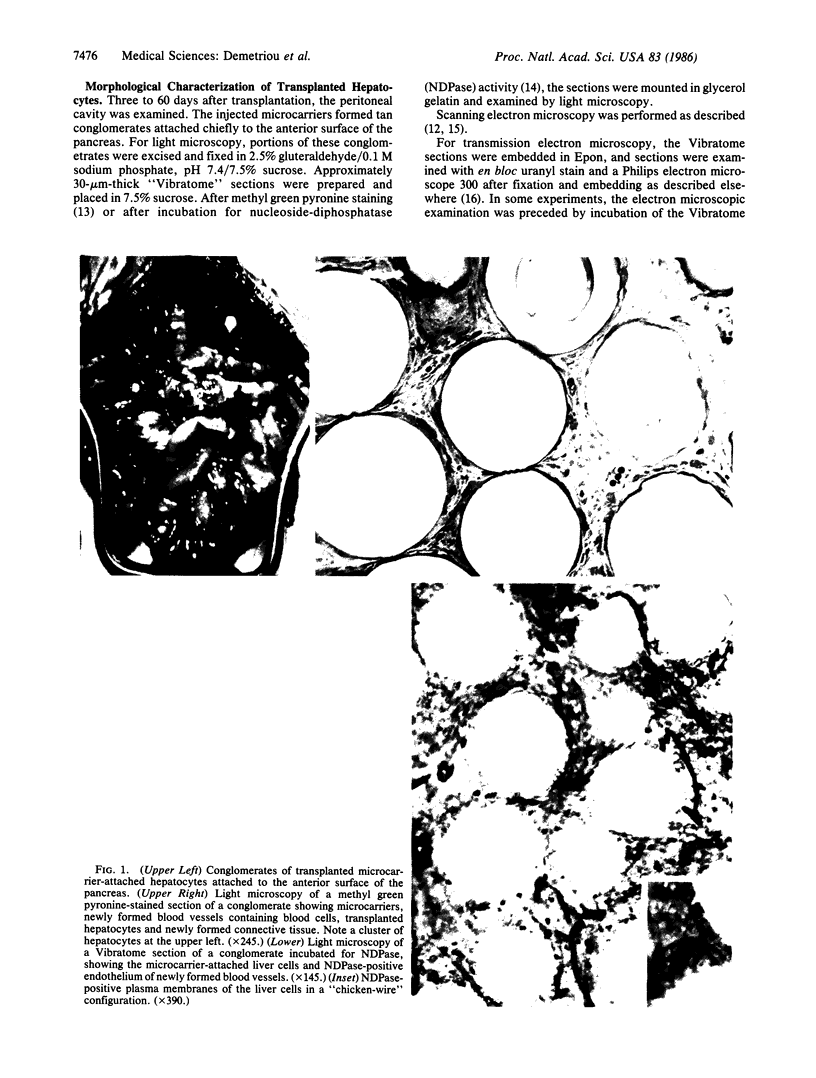
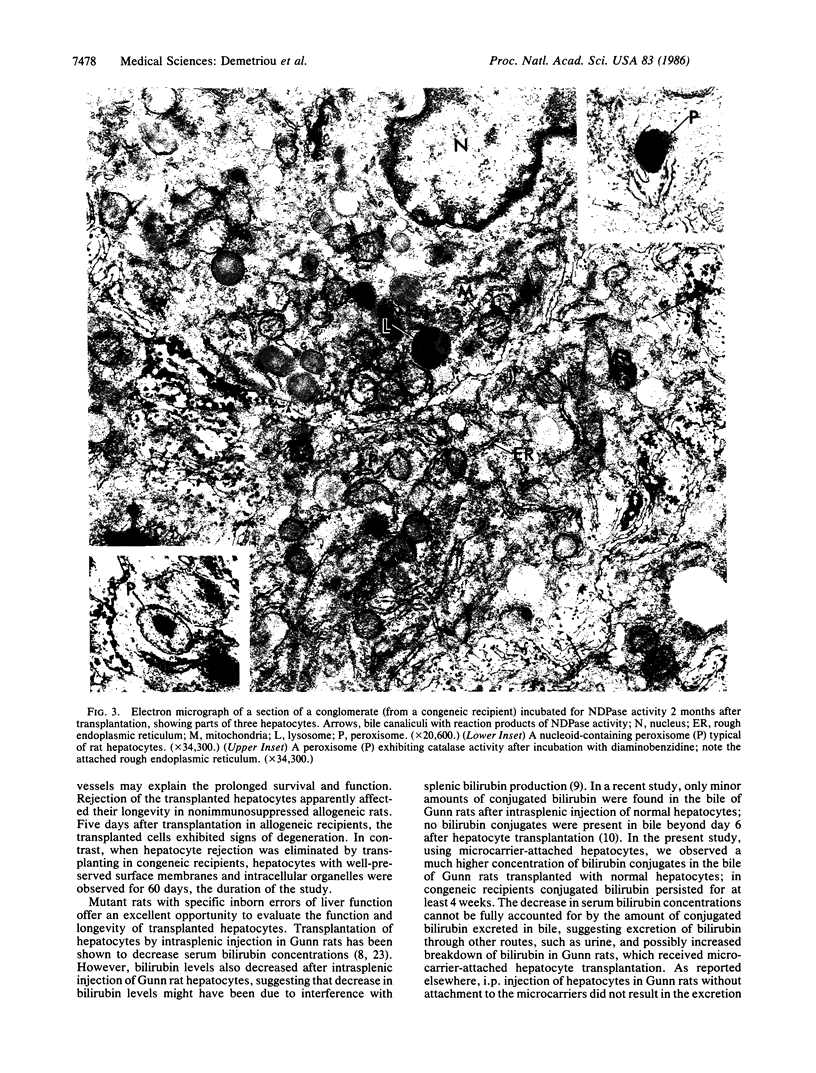
Abstract
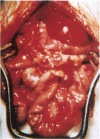
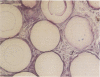
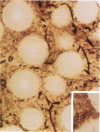
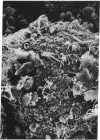
Hepatocytes harvested by collagenase perfusion of rat liver were attached to collagen-coated microcarriers and injected intraperitoneally into congeneic or allogeneic bilirubin-UDP-glucuronosyltransferase (EC 2.4.1.17)-deficient (Gunn) rats or allogeneic analbuminemic (NAR) rats. Five days later, the microcarriers were observed to have formed conglomerates chiefly on the anterior surface of the pancreas. Scanning electron microscopy showed hepatocytes attached to the granular collagen-coated surface of the microcarriers and newly formed connective tissue. Light microscopy revealed that the microcarriers formed a lattice with the collagen tissue; hepatocytes were seen within this lattice or on the surface of the microcarriers. Hepatocyte plasma membranes were nucleoside-diphosphatase (NDPase)-positive. Newly formed blood islands, blood vessels containing erythrocytes and leukocytes and NDPase-positive endothelium were observed in close proximity to the hepatocytes and fibroblasts. Transmission electron microscopic examination showed hepatocytes with microvilli and nucleoid-containing peroxisomes with catalase activity. Hepatocytes were present for up to 2 months in congeneic recipients, the longest period of observation after transplantation. After normal microcarrier-attached hepatocytes were transplanted into allogeneic Gunn rats, bilirubin glucuronides were present in bile for 6 days. When congeneic Gunn rat recipients were used, bilirubin glucuronides were present in bile throughout the study (28 days); this was accompanied by reduction of serum bilirubin concentrations to nearly normal levels. After injection of normal hepatocytes into allogeneic NAR rats, plasma albumin concentration progressively increased for 6 days and then declined. In NAR recipients which were immunosuppressed with cyclosporin A, peak plasma albumin levels were reached in 14 days and persisted nearly at that level throughout the study (28 days).
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgartner D., LaPlante-O'Neill P. M., Sutherland D. E., Najarian J. S. Effects of intrasplenic injection of hepatocytes, hepatocyte fragments and hepatocyte culture supernatants on D-galactosamine-induced liver failure in rats. Eur Surg Res. 1983;15(3):129–135. doi: 10.1159/000128344. [DOI] [PubMed] [Google Scholar]
- Beard M. E., Novikoff A. B. Distribution of peroxisomes (microbodies) in the nephron of the rat: a cytochemical study. J Cell Biol. 1969 Aug;42(2):501–518. doi: 10.1083/jcb.42.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
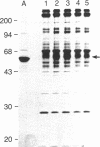
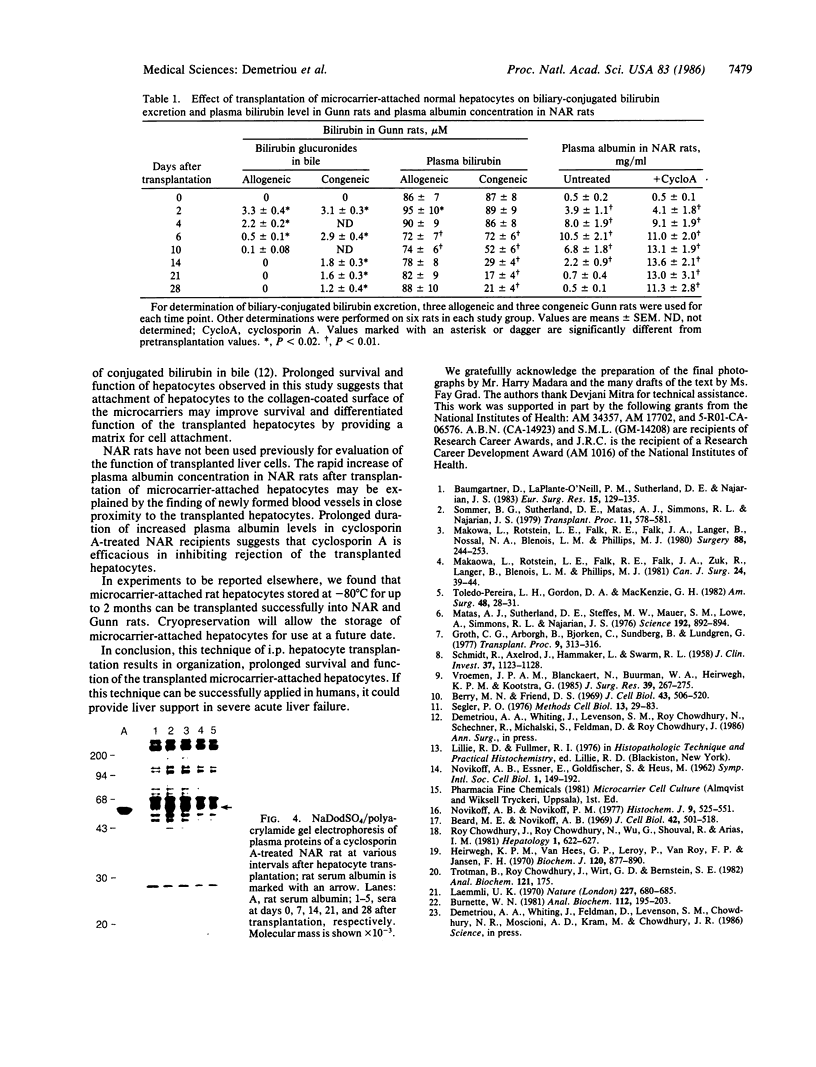
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chowdhury J. R., Chowdhury N. R., Wu G., Shouval R., Arias I. M. Bilirubin mono- and diglucuronide formation by human liver in vitro: assay by high-pressure liquid chromatography. Hepatology. 1981 Nov-Dec;1(6):622–627. doi: 10.1002/hep.1840010610. [DOI] [PubMed] [Google Scholar]
- Groth C. G., Arborgh B., Björkén C., Sundberg B., Lundgren G. Correction of hyperbilirubinemia in the glucuronyltransferase-deficient rat by intraportal hepatocyte transplantation. Transplant Proc. 1977 Mar;9(1):313–316. [PubMed] [Google Scholar]
- Heirwegh K. P., Van Hees G. P., Leroy P., Van Roy F. P., Jansen F. H. Heterogeneity of bile pigment conjugates as revealed by chromatography of their ethyl anthranilate azopigments. Biochem J. 1970 Dec;120(4):877–890. doi: 10.1042/bj1200877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Makowka L., Rotstein L. E., Falk R. E., Falk J. A., Langer B., Nossal N. A., Blendis L. M., Phillips M. J. Reversal of toxic and anoxic induced hepatic failure by syngeneic, allogeneic, and xenogeneic hepatocyte transplantation. Surgery. 1980 Aug;88(2):244–253. [PubMed] [Google Scholar]
- Makowka L., Rotstein L. E., Falk R. E., Falk J. A., Zuk R., Langer B., Blendis L. M., Phillips M. J. Studies into the mechanism of reversal of experimental acute hepatic failure by hepatocyte transplantation. 1. Can J Surg. 1981 Jan;24(1):39–44. [PubMed] [Google Scholar]
- Matas A. J., Sutherland D. E., Steffes M. W., Mauer S. M., Sowe A., Simmons R. L., Najarian J. S. Hepatocellular transplantation for metabolic deficiencies: decrease of plasms bilirubin in Gunn rats. Science. 1976 May 28;192(4242):892–894. doi: 10.1126/science.818706. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M. Cytochemical contributions to differentiating GERL from the Golgi apparatus. Histochem J. 1977 Sep;9(5):525–551. doi: 10.1007/BF01002901. [DOI] [PubMed] [Google Scholar]
- SCHMID R., AXELROD J., HAMMAKER L., SWARM R. L. Congenital jaundice in rats, due to a defect in glucuronide formation. J Clin Invest. 1958 Aug;37(8):1123–1130. doi: 10.1172/JCI103702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Sommer B. G., Sutherland D. E., Matas A. J., Simmons R. L., Najarian J. S. Hepatocellular transplantation for treatment of D-galactosamine-induced acute liver failure in rats. Transplant Proc. 1979 Mar;11(1):578–584. [PubMed] [Google Scholar]
- Toledo-Pereyra L. H., Gordon D. A., MacKenzie G. H. Immunologic response to liver cell allografts. Am Surg. 1982 Jan;48(1):28–31. [PubMed] [Google Scholar]
- Trotman B. W., Roy-Chowdhury J., Wirt G. D., Bernstein S. E. Azodipyrroles of unconjugated and conjugated bilirubin using diazotized ethyl anthranilate in dimethyl sulfoxide. Anal Biochem. 1982 Mar 15;121(1):175–180. doi: 10.1016/0003-2697(82)90572-3. [DOI] [PubMed] [Google Scholar]
- Vroemen J. P., Blanckaert N., Buurman W. A., Heirwegh K. P., Kootstra G. Treatment of enzyme deficiency by hepatocyte transplantation in rats. J Surg Res. 1985 Sep;39(3):267–275. doi: 10.1016/0022-4804(85)90152-0. [DOI] [PubMed] [Google Scholar]