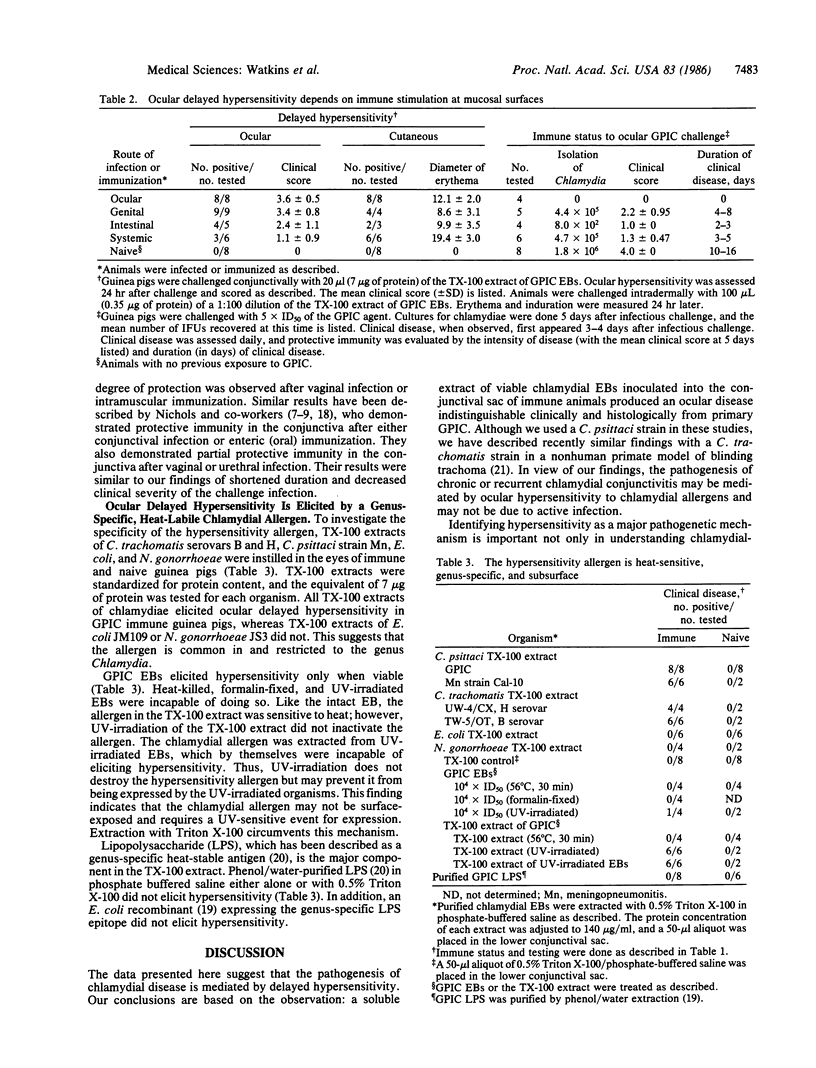
Abstract
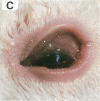
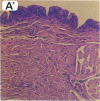
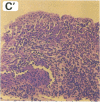
We used a naturally occurring, Chlamydia psittaci-caused eye disease in guinea pigs, guinea pig inclusion conjunctivitis, as an animal model to understand both the immune response and the pathogenesis of chlamydial eye infections. When instilled into the conjunctival sac of guinea pigs that had been previously infected and were immune, viable chlamydiae or a Triton X-100-soluble extract of them produced a short-lived (12-48 hr) eye disease indistinguishable clinically and histologically from that observed during primary chlamydial eye infection. The clinical and histologic findings were consistent with those of ocular delayed hypersensitivity. Ocular delayed hypersensitivity was induced by primary chlamydial infection at mucosal sites other than conjunctival, such as vaginal and intestinal. Preliminary characterization of the hypersensitivity allergen shows that it is heat sensitive and common to the genus Chlamydia. The allergen is apparently not surface-exposed on chlamydiae and requires viable but not replicating organisms for activity. Our observation should be useful in understanding pathogenetic mechanisms of Chlamydia trachomatis-caused infections in humans, in particular those that produce chronic inflammatory diseases, such as blinding trachoma and urogenital diseases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caldwell H. D., Hitchcock P. J. Monoclonal antibody against a genus-specific antigen of Chlamydia species: location of the epitope on chlamydial lipopolysaccharide. Infect Immun. 1984 May;44(2):306–314. doi: 10.1128/iai.44.2.306-314.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darougar S., Jones B. R. Trachoma. Br Med Bull. 1983 Apr;39(2):117–122. doi: 10.1093/oxfordjournals.bmb.a071801. [DOI] [PubMed] [Google Scholar]
- GOGOLAK F. M., ROSS M. R. The properties and chemical nature of the psittacosis virus hemagglutinin. Virology. 1955 Dec;1(5):474–496. doi: 10.1016/0042-6822(55)90038-6. [DOI] [PubMed] [Google Scholar]
- GRAYSTON J. T. SYMPOSIUM ON TRACHOMA. BIOLOGY OF THE VIRUS. Invest Ophthalmol. 1963 Oct;2:460–470. [PubMed] [Google Scholar]
- Grayston J. T., Wang S. P., Yeh L. J., Kuo C. C. Importance of reinfection in the pathogenesis of trachoma. Rev Infect Dis. 1985 Nov-Dec;7(6):717–725. doi: 10.1093/clinids/7.6.717. [DOI] [PubMed] [Google Scholar]
- HILLEMAN M. R., HAIG D. A., HELMOLD R. J. The indirect complement fixation hemagglutination and conglutinating complement absorption tests for viruses of the psittacosis-lymphogranuloma venereum group. J Immunol. 1951 Jan;66(1):115–130. [PubMed] [Google Scholar]
- Howard L. V., O'Leary M. P., Nichols R. L. Animal model studies of genital chlamydial infections. Immunity to re-infection with guinea-pig inclusion conjunctivitis agent in the urethra and eye of male guinea-pigs. Br J Vener Dis. 1976 Aug;52(4):261–265. doi: 10.1136/sti.52.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard L. V., O'Leary M. P., Nichols R. L. Animal model studies of genital chlamydial infections. Immunity to re-infection with guinea-pig inclusion conjunctivitis agent in the urethra and eye of male guinea-pigs. Br J Vener Dis. 1976 Aug;52(4):261–265. doi: 10.1136/sti.52.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lamont H. C., Semine D. Z., Leveille C., Nichols R. L. Immunity to vaginal reinfection in female guinea pigs infected sexually with Chlamydia of guinea pig inclusion conjunctivitis. Infect Immun. 1978 Mar;19(3):807–813. doi: 10.1128/iai.19.3.807-813.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L. W., Holmes K. K., Falkow S. Characterization of plasmid deoxyribonucleic acid from Neisseria gonorrhoeae. Infect Immun. 1974 Oct;10(4):712–717. doi: 10.1128/iai.10.4.712-717.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnickendam M. A., Darougar S., Tilbury A. M. Ocular and dermal delayed hypersensitivity reactions in guinea-pigs following infection with guinea-pig inclusion conjunctivitis agent (Chlamydia psittaci). Clin Exp Immunol. 1981 Apr;44(1):57–62. [PMC free article] [PubMed] [Google Scholar]
- Mount D. T., Barron A. L. Intrarectal infection of guinea pigs with the agent of guinea pig inclusion conjunctivitis. Proc Soc Exp Biol Med. 1976 Dec;153(3):388–391. doi: 10.3181/00379727-153-39552. [DOI] [PubMed] [Google Scholar]
- Nano F. E., Caldwell H. D. Expression of the chlamydial genus-specific lipopolysaccharide epitope in Escherichia coli. Science. 1985 May 10;228(4700):742–744. doi: 10.1126/science.2581315. [DOI] [PubMed] [Google Scholar]
- Nichols R. L., Murray E. S., Nisson P. E. Use of enteric vaccines in protection against chlamydial infections of the genital tract and the eye of guinea pigs. J Infect Dis. 1978 Dec;138(6):742–746. doi: 10.1093/infdis/138.6.742. [DOI] [PubMed] [Google Scholar]
- SIGEL M. M., POLLIKOFF R. Reduction of group reactivity of complement fixing antigen of meningopneumonitis virus by potassium periodate. Proc Soc Exp Biol Med. 1953 Dec;84(3):517–520. doi: 10.3181/00379727-84-20697. [DOI] [PubMed] [Google Scholar]
- Sabet S. F., Simmons J., Caldwell H. D. Enhancement of Chlamydia trachomatis infectious progeny by cultivation of HeLa 229 cells treated with DEAE-dextran and cycloheximide. J Clin Microbiol. 1984 Aug;20(2):217–222. doi: 10.1128/jcm.20.2.217-222.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senyk G., Kerlan R., Stites D. P., Schanzlin D. J., Ostler H. B., Hanna L., Keshishyan H., Jawetz E. Cell-mediated and humoral immune responses to chlamydial antigens in guinea pigs infected ocularly with the agent of guinea pig inclusion conjunctivitis. Infect Immun. 1981 Apr;32(1):304–310. doi: 10.1128/iai.32.1.304-310.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor H. R., Johnson S. L., Prendergast R. A., Schachter J., Dawson C. R., Silverstein A. M. An animal model of trachoma II. The importance of repeated reinfection. Invest Ophthalmol Vis Sci. 1982 Oct;23(4):507–515. [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T., Alexander E. R. Trachoma vaccine studies in monkeys. Am J Ophthalmol. 1967 May;63(5 Suppl):1615–1630. doi: 10.1016/0002-9394(67)94155-4. [DOI] [PubMed] [Google Scholar]
- Watson R. R., MacDonald A. B., Murray E. S., Modabber F. Z. Immunity to chlamydial infections of the eye. 3. Presence and duration of delayed hypersensitivity to guinea pig inclusion conjuctivitis. J Immunol. 1973 Aug;111(2):618–623. [PubMed] [Google Scholar]