Abstract
In general, alginate hydrogels are considered to be biologically inert and are commonly used for biomedical purposes that require minimum inflammation. However, Ca2+, which is commonly used to crosslink alginate, is a critical second messenger in immune cell signaling, and little has been done to understand its effect on immune cell fate when delivered as a component of alginate gels. We found that dendritic cells (DCs) encapsulated in Ca2+-crosslinked alginate (calcium alginate) secreted at least fivefold more of the inflammatory cytokine IL-1β when compared to DCs encapsulated in agarose and collagen gels, as well as DCs plated on tissue-culture polystyrene (TCPS). Plating cells on TCPS with the alginate polymer could not reproduce these results, whereas culturing DCs on TCPS with increasing concentrations of Ca2+ increased IL-1β, MHC class II and CD86 expression in a dose-dependent manner. In agreement with these findings, calcium alginate gels induced greater maturation of encapsulated DCs compared to barium alginate gels. When injected subcutaneously in mice, calcium alginate gels significantly upregulated IL-1β secretion from surrounding tissue relative to barium alginate gels, and similarly, the inflammatory effects of LPS were enhanced when it was delivered from calcium alginate gels rather than barium alginate gels. These results confirm that the Ca2+ used to crosslink alginate gels can be immunostimulatory and suggest that it is important to take into account Ca2+’s bioactive effects on all exposed cells (both immune and non-immune) when using calcium alginate gels for biomedical purposes. This work may strongly impact the way people use alginate gels in the future as well as provide insights into past work utilizing alginate gels.
Keywords: Alginate, Ca2+ signaling, Dendritic cell, Tissue engineering, IL-1β
1. Introduction
It is widely appreciated that Ca2+ is one of the most common second messengers in cell signaling, with important roles in transcription, apoptosis, cell adherence, activation, exocytosis, metabolism and proliferation [1–3]. White blood cells are examples of cells that are highly dependent on Ca2+ signaling for their function. For instance, dendritic cells (DCs) require Ca2+ signaling for cytokine secretion, maturation marker expression and phagocytosis [4,5], mast cells and neutrophils require calcium for degranulation and T cells require Ca2+ signaling for the production of IL-2 and IL-4 [6–8]. Calcium’s importance in the proper functioning of the immune system can be underscored by the fact that a single missense mutation in the gene encoding the Ca2+ release-activated Ca2+ channel, an important Ca2+ channel in the plasma membrane expressed by a number of immune cells, knocks out its function, causing severe combined immunodeficiency in humans [6]. Because of Ca2+’s importance in immune cell function, it has been proposed that Ca2+ channels and Ca2+ signaling pathways are promising therapeutic targets to control immune cell behavior [4–6,8,9].
Alginate, also known as alginic acid, is an anionic, linear and unbranched polysaccharide isolated from algae or bacterial biofilms. Alginate is composed of (1, 4)-linked, β-D-mannuronate (M) and α-L-guluronate (G) sugar monomers that are arranged in M blocks (MMMMMM), G blocks (GGGGGG) or alternating M and G residues (MGMGMG), with the exact M and G composition being dependent on the algae or bacteria source. Alginate polymers have a high affinity for divalent cations (in the order Mg2+ ≪ Ca2+ < Sr2+ < Ba2+) and can form a crosslinked network when these divalent cations associate with the G blocks in a proposed “egg-box” model to form crosslinks between the polymer chains [10,11]. Thus, alginate polymers rich in G blocks are able to create more ionic crosslinks and stiffer gels [10,12]. Ca2+-crosslinked alginate gels (calcium alginate) encapsulating growth factors, cells and/or cytokines have been used in vivo for a wide variety of applications such as type I diabetes treatment [13] and bone regeneration [14]. Interestingly, in a study where Ca2+-crosslinked alginate gels were used to deliver pro-angiogenic factors to enhance blood vessel formation [15], and in another study where they were used to deliver activated dendritic cells peritumorally to reduce tumor growth [16], alginate gels alone appeared to have a slight therapeutic effect, but none of these studies specifically examined the potential contribution of Ca2+ to the final outcome.
Contrary to the lack of studies examining the effects of calcium crosslinker, the inflammatory properties of alginate polysaccharides have been widely studied and disputed. For example, dissolved alginate polysaccharides (100–1000 μg ml−1) have been shown to activate monocytes and macrophages, depending on the molecular weight and the M and G ratio of the polymer [17–19]. However, other studies have shown that alginate polysaccharides can actually suppress inflammatory disease [20] or have demonstrated no effect at all [21].
Based on the importance of Ca2+ signaling in white blood cell activation, we hypothesized that the Ca2+ released from calcium alginate gels could promote inflammatory responses in vitro and in vivo. For in vitro studies, DCs were tested as a model leukocyte given their importance in dictating immune responses. To evaluate the immunostimulatory effects of alginate gels, calcium alginate was tested against three commonly used biomaterials (agarose, collagen and tissue culture polystyrene (TCPS)) for its ability to induce DC maturation and/or affect LPS-induced activation in vitro. The impact of both the alginate polymer itself and the Ca2+ used to crosslink the alginate gels was assessed. The cytokines IL-1β, IL-4, IL-6, IL-10, IL-12p70, IFN-γ and TNF-α and the activation markers CD86 and MHC class II were analyzed to gauge DC maturation. For in vivo studies, Ca2+- or Ba2+-crosslinked alginate gels, with or without LPS, were injected subcutaneously into C576BL/ 6J mice to determine their ability to induce local inflammatory cytokine secretion from surrounding tissue.
2. Materials and methods
2.1. Cell culture
Dendritic cells were generated from bone marrow isolated from 4–16 week old C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) as described by Lutz et al. [22].
2.2. Endotoxin testing
All polymer solutions, calcium crosslinkers and other ion-supplemented solutions used in this study were tested using the Limulus Amebocyte Lysate (LAL) Assay (Lonza, Walkersville, MD) according to the manufacturer’s instructions.
2.3. Comparing DC activation across TCPS, collagen, agarose and CaSO4-crosslinked alginate gels
For TCPS conditions, DCs were plated at a concentration of 250,000 cells/100 μl phosphate buffered saline (PBS)/well of a 48-well plate and incubated at 37 °C for 30 min. For collagen gel fabrication, rat tail collagen, type I (BD Biosciences, Franklin Lakes, NJ) was certified to be negative for bacteria, fungi and mycoplasma and used without further purification. DCs were harvested, washed once in PBS and resuspended at a concentration of 10 × 106 -cells ml−1 in a 3 mg ml−1 ice cold collagen solution prepared aseptically according to the manufacturer’s instructions. 100 μl (106 cells) were pipetted into the wells of a 48-well plate and allowed to cure at 37 °C for 30 min. For agarose gel fabrication, a 1.2% Sea-Plaque® agarose solution (Lonza, Allendale, NJ) in PBS was sterilized by autoclaving. For cell encapsulation, the solution was microwaved until fully dissolved. After cooling to 40 °C in a water bath, DCs were resuspended in agarose at a concentration of 10 × 106 cells ml−1, and 100 μl (106 cells) were immediately pipetted into wells of a 48-well plate. The agarose quickly cured at room temperature and was placed at 37 °C for 30 min. For alginate gel fabrication, PRONOVA™ Ultrapure medium viscosity alginate rich in α-L-guluronate residues (MVG) (FMC BioPolymer, Sandvika, Norway) was certified to be free of yeast, mold and bacteria and have an endotoxin content ≤100 EU g−1. MVG alginate was further sterilized by dissolving it in deionized water and filtering it through a 0.22 μm pore diameter membrane (Millipore, Billerica, MA). The sterile alginate solution was frozen, lyophilized and reconstituted aseptically in PBS to make a 2% solution. A CaSO4 · 2H2O slurry (183 mM) in deionized water was sterilized by autoclaving. DCs in PBS were mixed with the 2% alginate solution using two 1 ml syringes connected with a nylon female luer thread style coupler (Value Plastics, Fort Collins, CO) for a final concentration of 13 × 106 cells ml−1. 78 μl (106cells) of this suspension was added to wells of a 48-well plate using an 18 gauge needle, and 22 μl of thoroughly mixed CaSO4 slurry was quickly pipetted and stirred into the alginate in each well. The alginate was allowed to cure at 37 °C for 30 min. Final alginate gels contained 1.2% alginate, 40 mM CaSO4 and 106 cells in a 100 μl gel volume.
After DC plating and encapsulation in hydrogels, 350 μl of R10 medium was added to each well and allowed to equilibrate at 37°C and 5% CO2 for 1 h before activation. For activation, 50 μl of 1000 ng ml−1 LPS (E. coli 0111:B4; Sigma–Aldrich) in R10 was added to each well so that the final concentration was 100 ng ml−1; for LPS-free wells, 50 μl of R10 only was added. After 20–24 h of activation on an orbital shaker, supernatant was collected and frozen at −20 °C for cytokine analysis.
2.4. Soluble polymer studies with collagen, agarose and alginate
Sterile 1 mg ml−1 solutions of dissolved collagen, agarose and alginate were made in PBS. In addition to the polymers used for gel fabrication, additional alginate polymers tested included pure G and M blocks (provided by Dr. Kamal Bouhadir at the American University of Beirut, Lebanon) and PRONOVA™ Ultrapure medium viscosity (MV), low viscosity (LV) and very low viscosity (VLV) alginates rich in either G or M residues (FMC BioPolymer). Each polymer solution was then diluted 1:10 in R10 medium for a final concentration of 100 μg ml−1 (referred to as polymer R10). PBS was used for the control. To prevent any potential differences in protein adsorption from affecting cell attachment across the different conditions, 200 μl of R10 medium was added to wells of a 96-well plate and incubated overnight to coat the wells with serum proteins prior to cell plating. DCs were harvested, washed in PBS, resuspended in the 100 μg ml−1 polymer solutions above and plated at a density of 100,000 cells/180 μl/serum-coated well. After 1 h of incubation at 37 °C and 5% CO2, cells were stimulated with 20 μl of 1000 ng ml−1 LPS in basal R10 or polymer R10 to give a final concentration of 100 ng ml−1; for LPS-free wells, 20 μl of basal R10 or polymer R10 only was added. After 20–24 h, supernatant was collected and frozen at −20 °C for cytokine analysis.
2.5. Soluble Ca2+ dose studies
Sterile, high Ca2+-containing medium was made by adding CaCl2 · 2H2O to deionized water, filtering it through a 0.22 μM membrane and diluting it 1:100 in R10 so that the final Ca2+ concentration ([Ca2+]) equaled 3, 6 or 12 mM (basal R10 = 0.42 mM). Sterile deionized water was used for the control. To confirm that results were not due to a Ca2+ artifact, Mg2+, K+, Na+ and A23187 (Sigma–Aldrich), a Ca2+ ionophore that increases intracellular Ca2+ concentration ([Ca2+]i), were also screened for their ability to alter cytokine expression. Medium was supplemented with 400 ng ml−1 A23187 or each ion so that its final concentration ([Ca2+] = 3 mM; [Mg2+] = 1 mM; [K+] = 5.6 mM, [Na+] = 162 mM) was 10% higher than the physiological upper limit. DCs were harvested, washed once in PBS and resuspended in basal medium or R10 containing high Ca2+, Mg2+, K+, Na2+ or A23187 (referred to as high ion R10). For cytokine analysis, 100,000 cells/180 μl of basal or high ion R10 were plated per well of a 96-well plate and allowed to equilibrate at 37 °C and 5% CO2. After 1 h, 20 μl of 1000 ng ml−1 LPS in basal or high ion R10 was added to each well for a final concentration of 100 ng ml−1; for LPS-free wells, 20 μl of basal or high ion R10 only was added. After 20–24 h of activation supernatantwas collected and frozen at −20 °C. To measure surface marker expression, 106 cells/1.8 ml basal or high calcium R10 were plated per well of a 6-well plate and stimulated with 200 μl of activation or LPS-free medium as above. Cytokines and cell-surface markers were detected and analyzed as described below.
2.6. Comparing the effect of BaCl2-crosslinked, CaCl2-crosslinked and CaSO4-crosslinked alginate gels in vitro
To compare DCs encapsulated within barium and calcium alginate gels, DCs were harvested, washed once in PBS and resuspended in R10 at a concentration of 125 × 106 cells ml−1. 800 μl of a sterile 2.5% Ultrapure MVG alginate solution in PBS was mixed with 200 μl of cell suspension using two 5 ml syringes connected with a coupler to give a final concentration of 25 × 106 cells ml−1 of 2% alginate. A sterile beaker containing a sterile 10 mM BaCl2 or 100 mM CaCl2 solution with 0.1 M HEPES in ddH2O (pH 7.4) was stirred using a stir plate while the alginate was ejected into the bath using a 30 gauge needle to create uniform alginate beads ~2 mm in diameter (~4 μl volume) with ~100,000 cells per bead. The beads were allowed to stir for 10 min for complete gelation, poured into a 0.22 μM filter and washed twice with 50 ml of PBS. Ten beads (~106 cells) were placed in each well of a 48-well plate containing 500 μl medium and were allowed to equilibrate at 37 °C and 5% CO2. After 1 h, 60 μl of 1000 ng ml−1 LPS in R10 was added so that the final concentration in each well was 100 ng ml−1; for LPS-free wells, 60 μl of R10 only was added. Beads were incubated on an orbital shaker for 20–24 h, after which supernatant was collected and frozen at −20 °C. For cytokine and cell-surface marker analysis, see below.
To compare DCs encapsulated in beads crosslinked with 10 or 100 mM CaCl2, DCs were harvested, washed once in PBS and resuspended in PBS at a concentration of 100 × 106 cells ml−1. 750 μl of a sterile 2% Ultrapure MVG alginate solution in R10 was mixed with 250 μl of cell suspension using two 5 ml syringes connected with a coupler to give a final concentration of 25 × 106 cells ml−1 of 1.5% alginate. A sterile beaker containing a sterile 10 or 100 mM CaCl2 solution with 0.1 M HEPES in ddH2O (pH 7.4) was stirred using a stir plate while the alginate was ejected into the CaCl2 bath using a 30 gauge needle to create uniform alginate beads ~2 mm in diameter (~4 μl volume) with ~100,000 cells per bead. The beads were washed and cultured as above.
To compare DCs cultured in 10 or 40 mM CaSO4-crosslinked alginate discs, DCs were harvested, washed once in PBS and resuspended in R10 at a concentration of 128 × 106 cells ml−1. 1.2 ml of a sterile 2% Ultrapure MVG alginate solution in R10 was mixed with 300 μl of cell suspension using two 3 ml syringes connected with a coupler. The syringe containing the alginate–cell suspension was then connected to a third syringe containing 420 μl of 183 or 46 mM CaSO4 crosslinker. The two syringes were pumped back and forth quickly 8–10 times to homogeneously mix the alginate with the crosslinker, and the mixture was cast between two Teflon-coated aluminum plates (McMaster-Carr, Elmhurst, IL) with 1 mm spacers. The alginate was allowed to cure for 10–20 min, after which 8 mm diameter discs were punched using a disposable biopsy punch (Premier, Plymouth Meeting, PA). Final discs were 8 × 1 mm (~50 μl) and contained 1.25% alginate, 106 cells and either 10 or 40 mM CaSO4. Discs were placed in 490 μl R10 in 48-well plates and allowed to equilibrate at 37 °C and 5% CO2. After 1 h, 60 μl of 1000 ng ml−1 LPS in R10 was added so that the final concentration in each well was 100 ng ml−1; for LPS-free wells, 60 μl of R10 only was added. Discs were incubated on an orbital shaker for 20–24 h after which supernatant was collected and frozen at −20 °C.
To compare DCs cultured externally from gels, 106 DCs in 2 ml of medium were plated in 6-well plates. After 1 h of culture, 200 μl of Ba2+- or Ca2+-crosslinked alginate gels were added to wells. Gels were fabricated by mixing a 2.5% MVG alginate solution in R10 with 20 mM BaCl2 or 244 mM CaSO4 crosslinker solution in water in a 4:1 ratio using two syringes connected by a coupler. 200 μl was ejected through an 18 gauge needle onto a sterile plate and allowed to cure for 10 min. Final gels, containing 2% alginate and 4 mM barium or 48.8 mM calcium, were then added to wells using a sterile spatula. After 20–24 h of stagnant culture, supernatants were collected for IL-1β analysis. For surface marker analysis, the above procedure was repeated, except that gels were cast between two Teflon-coated aluminum plates and 8 mm discs were punched using a biopsy punch. Four gels were added per well and DCs were cultured on an orbital shaker. After 20–24 h, cells were scraped from wells for surface marker staining as described below.
2.7. In vivo studies
Female C57BL/6 J mice (Jackson Laboratory, Bar Harbor, ME), aged 4–16 weeks, were anesthetized with isoflurane, and their backs were shaved and wiped with ethanol. A 2.5% MVG alginate solution in PBS was mixed with a sterile 20 mM BaCl2 or 244 mM CaSO4 solution in water using two 1 ml syringes connected by a coupler. The volumes were mixed in a 4:1 ratio so that the final gel contained 2% alginate and 4 mM BaCl2 or 48.8 mM CaSO4. For alginate gels delivering LPS, MVG alginate was dissolved with PBS containing LPS so that final crosslinked gels contained 1 μg of LPS/50 μl gel. All gels were prepared aseptically. After mixing the dissolved alginate with the crosslinker, 50 μl of gel was injected subcutaneously with a 23 gauge needle in the center of the back. Mice were allowed to recover and consume food and water ad libitum. At the timepoints specified, mice were sacrificed and gels were removed. Samples were frozen at −20 °C until analysis. These studies were performed in compliance with the NIH Guide for Care and Use of Laboratory Animals and the Guidelines for the Use of Vertebrate Animals in Research and Teaching of the Faculty of Arts and Sciences of Harvard University.
2.8. Cell-surface marker analysis
For studies on TCPS, 1 ml of 50 mM EDTA in PBS (pH 7.4) was added to each well, and plates were placed in the incubator for 15 min to aid in cell detachment. Cells were then scraped from wells and washed with stain buffer (BD Pharmingen, Franklin Lakes, NJ), which contained standard PBS and 0.2% BSA. To retrieve DCs from the gels, 500 μl of 50 mM EDTA in PBS (pH 7.4) was added to each well. Gels were allowed to dissolve at 37 °C and cells were collected and washed in stain buffer. Collected cells were stained with APC-conjugated anti-mouse CD11c, FITC-conjugated anti-mouse MHC class II and PE-conjugated anti-mouse CD86 (eBioscience, San Diego, CA). Cell-surface antigen staining was analyzed using an LSR II or LSR Fortessa™ flow cytometer (BD Biosciences). Cell viability was determined using forward-(FSC) and side-scatter (SSC), and only viable cells were gated for surface marker analysis. A Live/Dead® Violet Fixable Dead Cell Stain Kit (Life Technologies) was used according to the manufacturer’s instructions to confirm cell viability.
2.9. Cytokine analysis
Cell culture supernatants were analyzed for mouse IL-1β, IL-4, IL-6, IL-10, IL-12p70, IFN-γ and TNF-α using the Bio-Plex Pro™ Magnetic Cytokine Assay System (Bio-Rad, Hercules, CA) or mouse IL-1β only using an Quantikine® Colorimetric Sandwich ELISA kit (R&D Systems, Minneapolis, MN). For in vivo assays, gels were digested with 100 μl of 10 unit ml−1 alginate lyase (Sigma) in a 37°C dry bath with occasional vortexing until fully dissolved. Digested alginate was analyzed with the Bio-Plex Pro™ Mouse Cytokine 23-plex Assay System and with an IL-1β Quantikine® Colorimetric Sandwich ELISA kit. An IL-1β control was resuspended in deionized H2O or deionized H2O supplemented with 12 mM Ca2+ and assayed via ELISA to confirm that high levels of Ca2+ in the supernatants were not affecting antibody detection of IL-1β.
2.10. Ca2+ release assay
For in vitro studies, alginate beads and discs were fabricated and incubated in R10 as above but without cells. Medium was collected at 1, 4 and 10 h, frozen at −20 °C. For in vivo studies, gels were harvested at the timepoints indicated, digested with alginate lyase and diluted 1:4 in PBS. All samples were assayed using the QuantiChrom™ Calcium Assay Kit (BioAssay Systems, Hayward, CA) according to the manufacturer’s instructions. Absorbance was read with a Synergy™ HT microplate reader (Bio-Tek, Winooski, VT).
2.11. Intracellular Ca2+ assay
To measure [Ca2+]i, cells were labeled with the fluorescent intracellular Ca2+ probe Fluo-4 AM (Life Technologies). DCs were harvested, washed in PBS and resuspended at a density of 106 cells ml−1 in 5 μM Fluo-4 AM in PBS for 30 min at room temperature. Labeled cells were then washed in PBS and allowed to sit for another 30 min to allow for complete de-esterification of the probe before plating. Immediately after plating, cells were imaged with an EVOS® fl microscope (AMG, Bothell, WA) using the FITC channel. For kinetic studies, DCs were plated on TCPS in increasing [Ca2+] as above and fluorescence was measured using a Synergy™ HT microplate reader (Ex. 488 nm, Em. 516) at multiple timepoints after plating. For all Fluo-4 studies, the anti-FITC antibody A889 (Life Technologies) was diluted in the medium 1:200 to quench background fluorescence.
2.12. Differential interference contrast (DIC) microscopy and photos of whole alginate gels
DIC images of cells were taken using an Olympus IX81® inverted microscope (Olympus, Center Valley, PA). Photos of whole alginate gels were taken with a Nikon COOLPIX® p90 camera (Nikon, Melville, NY).
2.13. Graphs and statistical analysis
Flow cytometry data were analyzed and plotted using FlowJo® software (Tree Star, Ashland, OR), and all other graphs were made using Kaleidagraph® software (Synergy Software, Reading PA). Statistical analysis was performed using Microsoft® Excel (Microsoft, Redmond, WA) or Kaleidagraph® software. A two-tailed Student’s t-test assuming equal variances was used when comparing two groups, and a one-way analysis of variance (ANOVA) followed by a post hoc Tukey test was used when comparing multiple groups. For all experiments n = 3–4 unless otherwise indicated. Data are reported as the mean ± standard deviation.
3. Results
3.1. Endotoxin testing
All polymer solutions, calcium crosslinkers and other ion-supplemented solutions used in this study tested ≤0.1 EU ml−1.
3.2. DC Comparison across materials
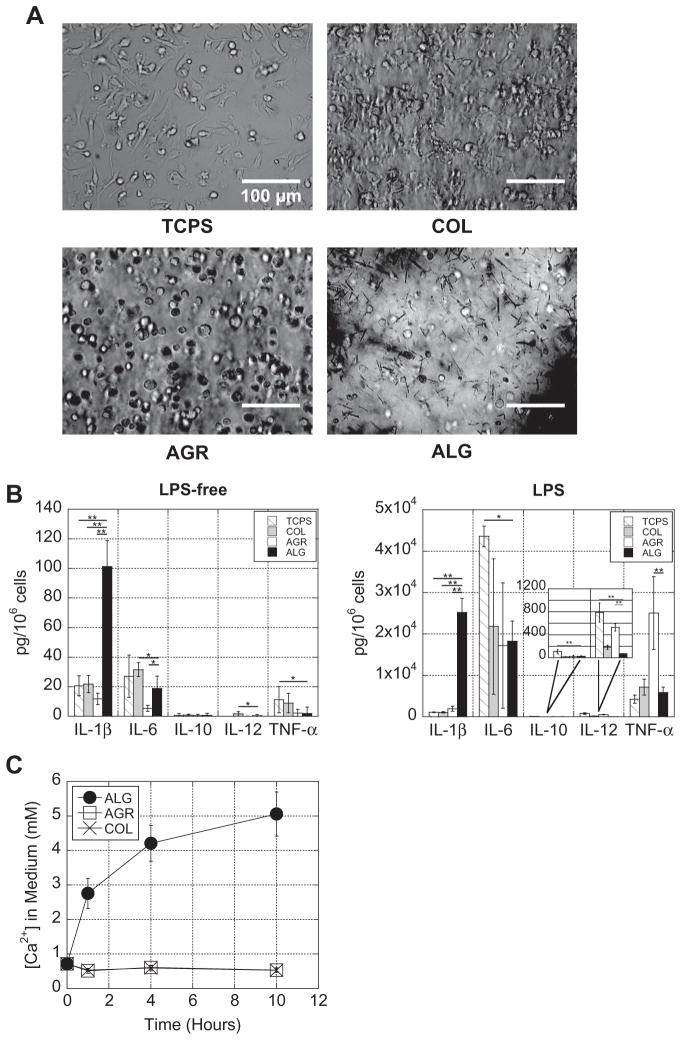
To determine if Ca2+-crosslinked alginate gels had a distinct effect on DC behavior, DCs encapsulated in 40 mM CaSO4 alginate gels were compared to DCs encapsulated in agarose and collagen gels and DCs plated on TCPS. Agarose was chosen because, like alginate, it is a polysaccharide that lacks integrin binding motifs and cannot be enzymatically degraded by mammalian cells; collagen and TCPS were chosen as standard biomaterial controls. In order to cast DCs in alginate gels at the bottom of wells consistently with collagen and agarose gels, CaSO4 was used as the crosslinker because it is less soluble in aqueous solution than CaCl2 (a more commonly used calcium crosslinker), leading to a slower gelation rate and the ability to mold alginate gels into the bottom of wells. DCs in alginate or agarose gels had a rounded appearance throughout the duration of the experiment, while cells on TCPS and in collagen were able to migrate, spread and make direct cell–cell contacts (Fig. 1A).
Fig. 1.
Alginate gels enhanced IL-1β secretion from encapsulated DCs. (A) Photomicrographs were taken of DCs adhered to TCPS or encapsulated in collagen (COL), agarose (AGR) or alginate (ALG) gels 1 h after encapsulation. (B) DCs were cultured without LPS or with LPS. After 24 h, supernatant was collected and analyzed for multiple cytokines. (C) The Ca2+ released into medium from each of the hydrogels was quantified over 10 h. *P ≤ 0.05; **P ≤ 0.001.
To determine if there were differences in DC activation across the materials, cell culture supernatants were bioplexed for various cytokines. DCs encapsulated in calcium-crosslinked alginate gels produced significantly higher levels of the inflammatory cytokine IL-1β when compared to DCs encapsulated in agarose and collagen gels and DCs plated on TCPS (Fig. 1B). Overall cytokine expression increased for all conditions when cells were stimulated with LPS, but IL-1β was even further enhanced with alginate gels (Fig. 1B). Interestingly, with LPS stimulation, DCs in agarose gels produced significantly higher levels of TNF-α, while DCs plated on TCPS produced significantly higher levels of IL-6. IL-4 and IFN-γ secretion were negligible for both LPS-free and LPS stimulated cells in all conditions and thus excluded from the rest of the study.
The impact of each gel on Ca2+ levels was next analyzed, since it is known that in physiological buffers, such as PBS, cell culture medium or serum, monovalent cations (e.g. Na+) compete with divalent cations crosslinking the alginate, causing the divalent cations to be released over time [23]. Over a 10 h period, Ca2+-cross-linked alginate gels increased the [Ca2+] in the surrounding medium to ~5 mM (indicating that ~43% of the calcium initially incorporated into the gel was released), while the other gels did not impact the Ca2+ concentration (Fig. 1C).
3.3. Testing polymer components of gels on TCPS-cultured DCs
The impact of the polymer components of the three hydrogels tested were next examined by culturing DCs on TCPS overnight with each of the three dissolved polymers. In the absence of LPS, IL-6 secretion for alginate was slightly higher than the no polymer (NP) condition (Fig. 2), and in the presence of LPS, IL-1β and IL-12p70 secretion for collagen and agarose, respectively, were slightly different from NP (Fig. 2). However, these differences were minor and did not account for the trends seen with intact gels. In addition to the polymers used to fabricate gels, various alginate polymers of different molecular weight and M and G ratio (Supplementary Table S.1) were also screened and did not induce or enhance cytokine expres-
Fig. 2.
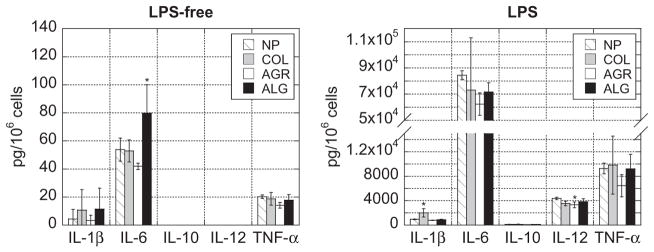
Soluble polymer components of gels were not responsible for trends seen with intact gels. DCs were cultured on TCPS with no polymer (NP), or dissolved collagen (COL), agarose (AGR) or alginate (ALG), in the absence of LPS or in the presence of LPS. After 24 h, supernatant was collected and multiplexed for cytokines. Asterisks indicate that the condition is significantly different from the NP condition. *P<0.05.
3.4. Testing Ca2+-supplemented medium on TCPS-cultured DCs
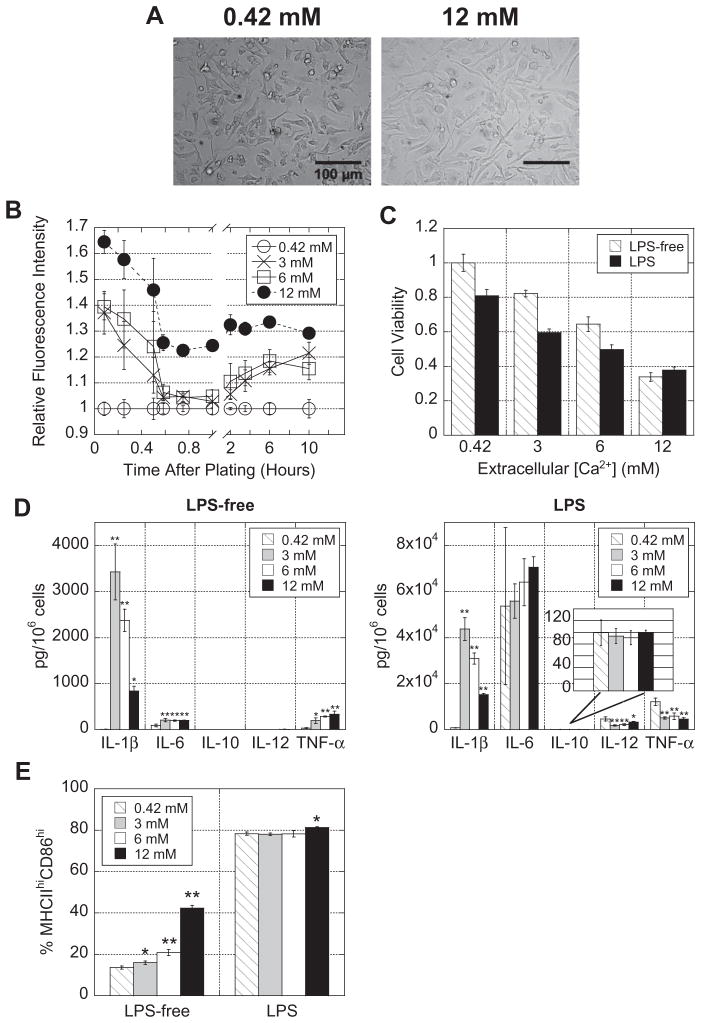
The impact of free Ca2+ on cytokine secretion was tested next. DCs were cultured on TCPS in medium supplemented with Ca2+ so that the final [Ca2+] equaled 3, 6 or 12 mM. Interestingly, DCs extended longer processes (indicative of activation) as [Ca2+] in the medium increased, particularly for 12 mM (Fig. 3A).
Fig. 3.
Ca2+ promotes DC maturation. DCs were plated in increasing [Ca2+] in the absence or presence of LPS for 24 h. (A) Photomicrographs were taken of DCs (only 0.42 and 12 mM are shown). (B) DCs were labeled with the intracellular Ca2+ probe Fluo-4 and fluorescence was quantified over a 10 h period using a plate reader. (C) Cells were collected and viability was quantified based on forward-/side-scatter measurements obtained using flow cytometry. (D) Supernatants were multiplexed for cytokines. (E) DCs cultured in increasing [Ca2+] were stained with PE-labeled anti-CD86 and FITC-labeled anti-MHC class II and analyzed using flow cytometry. Asterisks indicate that the Ca2+-supplemented condition is significantly different from the basal (0.42 mM) condition. *P ≤ 0.05; **P ≤ 0.001.
Fluo-4, a fluorescent intracellular calcium probe, was used to quantify the levels of [Ca2+]i for each of the conditions. Both photomicrographs (Supplementary Fig. S.2) and microplate readings (Fig. 3B) revealed that the [Ca2+]i levels rose with increasing extracellular [Ca2+]. Although [Ca2+]i were highest immediately after plating and fluctuated within the first hour, a sustained high [Ca2+]i was observed and maintained for at least 10 h (Fig. 3B).
Despite the fact that DCs in the 3, 6 and 12 mM [Ca2+] conditions looked equally as viable as the 0.42 mM conditions, forward-and side-scatter analysis using flow cytometry revealed that high [Ca2+] reduced cell viability in a dose-dependent manner and that LPS stimulation further reduced cell viability (Fig. 3C). The live/ dead populations gated using forward- and side-scatter were confirmed to be alive or dead using a live/dead fixable dye (Supplementary Fig. S.3).
Bioplex of cell culture supernatants showed that high [Ca2+] led to an increase in IL-1β both with and without LPS stimulation (with secretion peaking at 3 mM calcium) (Fig. 3D). These data were consistent with DCs cultured in calcium alginate gels. Interestingly, in the LPS-free condition, high extracellular [Ca2+] also upregulated IL-6 and TNF-α secretion, but in the presence of LPS, had no effect on IL-6 and actually downregulated IL-12p70 and TNF-α (Fig. 3D). To confirm that these trends were due to excess Ca2+ enhancing Ca2+ signaling and not an artifact of the ion, the medium was supplemented with high concentrations of other physiologically relevant ions (Mg2+, Na+ or K+) or the Ca2+ ionophore A23187, a small molecule that increases [Ca2+]i without changing the total [Ca2+] in the cell’s environment, and after 24 h, supernatants were bioplexed for cytokines. The increase in IL-1β, IL-6 and TNF-α observed with calcium was repeated only with the Ca2+ ionophore A23187 (Supplementary Fig. S.4A). Additionally, an IL-1β control (86–144 pg ml−1) was reconstituted in deionized H2O or deionized H2O supplemented with 12 mM Ca2+, and no difference in concentration was detected by ELISA (Supplementary Fig. S.4B).
DCs cultured in increasing [Ca2+] were stained with anti-CD86 and anti-MHC class II antibodies, and live cells were gated into populations of negative, low or high expression of each marker based on flow cytometry histograms (Supplementary Fig. S.5). In LPS-free medium, increasing extracellular [Ca2+] correlated with increasing MHC class IIhiCD86hi double-positive expression in a dose-dependent manner, while LPS stimulation caused MHC class IIhiCD86hi double-positive cells to plateau at ~80% for all conditions (Fig. 3E).
3.5. Testing Ba2+ and Ca2+ crosslinker in alginate gels
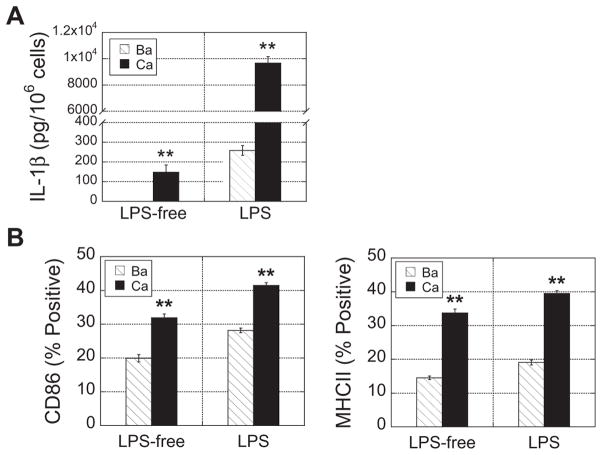
Since the data indicated that it was the calcium crosslinker and not the alginate polymer accounting for enhanced DC maturation, we hypothesized that DCs encapsulated in Ca2+-crosslinked alginate would have a greater degree of maturation than DCs encapsulated in Ba2+-crosslinked alginate. To test this, DCs were encapsulated in alginate beads crosslinked in a 10 mM BaCl2 or 100 mM CaCl2 bath, which is the most standard method for cross-linking alginate, and analyzed for their ability to induce DC maturation. 100 mM CaCl2 was chosen because it is a concentration typically used for biomedical applications, and 10 mM BaCl2 was selected because it yielded beads approximately the same size as 100 mM CaCl2. As hypothesized, DCs encapsulated in Ca2+-cross-linked alginate secreted significantly more IL-1β compared to DCs in Ba2+-crosslinked gels as detected by ELISA (Fig. 4A). LPS stimulation caused an overall increase in IL-1β, but the presence of Ca2+ even further enhanced its secretion. Likewise, for both LPS-free and LPS conditions, DCs extracted from Ca2+-crosslinked alginate gels had increased expression of CD86 and MHC class II compared to DCs extracted from Ba2+-crosslinked alginate gels (Fig. 4B).
Fig. 4.
Calcium crosslinker enhanced activation markers of encapsulated DCs compared to barium crosslinker. (A) After 24 h in culture, supernatants from DCs encapsulated in barium (10 mM) or calcium (100 mM) alginate beads, in the absence or presence of LPS, were collected and assayed for IL-1β. (B) DCs were extracted from beads and analyzed for CD86 and MHC class II using flow cytometry. Asterisks indicate that the calcium condition is significantly different from the barium condition. **P ≤ 0.001.
Since the calcium crosslinker was responsible for the enhanced IL-1β secretion and maturation marker expression observed with alginate gels, we then hypothesized that DCs encapsulated in alginate gels with increasing concentrations of calcium crosslinker would have a dose-dependent response. We found that increasing the CaCl2 crosslinking concentration in alginate beads from 10 to 100 mM raised the [Ca2+] of surrounding medium, increased [Ca2+]i, decreased cell viability, significantly enhanced IL-1β secretion, and increased the expression of MHC class II and CD86 (Supplementary Fig. S.6), which was consistent with results seen with two-dimensional plated DCs cultured in increasing [Ca2+] (Section 3.4). To confirm that these results were not unique to CaCl2-crosslinked alginate beads, we tested alginate discs cured with 10 or 40 mM CaSO4, and the same trends were observed (Supplementary Fig. S.7).
To establish that DCs cultured externally from gels could also be matured by the Ca2+ released from calcium alginate gels, DCs were plated on TCPS in the absence or presence of alginate gels cross-linked with 4 mM BaCl2 or 48.8 mM CaSO4. These crosslinkers and crosslinking concentrations were chosen because they yielded gels that could be injected for in vivo testing. Similar to results seen above, DCs cultured in the presence of calcium alginate gels secreted significantly higher concentrations of IL-1β (Fig. 5A) and expressed higher levels of CD86 and MHC class II (Fig. 5B) compared to control and barium alginate conditions.
Fig. 5.
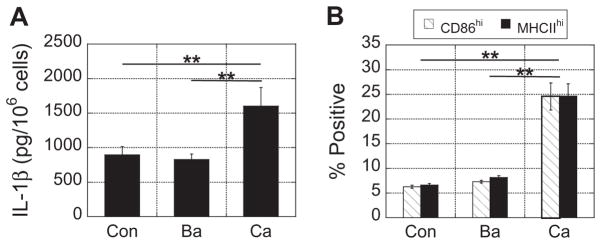
Calcium crosslinker enhanced activation markers of DCs cultured outside of gels compared to barium crosslinker. (A) After 24 h in culture, supernatants from DCs plated on TCPS and cultured in the presence of barium (4 mM) or calcium (48.8 mM) alginate gels were collected and assayed for IL-1β. (B) DCs were scraped from wells and analyzed for CD86 and MHC class II using flow cytometry. **P ≤ 0.001.
3.6. In vivo studies
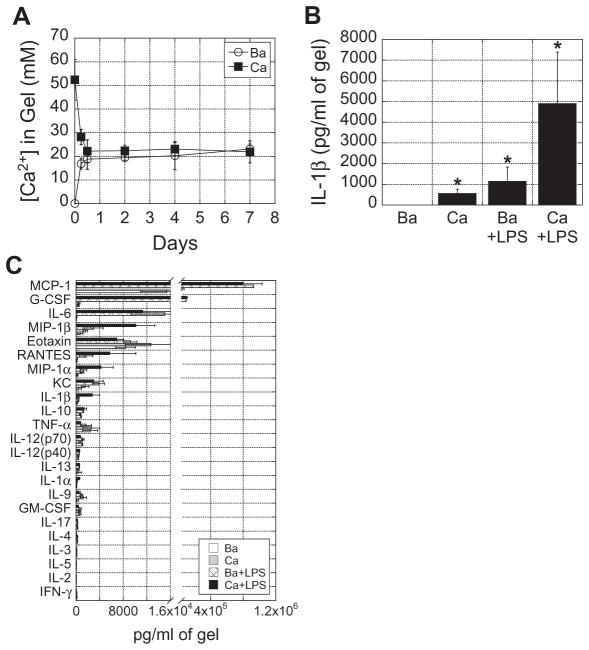
To determine if calcium alginate gels could enhance inflammatory responses as demonstrated in vitro, 50 μl alginate gels cross-linked with 4 mM BaCl2 or 48.8 mM CaSO4, with or without 1 μg LPS, were injected medially into mice. Because alginate gels were injected into the body, which is an open system where Ca2+ can diffuse freely in and out, we were highly interested in analyzing the [Ca2+] within the gels over time. More than 50% of the calcium originally incorporated within calcium alginate gels was released within the first 12 h (Fig. 6A), and the [Ca2+] within the gels was maintained at a steady state thereafter. Interestingly, the [Ca2+] in barium alginate gels increased over time and also reached steady state by ~12 h. Thus, by the time gels were analyzed at 24 h, [Ca2+] in both gels were identical.
Fig. 6.
Calcium alginate gels induce greater inflammatory cytokine secretion compared to barium alginate gels and enhanced LPS-induced inflammation in vivo. (A) 50 μl of barium (4 mM) and calcium (48.8 mM) alginate gels were injected into subcutaneous tissue of mice. At various timepoints, alginate gels were removed and analyzed for [Ca2+]. (B) 50 μl of barium and calcium alginate gels, with or without LPS, were injected into mice. After 24 h, gels were harvested and analyzed for IL-1β concentration and (C) other inflammatory cytokines and chemokines. Asterisks indicate that the condition is significantly different from the barium only condition. *P < 0.05.
Although the [Ca2+] in both gels were identical at the time of analysis, calcium alginate gels induced more IL-1β secretion compared to barium alginate gels, and LPS delivered from calcium alginate gels more than quadrupled IL-1β secretion relative to LPS delivered from barium alginate gels (Fig. 6B). Despite most of the Ca2+ being released within the first 12 h, IL-1β could be detected within the gels for at least 4 days (Supplementary Fig. S.8). To get a more comprehensive analysis of other inflammatory mediators that calcium alginate gels induced from surrounding tissue, gels were multiplexed for 23 various cytokines and chemokines. We found that chemokines (MCP-1, MIP-1β, eotaxin, RANTES, MIP-1α and KC) were generally secreted in higher concentrations, and LPS delivered from calcium gels upregulated MIP-1β, RANTES, MIP-1α, IL-1β, and IL-1α by ~3–5-fold greater than LPS delivered from barium gels (Fig. 6C). These data were consistent with in vitro data.
4. Discussion
The results of this study indicate that the Ca2+ used to crosslink alginate gels was released over time and promoted IL-1β, CD86 and MHC class II expression by DCs, whether they were encapsulated within gels or cultured externally from gels in vitro. In addition to increasing DC maturation, increasing extracellular [Ca2+] was also associated with increasing [Ca2+]i and reduced cell viability. Consistent with in vitro results, Ca2+ crosslinked alginate gels significantly promoted inflammatory cytokine secretion and enhanced the effects of LPS in vivo.
Contrary to published data showing that alginate polysaccharides could stimulate inflammatory cytokine production from monocyte populations, the various soluble alginate polysaccharides used in these experiments did not stimulate bone-marrow-derived DCs. Alginate has been suggested to activate monocytes and macrophages, potentially via the NF-κβ pathway [18,19,24–26], and several studies have reported the detection of antibodies against alginate in vivo [27–29]. Conflicting with this, alginate has been used as an anti-inflammatory to suppress experimental glomerulonephritis and ulcerative colitis [20,30]. The literature on alginate immunogenicity is abundant yet controversial, and many of these results must be interpreted with caution as alginates used in past studies may have contained impurities such as endotoxins, residual proteins or polyphenols, all of which affect the immunogenicity of alginate [21,31–33]. The alginate used in this study was of ultrapure grade, which may explain why no immunostimulatory effects were seen.
Although agarose polymers did not induce or affect DC activation, it was observed that whole agarose gels had a unique effect on DC activation. The gels themselves did not induce cytokine secretion from DCs but greatly enhanced TNF-α production when DCs were pulsed with LPS. It has been shown that heat shock proteins (HSPs) play an important role as molecular chaperones of the LPS-signaling pathway [34,35], and it is possible that the heated agarose, although cooled near body temperature before use, elicited the production of HSPs by DCs, subsequently enhancing TNF-α secretion when the cells were exposed to LPS. Although further testing would have to be done to confirm this, it is an interesting idea that could be used advantageously to activate white blood cells.
Calcium alginate gels increased the [Ca2+] in the medium up to 5 mM (2.5 times what is physiologically relevant) over a 10 h period, and Fluo-4 labeling revealed that the elevated level of extracellular [Ca2+] whether it be on TCPS or with alginate gels, resulted in a sustained increase in [Ca2+]i. The exact mechanism(s) of increased [Ca2+]i in this study was not determined, but it was likely due to a series of events leading to contributions from both intracellular Ca2+ stores and extracellular Ca2+ [36,37]. Given the intricately intertwined activities of ion channels, pumps, transporters, intracellular buffers, Ca2+-binding proteins and organelles, all of which affect [Ca2+]i [37], one would have to use more sophisticated techniques, such as patch clamp techniques, to precisely determine the mechanisms of increased [Ca2+]i in these experiments.
A critical finding of this study was that increasing [Ca2+] matured DCs and enhanced LPS-induced DC activation. Dendritic cells and other leukocytes are highly dependent on Ca2+ to carry out their effector functions, and several intracellular Ca2+-sensing and -signaling molecules have been identified as having necessary roles in their activation [3–5,8]. It is likely that the increases in [Ca2+]i induced by raising extracellular [Ca2+] activated these Ca2+ signaling molecules, leading to the upregulation of DC activation marker expression and inflammatory cytokine secretion. These results illustrate how one can amplify protein expression by increasing a second messenger (in this case Ca2+) that is downstream of a signaling event (such as TLR signaling). It is also plausible that the Ca2+ being released from the alginate gels was sensed externally by the G-protein-coupled extracellular Ca2+-sensing receptor, CaR, initiating various signaling pathways leading to expression of inflammatory markers [38].
One of the most striking findings was the increase in IL-1β observed with calcium alginate gels. IL-1β is a critical mediator of inflammation, with important roles in neutrophil mobilization, cellular adhesion to the endothelium and white blood cell infiltration [39,40]. Cleavage of pro-IL-1β into its functional, secreted form is predominantly mediated by the NRLP3 inflammasome, an important molecular platform expressed by myeloid cells in innate immune defense that can be activated by a number of danger signals and stress factors [41]. A connection has been made between Ca2+-induced mitochondrial damage and activation of the NLRP3 inflammasome, which likely explains the increase in IL-1β [41–43]. Inflammasome activation has been implicated in the success of adjuvants, such as alum [44,45], which suggests that Ca2+ induction of IL-1β (and potentially inflammasome activation) may have interesting implications in the field of vaccination.
The [Ca2+] levels in this study correlated with reduced cell viability in a dose-dependent manner. This is consistent with past reports showing that elevations in [Ca2+]i can directly and indirectly induce cell injury and death [2]. The largest contributor to Ca2+ toxicity is believed to be Ca2+-induced mitochondrial permeability transition (MPT), which is the formation of a large pore in the mitochondrial membrane during mitochondrial stress [2]. When cytosolic Ca2+ is elevated (>500 nM), mitochondria can be destabilized, initiating MPT, and resulting in the release of pro-apoptotic proteins. Cell death due to Ca2+ toxicity may have accounted for decreases in IL-1β seen with TCPS for [Ca2+] above 3 mM. Additionally, LPS-induced TNF-α secretion could have triggered apoptosis via the TNF pathway and accounted for the decreased viability seen with LPS-treated conditions [46].
When calcium alginate gels were injected in vivo, it was demonstrated that this could induce inflammatory cytokine secretion from surrounding tissue. Similar to Ca2+ release profiles in vitro, the majority of the Ca2+ was released within the first few hours. Interestingly, even though [Ca2+] in both calcium and barium alginate gels equilibrated within the first 12 h, we were still able to detect strong differences in IL-1β between the two gels at the time of analysis. These bioplex results, along with our bioplex data showing that only A23187 was able to reproduce the effects of supplementary Ca2+ and our ELISA data showing that antibody binding to IL-1β was not Ca2+-dependent, are strong evidence that the phenomena observed were due to Ca2+ signaling enhancing cytokine secretion and not due to a change in osmolality or an artifact of excess Ca2+ influencing antibody detection of the cytokines. Since IL-1β could be detected within alginate gels beyond the first day, it is possible that the gels sequestered cytokines induced by the burst release of Ca2+, but slowly released these cytokines as inflammation subsided. Consistent with results seen in vitro, Ca2+ and LPS released from alginate gels had a synergistic effect and activated the most IL-1β secretion observed in vivo. Aside from IL-1β, strong upregulations in other inflammatory cytokines and chemokines were also observed.
Although the majority of studies utilizing calcium alginate gels for biomedicalpurposes have not examined the effects of the calcium crosslinker, evidence in some studies is consistent with our findings. When activated dendritic cells were delivered in Ca2+-crosslinked alginate gels to reduce tumor size in mice, calcium alginate gels alone seemed to have a slight therapeutic effect. Consequently, it would be interesting to see if replacing the Ca2+ with Ba2+ would reduce vaccine efficacy [16]. Also mentioned earlier was that Ca2+-crosslinked alginate gels had a positive effect on angiogenesis in a mouse hindlimb ischemia model [15]. Although not statistically significant, alginate alone appeared to increase blood vessel density over untreated animals and animals injected with a bolus dose of vascular endothelial growth factor (VEGF). It is possible that the Ca2+ in the gels promoted the secretion of endogenous pro-angiogenic factors from immune and/or non-immune cells, leading to enhanced blood vessel formation. Lastly, alginate has been used for decades in the management of acute and chronic wounds, although its exact molecular and cellular effects are not well-understood. There has been a recent report that alginate promoted keratinocyte differentiation, which is critical for wound healing, and this was due to the Ca2+ released by the alginate [47]. These examples illustrate the importance of characterizing the effects of Ca2+ on other leukocytes and non-immune cells both in vitro and in vivo.
5. Conclusion
We found that the Ca2+ released from calcium alginate gels led to DC maturation and enhanced LPS-induced inflammatory cytokine secretion in vitro. Likewise, calcium alginate cells injected subcutaneously in mice were able to upregulate a number of inflammatory cytokines and chemokines relative to barium alginate, and the inflammatory effects of LPS on surrounding tissue were enhanced when LPS was delivered from calcium alginate vs. barium alginate. This work suggests that it is important to take into consideration the effects of calcium crosslinker when using alginate gels for biomedical applications and may have significant implications in future biomaterial design.
Supplementary Material
Acknowledgments
We would like to thank Beth Ann Lopez (University of New Mexico – Albuquerque) for her help with preliminary experiments and Dr. Kamal Bouhadir (American University at Beirut) for generously providing purified M and G block alginate polymers. This work was supported by the NIH (R01DE019917).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.actbio.2013.08.002.
References
- 1.Clapham DE. Calcium signaling. Cell. 2007;131:1047–58. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 2.Dong Z, Saikumar P, Weinberg JM, Venkatachalam MA. Calcium in cell injury and death. Annu Rev Pathol Mech. 2006;1:405–34. doi: 10.1146/annurev.pathol.1.110304.100218. [DOI] [PubMed] [Google Scholar]
- 3.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 4.Connolly SF, Kusner DJ. The regulation of dendritic cell function by calcium-signaling and its inhibition by microbial pathogens. Immunol Res. 2007;39:115–27. doi: 10.1007/s12026-007-0076-1. [DOI] [PubMed] [Google Scholar]
- 5.Shumilina E, Huber SM, Lang F. Ca2+ signaling in the regulation of dendritic cell functions. Am J Physiol Cell Physiol. 2011;300:C1205–14. doi: 10.1152/ajpcell.00039.2011. [DOI] [PubMed] [Google Scholar]
- 6.Parekh AB. Store-operated CRAC channels: function in health and disease. Nat Rev Drug Discov. 2010;9:399–410. doi: 10.1038/nrd3136. [DOI] [PubMed] [Google Scholar]
- 7.Brown AP, Ganey PE. Neutrophil degranulation and superoxide production induced by polychlorinated biphenyls are calcium dependent. Toxicol Appl Pharmacol. 1995;131:198–205. doi: 10.1006/taap.1995.1062. [DOI] [PubMed] [Google Scholar]
- 8.Li SW, Westwick J, Poll CT. Receptor-operated Ca2+ influx channels in leukocytes: a therapeutic target? Trends Pharmacol Sci. 2002;23:63–70. doi: 10.1016/s0165-6147(00)01897-6. [DOI] [PubMed] [Google Scholar]
- 9.Koski GK, Schwartz GN, Weng DE, Czerniecki BJ, Carter C, Gress RE, et al. Calcium mobilization in human myeloid cells results in acquisition of individual dendritic cell-like characteristics through discrete signaling pathways. J Immunol. 1999;163:82–92. [PubMed] [Google Scholar]
- 10.Braccini I, Pérez S. Molecular basis of Ca2+-induced gelation in alginates and pectins: the egg-box model revisited. Biomacromolecules. 2001;2:1089–96. doi: 10.1021/bm010008g. [DOI] [PubMed] [Google Scholar]
- 11.Mørch ÝA, Donati I, Strand BL. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules. 2006;7:1471–80. doi: 10.1021/bm060010d. [DOI] [PubMed] [Google Scholar]
- 12.Kuo CK, Ma PX. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials. 2001;22:511–21. doi: 10.1016/s0142-9612(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 13.Soonshiong P, Heintz RE, Merideth N, Yao QX, Yao ZW, Zheng TL, et al. Insulin independence in a type-1 diabetic patient after encapsulated islet transplantation. Lancet. 1994;343:950–1. doi: 10.1016/s0140-6736(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 14.Alsberg E, Anderson KW, Albeiruti A, Franceschi RT, Mooney DJ. Cell-interactive alginate hydrogels for bone tissue engineering. J Dent Res. 2001;80:2025–9. doi: 10.1177/00220345010800111501. [DOI] [PubMed] [Google Scholar]
- 15.Silva EA, Mooney DJ. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J Thromb Haemost. 2007;5:590–8. doi: 10.1111/j.1538-7836.2007.02386.x. [DOI] [PubMed] [Google Scholar]
- 16.Hori Y, Stern PJ, Hynes RO, Irvine DJ. Engulfing tumors with synthetic extracellular matrices for cancer immunotherapy. Biomaterials. 2009;30:6757–67. doi: 10.1016/j.biomaterials.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otterlei M, Ostgaard K, Skjak-Braek G, Smidsrod O, Soon-Shiong P, Espevik T. Induction of cytokine production from human monocytes stimulated with alginate. J Immunother. 1991;10:286–91. doi: 10.1097/00002371-199108000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Otterlei M, Sundan A, Skjak-Braek G, Ryan L, Smidsrod O, Espevik T. Similar mechanisms of action of defined polysaccharides and lipopolysaccharides: characterization of binding and tumor necrosis factor alpha induction. Infect Immun. 1993;61:1917–25. doi: 10.1128/iai.61.5.1917-1925.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwamoto M, Kurachi M, Nakashima T, Kim D, Yamaguchi K, Oda T, et al. Structure-activity relationship of alginate oligosaccharides in the induction of cytokine production from RAW264. 7 cells. FEBS Lett. 2005;579:4423–9. doi: 10.1016/j.febslet.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Razavi A, Khodadadi A, Eslami MB, Eshraghi S, Mirshafiey A. Therapeutic effect of sodium alginate in experimental chronic ulcerative colitis. Iran J Allergy Asthm. 2008;7:13–8. [PubMed] [Google Scholar]
- 21.Zimmermann U, Klöck G, Federlin K, Hannig K, Kowalski M, Bretzel RG, et al. Production of mitogen-contamination free alginates with variable ratios of mannuronic acid to guluronic acid by free flow electrophoresis. Electrophoresis. 1992;13:269–74. doi: 10.1002/elps.1150130156. [DOI] [PubMed] [Google Scholar]
- 22.Lutz MB, Kukutsch N, Ogilvie ALJ, Rößner S, Koch F, Romani N, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 23.LeRoux MA, Guilak F, Setton LA. Compressive and shear properties of alginate gel: effects of sodium ions and alginate concentration. J Biomed Mater Res. 1999;47:46–53. doi: 10.1002/(sici)1097-4636(199910)47:1<46::aid-jbm6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto Y, Kurachi M, Yamaguchi K, Oda T. Induction of multiple cytokine secretion from RAW264. 7 cells by alginate oligosaccharides. Biosci Biotech Biochem. 2007;71:238–41. doi: 10.1271/bbb.60416. [DOI] [PubMed] [Google Scholar]
- 25.Flo TH, Ryan L, Latz E, Takeuchi O, Monks BG, Lien E, et al. Involvement of toll-like receptor (TLR) 2 and TLR4 in cell activation by mannuronic acid polymers. J Biol Chem. 2002;277:35489–95. doi: 10.1074/jbc.M201366200. [DOI] [PubMed] [Google Scholar]
- 26.Yang D, Jones KS. Effect of alginate on innate immune activation of macrophages. J Biomed Mater Res A. 2009;90:411–8. doi: 10.1002/jbm.a.32096. [DOI] [PubMed] [Google Scholar]
- 27.Johansen HK, Hoiby N, Pedersen SS. Experimental immunization with Pseudomonas aeruginosa alginate induces IgA and IgG antibody responses. APMIS. 1991;99:1061–8. [PubMed] [Google Scholar]
- 28.Pressler T, Pedersen SS, Espersen F, Hoiby N, Koch C. IgG subclass antibody responses to alginate from Pseudomonas aeruginosa in patients with cystic fibrosis and chronic P. aeruginosa infection. Pediatr Pulmonol. 1992;14:44–51. doi: 10.1002/ppul.1950140109. [DOI] [PubMed] [Google Scholar]
- 29.Kulseng B, Skjak-Braek G, Ryan L, Andersson A, King A, Faxvaag A, et al. Transplantation of alginate microcapsules: generation of antibodies against alginates and encapsulated porcine islet-like cell clusters. Transplantation. 1999;67:978–84. doi: 10.1097/00007890-199904150-00008. [DOI] [PubMed] [Google Scholar]
- 30.Mirshafiey A, Borzooy Z, Abhari RS, Razavi A, Tavangar M, Rehm BHA. Treatment of experimental immune complex glomerulonephritis by sodium alginate. Vasc Pharmacol. 2005;43:30–5. doi: 10.1016/j.vph.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Orive G, Tam SK, Pedraz JL, Halle JP. Biocompatibility of alginate-poly-L-lysine microcapsules for cell therapy. Biomaterials. 2006;27:3691–700. doi: 10.1016/j.biomaterials.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 32.Orive G, Carcaboso AM, Hernández RM, Gascón AR, Pedraz JL. Biocompatibility evaluation of different alginates and alginate-based microcapsules. Biomacromolecules. 2005;6:927–31. doi: 10.1021/bm049380x. [DOI] [PubMed] [Google Scholar]
- 33.Dusseault J, Tam SK, Ménard M, Polizu S, Jourdan G, Yahia LH, et al. Evaluation of alginate purification methods: effect on polyphenol, endotoxin, and protein contamination. J Biomed Mater Res A. 2006;76A:243–51. doi: 10.1002/jbm.a.30541. [DOI] [PubMed] [Google Scholar]
- 34.Triantafilou K, Triantafilou M, Ladha S, Mackie A, Fernandez N, Dedrick RL, et al. Fluorescence recovery after photobleaching reveals that LPS rapidly transfers from CD14 to hsp70 and hsp90 on the cell membrane. J Cell Sci. 2001;114:2535–45. doi: 10.1242/jcs.114.13.2535. [DOI] [PubMed] [Google Scholar]
- 35.Triantafilou M, Triantafilou K. Heat-shock protein 70 and heat-shock protein 90 associate with Toll-like receptor 4 in response to bacterial lipopolysaccharide. Biochem Soc Trans. 2004;32:636–9. doi: 10.1042/BST0320636. [DOI] [PubMed] [Google Scholar]
- 36.Hsu S, O’Connell PJ, Klyachko VA, Badminton MN, Thomson AW, Jackson MB, et al. Fundamental Ca2+ signaling mechanisms in mouse dendritic cells: CRAC is the major Ca2+ entry pathway. J Immunol. 2001;166:6126–33. doi: 10.4049/jimmunol.166.10.6126. [DOI] [PubMed] [Google Scholar]
- 37.Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–7. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 38.Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol. 2003;4:530–8. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- 39.Allantaz F, Chaussabel D, Banchereau J, Pascual V. Microarray-based identification of novel biomarkers in IL-1-mediated diseases. Curr Opin Immunol. 2007;19:623–32. doi: 10.1016/j.coi.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–147. [PubMed] [Google Scholar]
- 41.Davis BK, Wen HT, Ting JPY. The Inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–35. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, et al. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci USA. 2012;109:11282–7. doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou RB, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–5. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 44.Li HF, Willingham SB, Ting JPY, Re F. Cutting edge: Inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–U13. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stenvik J, Sletta H, Grimstad Ø, Pukstad B, Ryan L, Aune R, et al. Alginates induce differentiation and expression of CXCR7 and CXCL12/SDF-1 in human keratinocytes—the role of calcium. J Biomed Mater Res A. 2012;100A:2803–12. doi: 10.1002/jbm.a.34219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.