Abstract
Several cDNA clones coding for the high molecular weight (alpha) subunit of the voltage-sensitive Na channel have been selected by immunoscreening a rat brain cDNA library constructed in the expression vector lambda gt11. As will be reported elsewhere, the amino acid sequence translated from the DNA sequence shows considerable homology to that reported for the Electrophorus electricus electroplax Na channel. Several of the cDNA inserts hybridized with a low-abundance 9-kilobase RNA species from rat brain, muscle, and heart. Sucrose-gradient fractionation of rat brain poly(A) RNA yielded a high molecular weight fraction containing this mRNA, which resulted in functional Na channels when injected into oocytes. This fraction contained undetectable amounts of low molecular weight RNA. The high molecular weight Na channel RNA was selected from rat brain poly(A) RNA by hybridization to a single-strand antisense cDNA clone. Translation of this RNA in Xenopus oocytes resulted in the appearance of tetrodotoxin-sensitive voltage-sensitive Na channels in the oocyte membrane. These results demonstrate that mRNA encoding the alpha subunit of the rat brain Na channel, in the absence of any beta-subunit mRNA, is sufficient for translation to give functional channels in oocytes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnew W. S., Levinson S. R., Brabson J. S., Raftery M. A. Purification of the tetrodotoxin-binding component associated with the voltage-sensitive sodium channel from Electrophorus electricus electroplax membranes. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2606–2610. doi: 10.1073/pnas.75.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnew W. S., Moore A. C., Levinson S. R., Raftery M. A. Identification of a large molecular weight peptide associated with a tetrodotoxin binding protein from the electroplax of Electrophorus electricus. Biochem Biophys Res Commun. 1980 Feb 12;92(3):860–866. doi: 10.1016/0006-291x(80)90782-2. [DOI] [PubMed] [Google Scholar]
- Barchi R. L., Cohen S. A., Murphy L. E. Purification from rat sarcolemma of the saxitoxin-binding component of the excitable membrane sodium channel. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1306–1310. doi: 10.1073/pnas.77.3.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneski D. A., Catterall W. A. Covalent labeling of protein components of the sodium channel with a photoactivable derivative of scorpion toxin. Proc Natl Acad Sci U S A. 1980 Jan;77(1):639–643. doi: 10.1073/pnas.77.1.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadei J. M., Gordon R. D., Lampson L. A., Schotland D. L., Barchi R. L. Monoclonal antibodies against the voltage-sensitive Na+ channel from mammalian skeletal muscle. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6227–6231. doi: 10.1073/pnas.81.19.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Contreras R., Cheroutre H., Fiers W. A simple apparatus for injection of nanoliter quantities into Xenopus laevis oocytes. Anal Biochem. 1981 May 1;113(1):185–187. doi: 10.1016/0003-2697(81)90063-4. [DOI] [PubMed] [Google Scholar]
- Costa M. R., Catterall W. A. Cyclic AMP-dependent phosphorylation of the alpha subunit of the sodium channel in synaptic nerve ending particles. J Biol Chem. 1984 Jul 10;259(13):8210–8218. [PubMed] [Google Scholar]
- Darbon H., Jover E., Couraud F., Rochat H. Photoaffinity labeling of alpha- and beta- scorpion toxin receptors associated with rat brain sodium channel. Biochem Biophys Res Commun. 1983 Sep 15;115(2):415–422. doi: 10.1016/s0006-291x(83)80160-0. [DOI] [PubMed] [Google Scholar]
- Dierks P., van Ooyen A., Mantei N., Weissmann C. DNA sequences preceding the rabbit beta-globin gene are required for formation in mouse L cells of beta-globin RNA with the correct 5' terminus. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1411–1415. doi: 10.1073/pnas.78.3.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Messenger RNA from human brain induces drug- and voltage-operated channels in Xenopus oocytes. 1984 Mar 29-Apr 4Nature. 308(5958):421–424. doi: 10.1038/308421a0. [DOI] [PubMed] [Google Scholar]
- Hartshorne R. P., Catterall W. A. Purification of the saxitoxin receptor of the sodium channel from rat brain. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4620–4624. doi: 10.1073/pnas.78.7.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne R. P., Messner D. J., Coppersmith J. C., Catterall W. A. The saxitoxin receptor of the sodium channel from rat brain. Evidence for two nonidentical beta subunits. J Biol Chem. 1982 Dec 10;257(23):13888–13891. [PubMed] [Google Scholar]
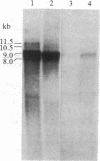
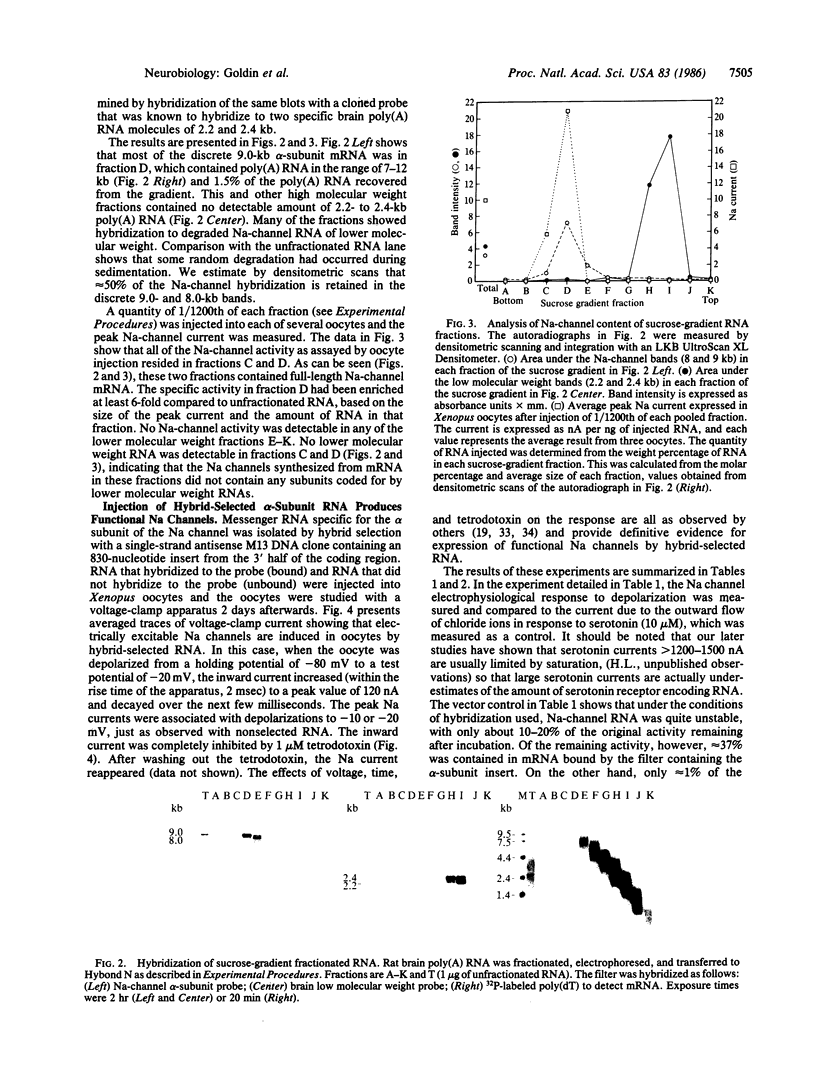
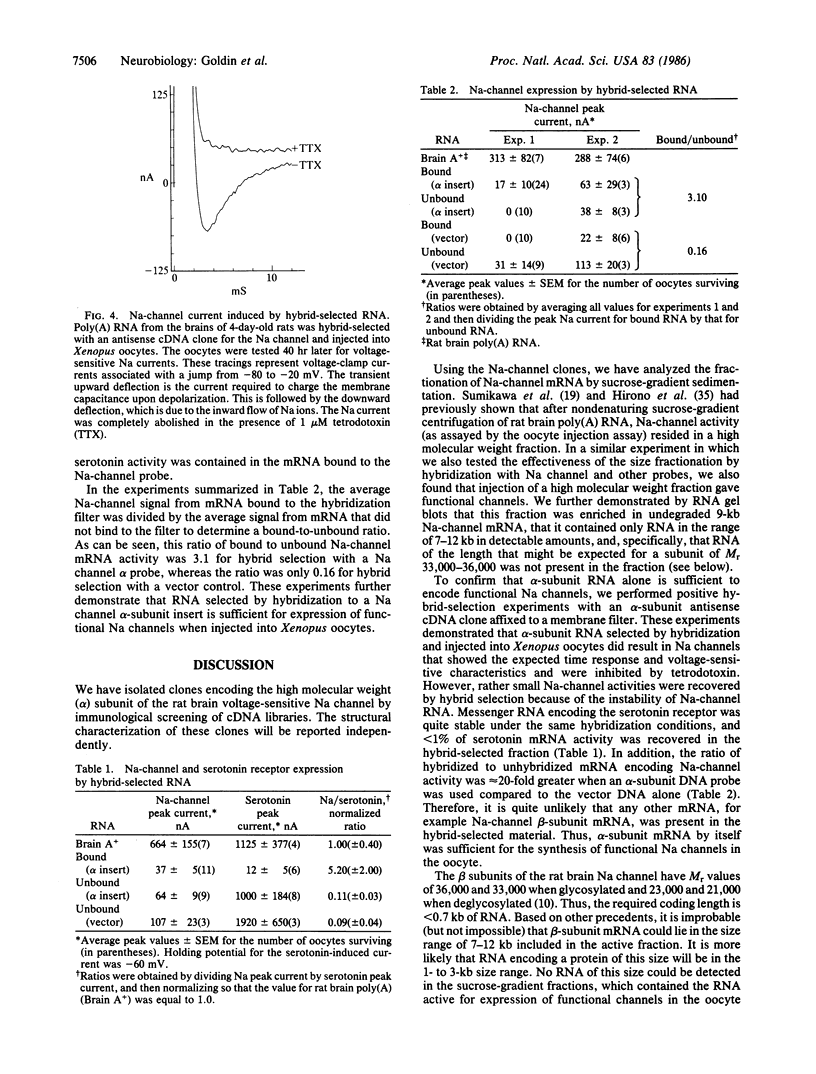
- Hirono C., Yamagishi S., Ohara R., Hisanaga Y., Nakayama T., Sugiyama H. Characterization of mRNA responsible for induction of functional sodium channels in Xenopus oocytes. Brain Res. 1985 Dec 16;359(1-2):57–64. doi: 10.1016/0006-8993(85)91412-x. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegel D. R., Wolf B. D., Sheridan R. E., Lester H. A. Software for electrophysiological experiments with a personal computer. J Neurosci Methods. 1985 Feb;12(4):317–330. doi: 10.1016/0165-0270(85)90016-0. [DOI] [PubMed] [Google Scholar]
- Lombet A., Lazdunski M. Characterization, solubilization, affinity labeling and purification of the cardiac Na+ channel using Tityus toxin gamma. Eur J Biochem. 1984 Jun 15;141(3):651–660. doi: 10.1111/j.1432-1033.1984.tb08241.x. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner D. J., Catterall W. A. The sodium channel from rat brain. Role of the beta 1 and beta 2 subunits in saxitoxin binding. J Biol Chem. 1986 Jan 5;261(1):211–215. [PubMed] [Google Scholar]
- Messner D. J., Catterall W. A. The sodium channel from rat brain. Separation and characterization of subunits. J Biol Chem. 1985 Sep 5;260(19):10597–10604. [PubMed] [Google Scholar]
- Miller J. A., Agnew W. S., Levinson S. R. Principal glycopeptide of the tetrodotoxin/saxitoxin binding protein from Electrophorus electricus: isolation and partial chemical and physical characterization. Biochemistry. 1983 Jan 18;22(2):462–470. doi: 10.1021/bi00271a032. [DOI] [PubMed] [Google Scholar]
- Nakayama H., Withy R. M., Raftery M. A. Use of a monoclonal antibody to purify the tetrodotoxin binding component from the electroplax of Electrophorus electricus. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7575–7579. doi: 10.1073/pnas.79.23.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Ikeda T., Kayano T., Suzuki H., Takeshima H., Kurasaki M., Takahashi H., Numa S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature. 1986 Mar 13;320(6058):188–192. doi: 10.1038/320188a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Shimizu S., Tanabe T., Takai T., Kayano T., Ikeda T., Takahashi H., Nakayama H., Kanaoka Y., Minamino N. Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature. 1984 Nov 8;312(5990):121–127. doi: 10.1038/312121a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. L., Tomiko S. A., Agnew W. S. Reconstitution of neurotoxin-modulated ion transport by the voltage-regulated sodium channel isolated from the electroplax of Electrophorus electricus. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1239–1243. doi: 10.1073/pnas.81.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. L., Tomiko S. A., Agnew W. S. Single-channel properties of the reconstituted voltage-regulated Na channel isolated from the electroplax of Electrophorus electricus. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5594–5598. doi: 10.1073/pnas.81.17.5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. W., Catterall W. A. Biosynthesis and processing of the alpha subunit of the voltage-sensitive sodium channel in rat brain neurons. Cell. 1986 Aug 1;46(3):437–444. doi: 10.1016/0092-8674(86)90664-1. [DOI] [PubMed] [Google Scholar]
- Schmidt J., Rossie S., Catterall W. A. A large intracellular pool of inactive Na channel alpha subunits in developing rat brain. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4847–4851. doi: 10.1073/pnas.82.14.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumikawa K., Parker I., Miledi R. Partial purification and functional expression of brain mRNAs coding for neurotransmitter receptors and voltage-operated channels. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7994–7998. doi: 10.1073/pnas.81.24.7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talvenheimo J. A., Tamkun M. M., Catterall W. A. Reconstitution of neurotoxin-stimulated sodium transport by the voltage-sensitive sodium channel purified from rat brain. J Biol Chem. 1982 Oct 25;257(20):11868–11871. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W., Dumont J. N., Sega M. W. Protein incorporation by isolated amphibian oocytes. 3. Optimum incubation conditions. J Exp Zool. 1973 Jun;184(3):321–333. doi: 10.1002/jez.1401840305. [DOI] [PubMed] [Google Scholar]
- Weigele J. B., Barchi R. L. Functional reconstitution of the purified sodium channel protein from rat sarcolemma. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3651–3655. doi: 10.1073/pnas.79.11.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. M., Mayne K. M., Lester H. A., Davidson N. Mouse-Torpedo hybrid acetylcholine receptors: functional homology does not equal sequence homology. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4852–4856. doi: 10.1073/pnas.82.14.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]