin. Data represent 1 of 3 independent experiments.
Production of IFN-γ by T lymphocytes influenced by pDCs was measured by intracellular staining. IFN-γ was produced by T cells primed with pDCs activated with CpG 2006 or loxoribine. Its production was further enhanced by the presence of TSLP (Figure 5B).
Discussion
TSLP is a crucial regulator of Th2-driven allergic responses [4,8]. In the search for molecular events underlying the regulatory function of TSLP, DCs were confirmed as a primary target of TSLP function [4,8]. TSLP was described to activate myeloid DCs and induce subsequently naïve T cell differentiation to pro-allergenic effectors. The pro-allergenic role of TSLP-DCs, their importance in Th2 differentiation, and maintenance of allergic reaction is generally accepted. However, there is no consensus on the mechanisms of such TSLP-driven Th2 inflammation. According to initial studies, TSLP does not stimulate mDCs, its primary target, to produce Th1-polarizing cytokine IL-12 [12]. Instead, TSLP triggers DCs to produce a number of chemokines important in the recruitment of eosinophils and neutrophils to augment allergic inflammation [12]. TSLP thus contributes to the formation of an allergic microenvironment in tissues where it also mediates the crosstalk between epithelial cells and DCs [8,12]. The situation, however, is not entirely clear, as there are other reports [13] showing that TSLP-DCs might produce substantial amounts of IL-12 but still maintain a Th2-permissive environment in some circumstances. Allergic microenvironments in target organs are complex milieu in which major players include epithelial cells, dominant producers of TSLP, DCs and T cells, together with triggers of immune reaction permissive for allergic reactions. Such triggers and modifiers, particularly on mucosal sites, might be represented by infection and therefore engagement of TLR ligands. Several reports were recently published on the role of infection-induced TSLP and its influence on DCs and subsequent immune response [14,15].
Thus far, it is unclear how DCs respond to combined simultaneous stimulation by TSLP and TLR ligands. This question was addressed for mDCs showing synergy between TSLP and TLR ligands in Th polarization [16–18]. No studies to date have investigated combined TSLP-TLR ligand engagement of pDCs. We therefore decided to design a study for systematic investigation of the influence of TSLP and/or TLR ligands on DCs. Inn addition to mDCs, we also studied other relevant DC populations, particularly pDCs. Plasmacytoid DCs are major producers of type I interferons, indispensable in viral infection. They are also found in increased numbers at sites of allergic inflammation [9]. Furthermore, the recently described potential of TSLP-activated pDCs in the thymus to generate FOX-p3 regulatory T cells documented the importance of TSLP-pDC interaction [19]. The role of TSLP-pDCs in the periphery in allergic inflammation has not yet been studied. In our experiments we confirmed some basic, already known, effects of TSLP. Both mDCs and pDCs expressed TSLPR and augmented its expression upon relevant stimulation (Figure 1). In agreement with previous reports, TSLP had a direct effect on the phenotype of mDCs and led to an increase of CD86, CD83, and OX40L. All these markers were also increased by TLR ligands. TSLP even augmented TLR ligand-induced upregulation of these markers, with the exception of CD83. TSLP surprisingly and significantly diminished TLR ligand induced CD83 expression (Figure 2). This phenomenon has not yet been described and its significance is currently not clear. In contrast to the known effects (and in our study, the confirmed effects) on mDCs, TSLP did not affect the phenotype of pDCs.
TSLP had no or minimal effect on the direct production of cytokines by DCs. It did not induce the production of any measured cytokine by DCs and had no additional influence on cytokine production induced by TLR ligands (Figure 3).
However, TSLP-DCs showed remarkable effect on naïve CD4+ T cells. CD4+ T cells influenced by TSLP-mDCs produced significant amounts of IL-4, IL-5, IL-13, and TNF-α, and a small amount of IL-10 (Figure 4). In contrast to previous reports, we observed TSLP potential to induce IFN-γ by TSLP-mDCs – primed T cells. Production of IFN-γ by both TSLP-mDC and TSLP-pDC has already been described by others [20]. This observation only documents the very fine regulatory role of TSLP that is specifically dependent on its current inflammatory microenvironment.
Very novel results were observed when naïve CD4+ T were primed with TSLP-pDCs. TSLP-pDCs alone had no effect on T cells. However, the situation dramatically changed with TSLP+TLR ligand-pDCs. pDCs cultured with TSLP and CpG 2006 showed the highest capacity to induce Th2 characteristic cytokines IL-4, IL-5, IL-13 and TNF-α by CD4+ T cells. In contrast, CpG 2216 had no effect on any cytokine production (Figure 5). This is a very interesting observation, which puts pDCs in a central position in TSLP-induced Th2 polarization of immune response. The differential roles of class A and class B oligonucleotides are also noteworthy. Class A CpG-like CpG 2216 are potent inducers of IFN type I by pDCs. In contrast, class B oligonucleotides such as CpG 2006 do not induce type I IFNs, but are potent inducers of proinflammatory cytokines [20]. Indeed, in our experiments we confirmed the various effects of these distinct oligonucleotide classes. While class A CpG 2216 induced high levels of IFN-γ production in pDCs, class B CpG 2006 had no effect in this situation (Figure 3). However, in subsequent steps when TSLP-pDCs polarized T cells towards inflammatory cytokine production, we observed a negligible effect of class A CpG 2216, but a significant effect of class B CpG 2006 (Figure 5). Our results clearly demonstrate the robust synergy between TSLP and TLR ligands and potency of pDCs influenced by both TSLP and CpG 2006 to polarize Th-naïve cells to Th2 phenotype (Figure 5). TSLP-augmented effects of CpG primed pDCs on T cells have not yet been described and are of special importance in allergic inflammation.
Conclusions
We show that both major naturally occurring DC populations – mDCs and pDCs – respond to TSLP and have the potential to polarize T cells towards Th2 phenotype. We further demonstrate the synergistic effect of TSLP and TLR ligands on DCs of both types. We specifically present novel results showing that TSLP-CpG 2006 particularly influenced pDCs to polarize Th cells towards Th2 phenotype.
Footnotes
Statement
We disclose any financial relationship with biotechnology and pharmaceutical firms with an interest in our research.
Source of support: This work was supported by project GAUK 45709 provided by Charles University of Prague, and by project GACR 310/08/H077 provided by the Grant Agency of the Czech Republic
References
- 1.Liu YJ, Soumelis V, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 2.Ziegler SF, Roan F, Bell BD, et al. The biology of thymic stromal lymphopoietin (TSLP) Adv Pharmacol. 2013;66:129–55. doi: 10.1016/B978-0-12-404717-4.00004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friend SL, Hosier S, et al. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol. 1994;22(3):321–28. [PubMed] [Google Scholar]
- 4.Wang YH, Liu YJ. Thymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responses. Clin Exp Allergy. 2009;39(6):798–806. doi: 10.1111/j.1365-2222.2009.03241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang Q, Su H, et al. Delayed functional maturation of natural regulatory T cells in the medulla of postnatal thymus: role of TSLP. BMC Immunol. 2006;7:6. doi: 10.1186/1471-2172-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esnault S, Rosenthal LA, et al. Thymic stromal lymphopoietin (TSLP) as a bridge between infection and atopy. Int J Clin Exp Pathol. 2008;1(4):325–30. [PMC free article] [PubMed] [Google Scholar]
- 7.Li H, Zhao H, Yu J, et al. Increased prevalence of regulatory T cells in the lung cancer microenvironment: a role of thymic stromal lymphopoietin. Cancer Immunol Immunother. 2011;60(11):1587–96. doi: 10.1007/s00262-011-1059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reche PA, Soumelis V, et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. 2001;167(1):336–43. doi: 10.4049/jimmunol.167.1.336. [DOI] [PubMed] [Google Scholar]
- 9.Liu YJ. TSLP in epithelial cell and dendritic cell cross talk. Adv Immunol. 2009;101:1–25. doi: 10.1016/S0065-2776(08)01001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farkas L, Kvale EO, et al. Plasmacytoid dendritic cells activate allergen-specific TH2 memory cells: modulation by CpG oligodeoxynucleotides. J Allergy Clin Immunol. 2004;114(2):436–43. doi: 10.1016/j.jaci.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 11.Zhu DD, Zhu XW, et al. Thymic stromal lymphopoietin expression is increased in nasal epithelial cells of patients with mugwort pollen sensitive-seasonal allergic rhinitis. Chin Med J (Engl) 2009;122(19):2303–7. [PubMed] [Google Scholar]
- 12.Ito T, Liu YJ, Arima K. Cellular and molecular mechanisms of TSLP function in human allergic disorders-TSLP programs the “Th2 code” in dendritic cells. Allergol Int. 2012;61(1):35–43. doi: 10.2332/allergolint.11-RAI-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka J, et al. Human TSLP and TLR3 ligands promote differentiation of Th17 cells with a central memory phenotype under Th2-polarizing conditions. Clin Exp Allergy. 2009;39(1):89–100. doi: 10.1111/j.1365-2222.2008.03151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torii Y, Ito T, Amakawa R, et al. Imidazoquinoline acts as immune adjuvant for functional alteration of thymic stromal lymphopoietin-mediated allergic T cell response. J Immunol. 2008;181(8):5340–49. doi: 10.4049/jimmunol.181.8.5340. [DOI] [PubMed] [Google Scholar]
- 15.Yadava K, Sichelstiel A, Luescher IF, et al. TSLP promotes influenza-specific CD8+ T-cell responses by augmenting local inflammatory dendritic cell function. Mucosal Immunol. 2013;6(1):83–92. doi: 10.1038/mi.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhowmick S, Chatterjee D, Chaudhuri K. Human epithelial cells stimulated with Vibrio cholerae produce thymic stromal lymphopoietin and promote dendritic cell-mediated inflammatory Th2 response. Int J Biochem Cell Biol. 2012;44(11):1779–90. doi: 10.1016/j.biocel.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 17.Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med. 2006;203(2):269–73. doi: 10.1084/jem.20051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanabuchi S, Watanabe N, Liu YJ. TSLP and immune homeostasis. Allergol Int. 2012;61(1):19–25. doi: 10.2332/allergolint.11-RAI-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Zhou X, Zhou B. DC-derived TSLP promotes Th2 polarization in LPS-primed allergic airway inflammation. Eur J Immunol. 2012;42(7):1735–43. doi: 10.1002/eji.201142123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe N, Hanabuchi S, et al. Human TSLP promotes CD40 ligand-induced IL-12 production by myeloid dendritic cells but maintains their Th2 priming potential. Blood. 2005;105(12):4749–51. doi: 10.1182/blood-2004-09-3622. [DOI] [PubMed] [Google Scholar]