Abstract
The biological role of installing a critical exocyclic enone into the structure of the alkaloid, (−)-eburnamonine, and characterization of the new chemical reactivity by quantitative NMR without using deuterated solvents are described. This selective modification to a natural product imparts potent anticancer activity as well as bestows chemical reactivity toward nucleophilic thiols, which was measured by quantitative NMR. The synthetic strategy provides an overall conversion of 40%. In the key synthetic step, a modified Peterson olefination was accomplished through the facile release of trifluoroacetate to create the requisite enone in the presence of substantial steric hindrance.
Keywords: Alkaloid, Anticancer agent, NMR, Covalent, Mechanism of action
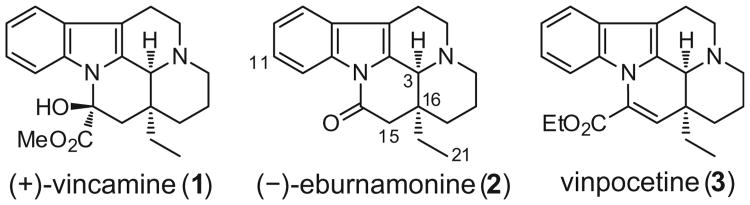
Natural products have long been a source of inspiration for drug discovery, especially in the area of cancer research.1 Indeed, from the period of 1981 to 2010, 75% of all FDA-approved new chemical entities (NCE) for treatment of cancer were either derived from or inspired by natural products.1 One of the richest sources of naturally derived chemotherapies is the Madagascar periwinkle (Catharanthus roseus) which produces the cytotoxic vinca alkaloids, vinblastine and vincristine.2,3 Vindesine and vinorelbine are two semi-synthetic drugs that are built from the naturally occurring scaffolds. Also, the Madagascar periwinkle is a source of the eburnamine-vincamine alkaloids, vincamine (1) and (−)-eburnamonine (2) (Fig. 1).4 These alkaloids are unique because they display their biological activity in the CNS. Another important member of this class of alkaloid is vinpocetine (3) which is a synthetic derivative of 1.4,5
Figure 1.
Structures of the eburnamine alkaloids: (+)-vincamine (1), (−)-eburnamonine (2), and vinpocetine (3).
Although vincamine 1 is generally considered to be the mother compound of these cerebrally active alkaloids, eburnamonine 2 has been shown to improve cerebrovascular disorders in patients.4,6 Moreover, in studies in canines, it can enhance both oxygen and glucose consumption in the brain as well as improve the brain's oxygen supply by increasing vertebral blood flow.4 Additional studies in rats have revealed that eburnamonine 2 can help alleviate learning and memory impairments in different experimental models of amnesia7 and can improve cerebrovascular function.8 Even though the mechanism of action has not been fully elucidated, it has been established that 2 has a relatively high affinity for human muscarinic receptors.9,10
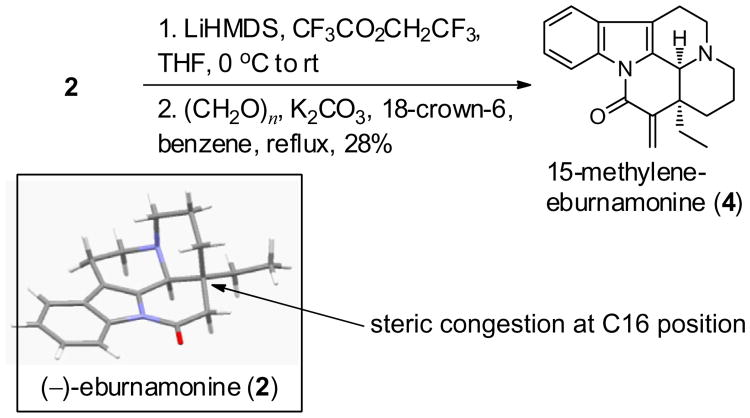
Due to the array of biological activities displayed by these alkaloids, they have been a target of synthetic and medicinal chemists. For example, a total synthesis of (−)-eburnamonine 2 and of (+)-epi-eburnamonine was accomplished by Wee.11 The most efficient total synthesis of racemic eburnamonine was published by Ghosh with an excellent overall yield of 35%.12 Eburnamonine (2) can also be accessed directly from vincamine (1) using a one-pot two-step procedure13 which was later optimized.14 Analogues of eburnamonine have been prepared by hydroxylation,15,16 trifluoromethylation,17 and nitration,16 but biological evaluations of these derivatives have not been reported. Amide derivatives of 2 at the C11-position have been synthesized and were investigated as protective agents against tin-induced brain edema.18 More recently, vinpocetine analogues were prepared and examined as potential antioxidants and cognitive enhancing agents.5 In 2011, Colby and co-workers synthesized 15-methylene-eburnamonine (4) by using an α-methylenation strategy that is promoted by the release of trifluoroacetate (Scheme 1).19 Analogue 4 displays antiproliferative activity in HL-60 (human leukemia) and cytotoxicity in MDA-MB-231 (breast cancer) cells (LC50 = 14.1 μM); however, the parent natural product 2 is nearly inactive in both cell lines. Even though the conversion of eburnamonine 2 into 15-methylene-eburnamonine 4 was accomplished in two steps, the yield was quite low (i.e., 28%). The steric congestion at the C16 position of 2 is apparent in its X-ray structure20 and prevents the installation of the α-methylene group using many other standard protocols; therefore the trifluoroacetate-release olefination approach was required to access the target 4. In order to conduct a more extensive biological evaluation of 4 in malignant cells as well as define its mechanism of action, an improved synthesis was clearly needed. Herein, we describe an efficient and scalable synthesis of 4 from 1 that enabled an investigation of its antiproliferative in additional cell lines and characterization of its mechanism of action by quantitative NMR.
Scheme 1.
Prior synthesis of 15-methylene-eburnamonine (4) from (−)-eburnamonine (2) and X-ray structure of the latter.
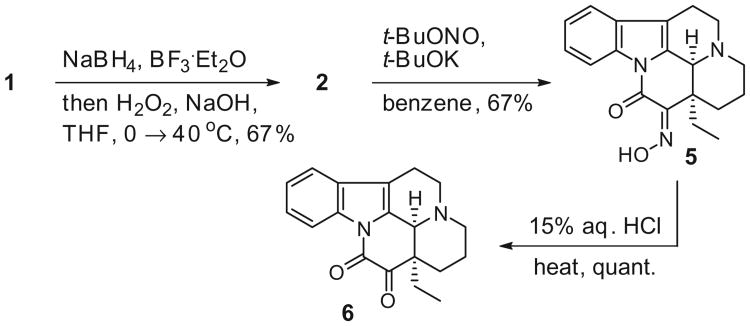
Even though the target 4 was originally synthesized from eburnamonine 2, a more plentiful starting material was required to enable the production of larger quantities of 4; therefore, the aforementioned one-pot two-step synthesis from vincamine 1 was selected (Scheme 2).13 Accordingly, the alkaloid 1 was reduced with NaBH4 and BF3·OEt2 at 0 °C and then treated with a mixture of aqueous H2O2 and NaOH. Following purification, gram quantities of eburnamonine 2 were accessed. Next, 2 was transformed into oxime 5 (as a pair of rotamers) using t-BuOK and t-BuONO by the literature protocol.21 Acid-promoted hydrolysis with 15% aqueous HCl at 110 °C provided the known dione 6 in 64% yield (from 2).21 All of the new and previously reported compounds were characterized by 1H NMR, 13C NMR, IR, HRMS, and optical rotation.
Scheme 2.
Synthesis of the dione 6 from (+)-vincamine (1).
Studies to convert the dione 6 into the target 4 were undertaken using a series of Wittig reactions that are listed in Table 1. Excess quantities of t-BuOK and PPh3CH3I were used in all of the reactions to promote this olefination process, but regardless of temperature, base, or solvent, only unreacted starting material was isolated. These results were disappointing because high temperatures can aid in the olefination of sterically hindered carbonyl groups using Wittig reactions. Next, titanium methylene complexes were screened,22 and only the highly reactive Tebbe reagent could overcome this critical issue of steric congestion at the C16 position and produce 4 in an optimized 16% yield.
Table 1. Conversion of the dione 6 to 15-methylene-eburnamonine (4).
|
| ||||
|---|---|---|---|---|
|
| ||||
| Reagent | Temp | Solvent | Yielda (%) | |
| PPh3MeI, t-BuOK | rt | THF | 0 | |
| PPh3MeI, t-BuOK | 80 °C | Benzene | 0 | |
| PPh3MeI, t-BuOK | 110 °C | Toluene | 0 | |
| Mg, TiCl4, CH2Cl2 | 0 °C → rt | THF | 0 | |
| Zn, TiCl4, CH2I2, PbI2 | rt → 40 °C | THF/CH2Cl2 | 0 | |
| Zn, TiCl4, CH2I2 | rt → 40 °C | THF/CH2Cl2 | Trace | |
| Tebbe | 0 °C | THF | 16 | |
Isolated yield.
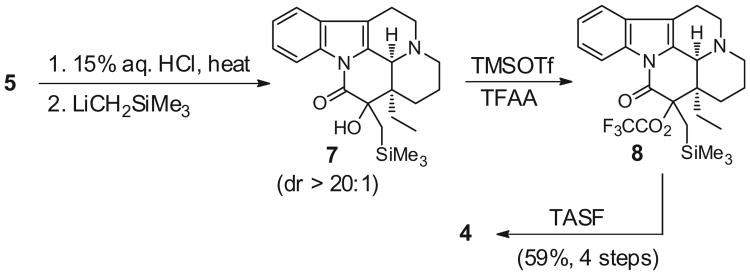
A Peterson-type methylenation was then selected and trimethylsilylmethyl lithium was reacted with the ketone 6 to give the stable tertiary alcohol 7 (Scheme 3).23 Next, protodesilylation/elimination was attempted using many conditions, but this approach was not successful. For example, the alcohol 7 was completely inert to BF3·OEt2 and merely desilylated following exposure to TFA Therefore, the sterically encumbered tertiary alcohol 7 was trifluoroacetylated to 8 with TFAA and TMSOTf,24 which were required over the less-reactive, traditional mixture of TFAA with DMAP. The intermediate trifluoroacetyl ester 8 was treated with TASF to liberate the silyl group and eliminate trifluoroacetate; the target 15-methylene-eburnamonine 4 was finally accessed. The four-step sequence from the oxime 5 provides the product 4 in 59% yield.25 Although the highest yields in the final step were obtained with TASF, this reagent was replaced with CsF in DMF on a larger scale with only a modest decrease of isolated yields. The overall yield from vincamine 1 is 40%. Accordingly, we have synthesized up to 115 mg of 15-methylene-eburnamoine 4 for biological and mechanistic evaluations.
Scheme 3.
Four-step synthesis of 15-methylene-eburnamonine (4) from oxime 5.
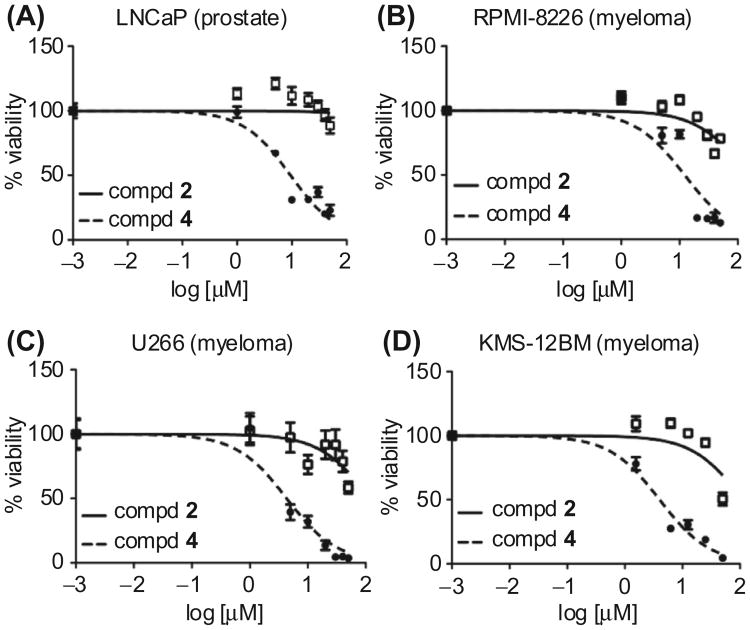
15-Methylene-eburnamonine 4 and eburnamonine 2 were tested side-by-side for cytotoxic activity in four additional cell lines: LNCaP (prostate cancer), RPMI-8226 (multiple myeloma), U266 (multiple myeloma), and KMS-12BM (multiple myeloma) cells (Table 2).26 Our rationale for choosing these additional cell lines is to compare prostate cancer cells to breast cancer cells and apply our prior discovery that enone-based anti-leukemic agents are also active against multiple myeloma cell lines.27 Agent 4 displays micromolar activity against the four cell lines and the natural product 2 is nearly inactive even at the high dose of 100 μM. Compound 4 displays higher cytotoxicity in prostate and myeloma cells compared to MDA-MB-231 (breast cancer) cells.19 Its most potent effects were LC50 = 4.3 μM (U266) and LC50 = 4.0 μM (KMS-12BM), and these low micromolar activities were an excellent validation of the aforementioned synthetic studies. The cell viability curves for four cancer cell lines are depicted in Figure 2 and present additional perspective on the side-by-side comparisons between 2 and 4. These viability curves, along with the previously published curves in MDA-MB-231 cells,19 display the repeated difference between the two agents across the five cells in that only compound 4 eliminates cancer cells in the low micromolar range. An additional antiproliferative study was conducted with agent 4 in an immortalized non-cancerous cell line to examine its potential selectivity for cancer cells. Indeed, highly selective killing of cancer cells has been demonstrated by the natural product, piperlongumine, which bears a similar α,β-unsaturated amide.28 The LC50 value of 4 in FnMSC cells, an immortalized mesenchymal stem cell line derived from the bone marrow, is 16.71 μM.29 The direct comparison of this data to cancer cells is to the LC50 value of 4.0 μM in KMS-12BM cells, and these data demonstrate decrease in potency in the non-cancerous cells compared to cancer cells.
Table 2. Biological evaluation in MDA-MB-231 (breast cancer), LNCaP (prostate cancer), RPMI-8226 (myleoma), U266 (myeloma), and KMS-12BM (myeloma) cell lines.
| Cell line | LC50 (μM)a | |
|---|---|---|
|
| ||
| (−)-Eburnamonine | 15-Methylene-eburnamonine (4) | |
| MDA-MB-231 | 41.8 (30–58) | 14.1 (10–17) |
| LNCaP | >100 | 9.5 (7.9–11.4) |
| RPMI-8226 | >100 | 12.2 (9.3–16.0) |
| U266 | >100 | 4.3 (3.1–6.0 |
| KMS-12BM | >100 | 4.0 (3.3–4.8) |
Values are given with 95% confidence interval in parentheses.
Figure 2.
Cell viability curves for (−)-eburnamonine (2) and 15-methylene-eburnamonine (4). (A) LNCaP cells. (B) RPMI-8226 cells. (C) U266 cells. (D) KMS-12BM cells.
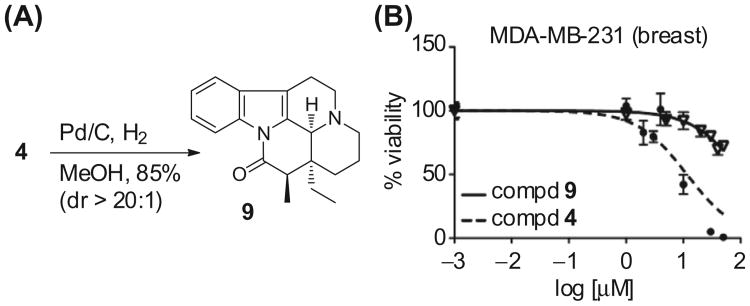
The incorporation of an α-methylene group on eburnamonine 2 (thus forming 4) clearly imparted cytotoxic activity against the cancer cell lines. It is well known that α,β-unsaturated carbonyl groups may produce cytotoxic effects following a Michael addition from nucleophiles such as biological thiols.30 To define the structure–activity relationship of the enone of 4, the α,β-unsaturation was reduced with Pd/C and H2 and analogue 9 was produced in 85% yield as a single diastereomer (Fig. 3).31 The axial assignment of the stereochemical configuration at the C15-site (eburnamonine numbering, see Fig. 1) was accomplished after acquisition of COSY and NOESY. On derivative 9, the proton at the C15 position is a quartet (J = 7.0 Hz) at 2.83 ppm and displays a NOESY crosspeak with the axial methine proton at the C3 position. This proton (see also Scheme 1) is a singlet at 4.16 ppm and exhibits a key NOESY crosspeak with the axial C21 methyl group (t, J = 7.5 Hz) at 0.98 ppm. These data support the axial assignment, because each is on the same face of the molecule. Next, a comparison of the cytotoxic effects of 9 and 4 in MDA-MB-231 cells demonstrates the expected loss of biological activity from removing the unsaturation at C15-position (Fig. 3B). These data validate the critical nature of the enone and this important structure–activity relationship.
Figure 3.
Structure–activity investigation of the enone group of 4. (A) Reduction of enone 4 to derivative 9. (B) Biological evaluation of 4 and 9 in MDA-MB-231 (breast) cancer cells.
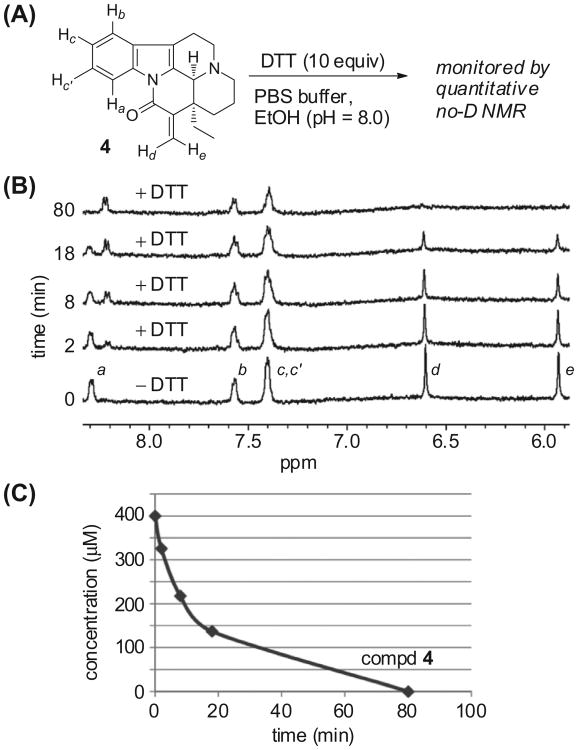
To establish the reactivity of the enone towards thiol-based nucleophiles,32 quantitative NMR studies were conducted.30,33 Quantitative NMR is a powerful analytical tool, and although the utility of NMR for structure determination is well established, its potential application in quantitation has not been fully appreciated in bio-organic and medicinal chemistry. Specifically, almost all molecules readily generate unique NMR signals, and the amplitude (integration) of NMR signals can be acquired proportional to the concentration. This technique eliminates the requirement of an internal standard to calibrate concentration. Knowing the exact concentration of a biologically active molecule and calculating the change in concentration in relationship to time provides valuable kinetic data. This point is fundamental in the reactivity of enones towards thiol-based nucleophiles, because the structure of the enone and the nature of the solvent can cause substantial differences in reactivity.32 Dithiothreitol (DTT) was chosen as a representative thiol for investigation with 15-methylene-eburanmonine 4 without any deuterated solvent.34 Accordingly, in an NMR tube, DTT (10 equiv) was added in one portion to a solution of 4 in 80% PBS buffer (pH 8.0) and 20% ethanol. The NMR sample was immediately placed in the spectrometer and spectra were acquired at intervals of 2, 8, 18, and 80 min. (Fig. 4). The 1H NMR peaks corresponding to the methylene groups disappeared after the addition of DTT; these data indicate that the thiol was adding into the enone. The changes in the concentration of 4 were simultaneously quantified by NMR at each time interval using our previously published no-D NMR methods (Fig. 4B).33,35 At t = 0 min, the concentration of 4 was 400 μM. Two minutes after treatment with DTT, the concentration of 4 decreased to 330 μM. After 18 min had elapsed, the concentration of 4 was 220 μM, and after 80 min, the compound was not detected (Fig. 4C).
Figure 4.
Nucleophilic addition of DTT to 15-methylene-eburnamonine 4. (A) Addition of DTT (10 equiv) to 4 in PBS buffer and 20% EtOH. (B) No-D NMR spectra (8.4–5.9 ppm) at 0 min (no DTT) and time-course across 80 min in the presence of DTT. (C) Quantitative concentration measurements of 4 by NMR.
These studies demonstrate the utility of installing an exocyclic enone into the structure of a natural product to impart anticancer activity as well as chemical reactivity toward nucleophilic thiols. The application of these structure–activity data is a key one, because biological activity (i.e., micromolar levels) can be efficiently realized. Also, a modified Peterson olefination was accomplished through the facile release of trifluoroacetate to create the requisite enone in the presence of substantial steric hindrance. Moreover, exploiting the release of trifluoroacetate enabled this selective modification to the structure of the natural product, eburnamonine. Lastly, the robustness and simplicity of quantitative analysis by NMR, which can be done readily without the use of deuterated solvents, were demonstrated by in situ monitoring of the consumption of reactant and formation of product. This NMR approach can gather mechanistic and kinetic data simultaneously, and presents a unique opportunity to examine complex systems at the interface of chemistry and biology.
Supplementary Material
Acknowledgments
Support for this work was provided by Purdue University and the Purdue Center for Cancer Research and by the National Institutes of Health, National Cancer Institute R25CA128770 Cancer Prevention Internship Program (M. O'Banion and M. M. Zheng), administered by the Oncological Sciences Center and the Discovery Learning Research Center at Purdue University.
Footnotes
Supplementary data: Supplementary data associated with (1H and 13C NMR, COSY, and NOESY spectra) this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmcl.2013.08.095.
References and notes
- 1.Newman DJ, Cragg GM. J Nat Prod. 2012;75:311. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roepke J, Salim V, Wu M, Thamm AMK, Murata J, Ploss K, Boland W, Luca VD. Proc Natl Acad Sci U S A. 2010;107:15287. doi: 10.1073/pnas.0911451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishikawa H, Colby DA, Seto S, Va P, Tam A, Kakei H, Rayl TJ, Hwang I, Boger DL. J Am Chem Soc. 2009;131:4904. doi: 10.1021/ja809842b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vas A, Gulyás B. Med Res Rev. 2005;25:737. doi: 10.1002/med.20043. [DOI] [PubMed] [Google Scholar]
- 5.Nemes A, Czibula L, Szántay C, Jr, Gere A, Kiss B, Laszy J, Gyertán I, Szombathelyi Z, Szántay C. J Med Chem. 2008;51:479. doi: 10.1021/jm070618k. [DOI] [PubMed] [Google Scholar]
- 6.Manna V, Conti L, Agnoli A. Curr Ther Res. 1982;32:740. [Google Scholar]
- 7.Drago F, Grassi M, Valerio C, Spadaro F, D'Agata V, Lauria N. Pharmacol Biochem Behav. 1990;37:53. doi: 10.1016/0091-3057(90)90040-o. [DOI] [PubMed] [Google Scholar]
- 8.Louajri A, Harraga S, Toubin G, Kantelip JP. Biol Pharm Bull. 1999;22:773. doi: 10.1248/bpb.22.773. [DOI] [PubMed] [Google Scholar]
- 9.Maksay G, Bíró T, Kiss B. Eur J Pharmacol. 2004;483:229. doi: 10.1016/j.ejphar.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 10.Jakubík J, Bačáková L, El-Fakahany EE, Tuček S. Mol Pharmacol. 1997;52:172. doi: 10.1124/mol.52.1.172. [DOI] [PubMed] [Google Scholar]
- 11.Wee AGH, Yu Q. J Org Chem. 2001;66:8935. doi: 10.1021/jo010751z. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh AK, Kawahama R. J Org Chem. 2000;65:5433. doi: 10.1021/jo000507s. [DOI] [PubMed] [Google Scholar]
- 13.Nárai G, Sohár P, Csámpai A, Zsadon B. Heterocycles. 1998;48:151. [Google Scholar]
- 14.Nemes A, Szántay C, Czibula L, Greiner I. Heterocycles. 2007;71:2347. [Google Scholar]
- 15.Adachi T, Saito M, Sasaki J, Karasawa Y, Araki H, Hanada K, Omura S. Chem Pharm Bull. 1993;41:611. doi: 10.1248/cpb.41.611. [DOI] [PubMed] [Google Scholar]
- 16.Sarlet P, Hannart J. Bull Soc Chim Belg. 1979;88:93. [Google Scholar]
- 17.Debarge S, Kassou K, Carreyre H, Violeau B, Jouannetaud MP, Jacquesy JC. Tetrahedron Lett. 2004;45:21. [Google Scholar]
- 18.Akendengue B, Uriac P, Huet J. Eur J Med Chem. 1987;22:511. [Google Scholar]
- 19.Riofski MV, John JP, Zheng MM, Kirshner J, Colby DA. J Org Chem. 2011;76:3676. doi: 10.1021/jo102114f. [DOI] [PubMed] [Google Scholar]
- 20.Toh-Seok T, Poh-Suan T, Chen W. Phytochem. 1993;33:921. [Google Scholar]
- 21.Sápi J, Szabó L, Baitz-Gács E, Kalaus G, Szántay C, Karsai-Bihátsi E. Liebigs Ann Chem. 1985:1794. [Google Scholar]
- 22.Yan TH, Tsai CC, Chien CT, Cho CC, Huang PC. Org Lett. 2004;6:4961. doi: 10.1021/ol0478887. [DOI] [PubMed] [Google Scholar]
- 23.Tertiary alcohol 7. To a solution of crude (−)-eburnane-14,15-dione (6) (9.5 mg, 0.031 mmol) in THF (35 μL) was added (trimethylsilyl)methyllithium (160 μL, 1.0 M in pentanes) at −78 °C. After 3 h at −78 °C, saturated NH4Cl (2 mL) was added, and the resulting mixture was extracted with CH2Cl2 (3 × 10 mL). The organics were dried over Na2SO4 and concentrated under reduced pressure. Preparatory TLC (5:1 toluene/methanol) afforded the tertiary alcohol 7 as a white solid (7.9 mg) in 64% yield: mp 125-126 °C; 1H NMR (500 MHz CDCl3) δ 8.26 (d, J = 8.1 Hz, 1H), 7.45 (d, J = 7.2 Hz, 1H), 7.37–7.29 (m, 2H), 3.97 (s, 1H), 3.78 (s, 1H), 3.39–3.24 (m, 2H), 2.89 (m, 1H), 2.58–2.50 (m, 2H), 2.30 (m, 1H), 2.22 (td, J = 12.6, 2.8 Hz, 1H), 2.02 (d, J = 14.0 Hz, 1H), 1.89 (dq, J = 15.3, 7.6 Hz, 1H), 1.67 (m, 1H), 1.48 (d, J = 14.6 Hz, 1H), 1.39 (d, J = 13.4 Hz, 1H), 1.06 (t, J = 7.7 Hz, 3H), 1.01 (d, J = 14.5 Hz, 1H), 0.55 (td, J = 13.4, 3.5 Hz, 1H), 0.05 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 174.1, 134.3, 130.9, 130.4, 124.6, 124.2, 118.5, 116.2, 113.4, 81.2, 58.1, 50.5, 46.1, 44.3, 29.7, 23.8, 22.9, 20.7, 17.0, 9.1, 0.3 (3C); IR(film) νmax3480, 1695, 1626, 1457, 1329 cm−1; HRMS (EI) m/z calcd for C23H32N2O2Si (M+H)+ 397.2311 found, 397.2303; −20.4° (c 0.23, CHCl3).
- 24.Procopiou PA, Baugh SPD, Flack SS, Inglis GGA. J Org Chem. 1998;63:2342. [Google Scholar]
- 25.15-Methylene-eburnamonine 4. Crude 15-(hydroxyimino)eburnan-14-one 5 (48 mg, 0.15 mmol) was stirred in an aqueous 15% HCl solution (8.2 mL) at 110 °C for 2 h. The solution was cooled to rt and concentrated NH4OH was added until pH 9.0. The alkaline solution was extracted with CH2Cl2 (3 × 10 mL). The organics were dried with Na2SO4 and concentrated under reduced pressure. A portion of the crude product (18.9 mg, 0.061 mmol) was suspended in THF (700 μL) and TMSCH2Li (0.074 mmol, 120 μL) was added at rt. The mixture stirred for 1 h at rt. Next, the solution was quenched with saturated NH4Cl (3 mL) and extracted with CH2Cl2 (3 × 10 mL). The organics were dried over Na2SO4 and concentrated under reduced pressure. The crude product was suspended in dry CH2Cl2 (800 μL) and cooled to 0 °C in an ice bath. Then, TFAA (0.061 mmol, 86 μL) was added followed by TMSOTf (0.061 mmol, 11 μL). The reaction vessel was immediately removed from the ice bath and stirred for 1 h at rt. The reaction mixture was concentrated under reduced pressure and the resulting brown oil was dissolved into THF (2 mL). This solution was added in three portions to a separate flask containing TASF (0.061 mmol, 168 mg). The resultant mixture was heated to reflux for 90 min, cooled to rt and extracted with CH2Cl2 (3 × 10 mL). The organics were dried with Na2SO4 and concentrated under reduced pressure. Purification by SiO2 PTLC (5:1 toluene/methanol) afforded 4 as a yellow oil (11.1 mg) in 59% yield. All characterization data were identical with the reported data in Ref. 19.
- 26.Cell culture. MDA-MB-231, U266, and RPMI-8226 cells were obtained from ATCC. KMS-12BM cells were a kind gift from Dr. Linda Pilarski (University of Alberta). LNCaP cells were a generous gift from Dr. Scott Crist (Purdue University). MDA-MB-231 cells were cultured in DMEM medium supplemented with 10% FBS, and 1% penicillin/streptomycin. U266, RPMI-8226, KMS-12BM, and LNCaP cells were cultured in RPMI-1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin. All cell lines were incubated at 37 °C in a humidified 5% CO2 tissue culture incubator. Antiproliferative assay. MDA-MB-231 cells were seeded at 20,000 cells/well in a 96 well plate. U266, RPMI-8226, and KMS-12BM cells were seeded at 25,000 cells/well, and LNCaP cells were seeded at 5000 cells/well. Cells were treated with either DMSO vehicle control or varying concentrations of drugs for 96 h. MTS assay was performed using CellTiter 96 AQueous Non-radioactive Cell Proliferation Assay (MTS) assay kit (Promega) per manufacturer instructions. The OD was read at 492 nm on a Multiscan Ascent plate reader. Data are presented as mean ± SEM of at least three independent experiments performed in triplicate. LC50 values were calculated by four-parameter logistic regression applied to log-transformed OD readings normalized to the signal from the vehicle control. Significance was analyzed by a two-way analysis of variance (ANOVA) and Bonferroni's correction as post-test to compare experiments with multiple parameters. P-values below 0.05 were considered statistically significant. All analyses were performed using Prism 5.0 software by GraphPad Inc. See also Ref. 19.
- 27.Gunn EJ, Williams JT, Huynh DT, Iannotti MJ, Han C, Barrios FJ, Kendall S, Glackins CA, Colby DA, Kirshner J. Leuk Lymphoma. 2011;52:1085. doi: 10.3109/10428194.2011.555891. [DOI] [PubMed] [Google Scholar]
- 28.Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF, Stern AM, Mandinova A, Schreiber SL, Lee SW. Nature. 2011;475:231. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Non-malignant, immortalized bone marrow mesenchymal stem cell line (FnMSC cells), a kind gift from Dr. Carlotta Glackin (Beckman Research Institute, City of Hope National Medical Center), was cultured in MEM medium with alpha modification supplemented with 10% FBS, 1% penicillin/streptomycin, and 1% l-glutamine (Sigma) at 37 °C in a humidified 5% CO2 tissue culture incubator. For experimental conditions, see Ref. 26.
- 30.Woods JR, Mo H, Bieberich AA, Alavanja T, Colby DA. MedChemComm. 2012;4:27. [Google Scholar]
- 31.Reduction product 9. A mixture of 15-methyelne-eburnamonine 4 (10.6 mg, 0.034 mmol) and Pd/C (2.0 mg, 0.017 mmol) in MeOH (800 μL) and CH2Cl2 (10 μL) was vigorously stirred under an atmosphere of hydrogen gas for 24 h at rt. Additional Pd/C (2.0 mg) was added mixture was stirred for 48 h at rt. The mixture was filtered and concentrated under reduced pressure. The title compound 9 as isolated as a colorless solid (8.9 mg) in 85% yield: mp 134–136 °C; 1H NMR (500 MHz CDCl3) δ 8.37 (d, J = 7.3 Hz, 1H), 7.43 (d, J = 7.0 Hz, 1H), 7.32–7.26 (m, 2H), 4.16 (s, 1H), 3.35–3.24 (m, 2H), 2.87–2.94 (m, 1H), 2.83 (q, J = 7.0 Hz, 1H), 2.58–2.36 (m, 4H), 1.82 (qt, J = 13.9, 4.4 Hz, 1H), 1.46 (d, J = 13.3 Hz, 1H), 1.39 (d, J = 13.1 Hz, 1H), 1.34–1.29 (m, 1H) 1.27 (d, J = 7.1 Hz, 3H), 0.98 (t, J = 7.5 Hz, 3H), 0.94–0.90 (td, J = 13.4, 4.2 Hz 1H); 13C NMR (125 MHz, CDCl3)δ 171.2, 134.3, 131.6, 130.0, 124.2, 123.6, 118.0, 116.2, 112.1, 54.6, 50.7, 44.4, 43.9, 41.5, 26.7, 24.9, 20.3, 16.7, 8.0, 7.6; IR (film) νmax 2939, 1701, 1625, 1454, 1376 cm−1; HRMS (EI) m/z calcd for C20H24N2O (M+H)+ 309.1967 found 309.1966; −20.4° (c 0.23, CHCl3).
- 32.Avonto C, Taglialatela-Scafati O, Pollastro F, Minassi A, Di Marzo V, De Petrocellis L, Appendino G. Angew Chem, Int Ed. 2011;50:467. doi: 10.1002/anie.201005959. [DOI] [PubMed] [Google Scholar]
- 33.Woods JR, Mo H, Bieberich AA, Alavanja T, Colby DA. J Med Chem. 2011;54:7934. doi: 10.1021/jm201114t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quantitative NMR studies. All 1D NMR data were acquired on a Bruker Avance 500 MHz spectrometer equipped with a proton gradient probe operating at room temperature and with deuterium lock turned-off. Strong solvent proton signals were utilized for spectrometer shimming; however, they were suppressed by a modified WET sequence that included multiple selective pulses. Total acquisition time for each spectrum was 2.5 min. Resonance peaks near 6.6 ppm and 5.9 ppm were attributed to the methylene protons d and e of 4 prior to reduction by thiols (see Fig. 4). The integration of these signals after polynomial baseline corrections represents the concentration of 4. Also see Refs. 30 and 32 and for additional details.
- 35.Mo H, Balko KM, Colby DA. Bioorg Med Chem Lett. 2010;20:6712. doi: 10.1016/j.bmcl.2010.08.145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.