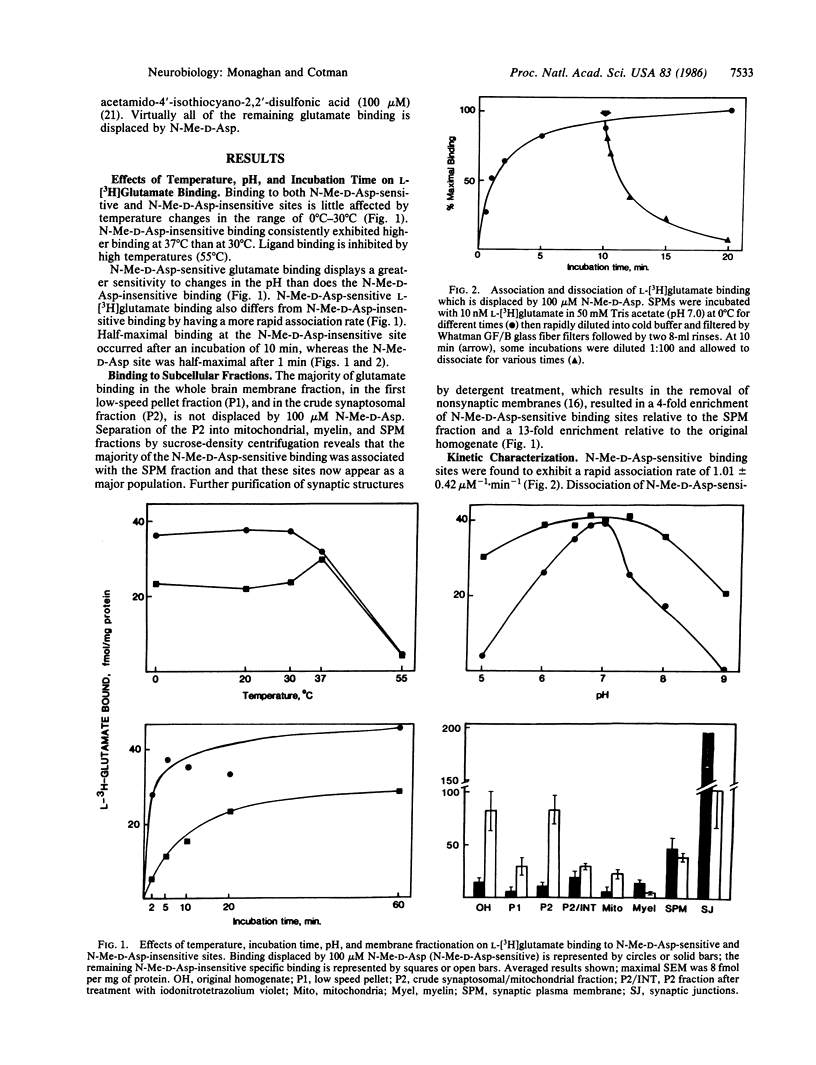
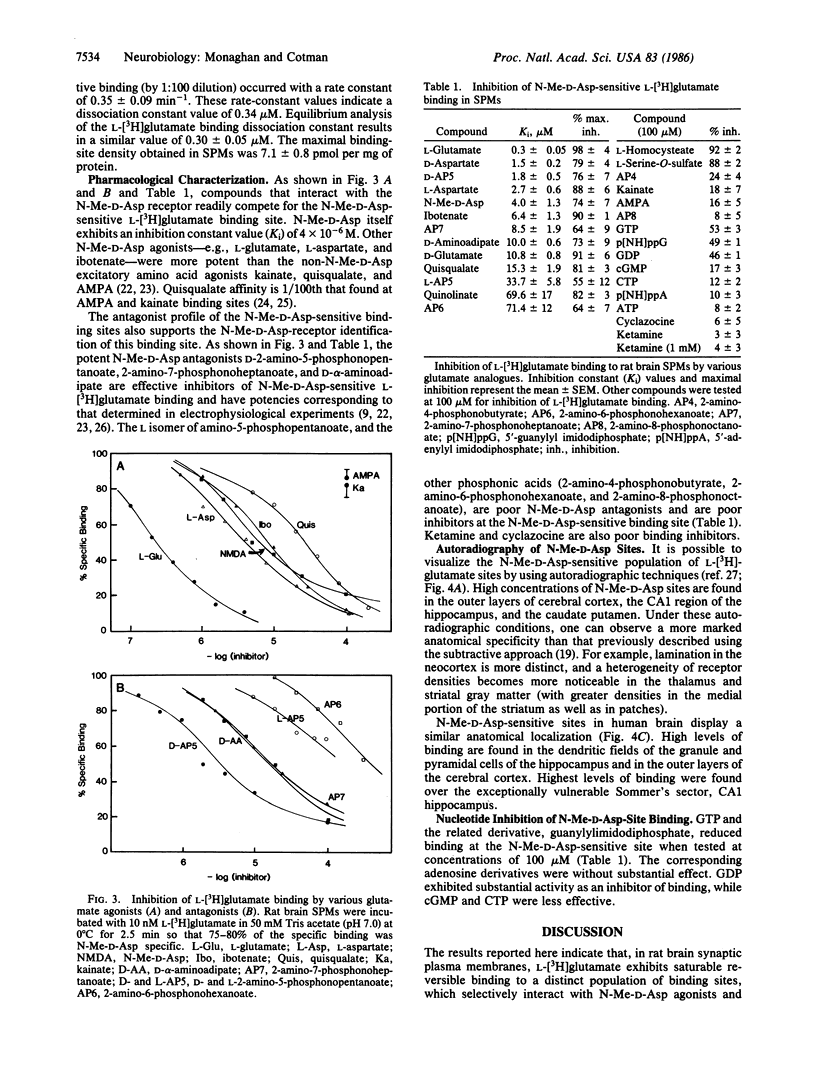
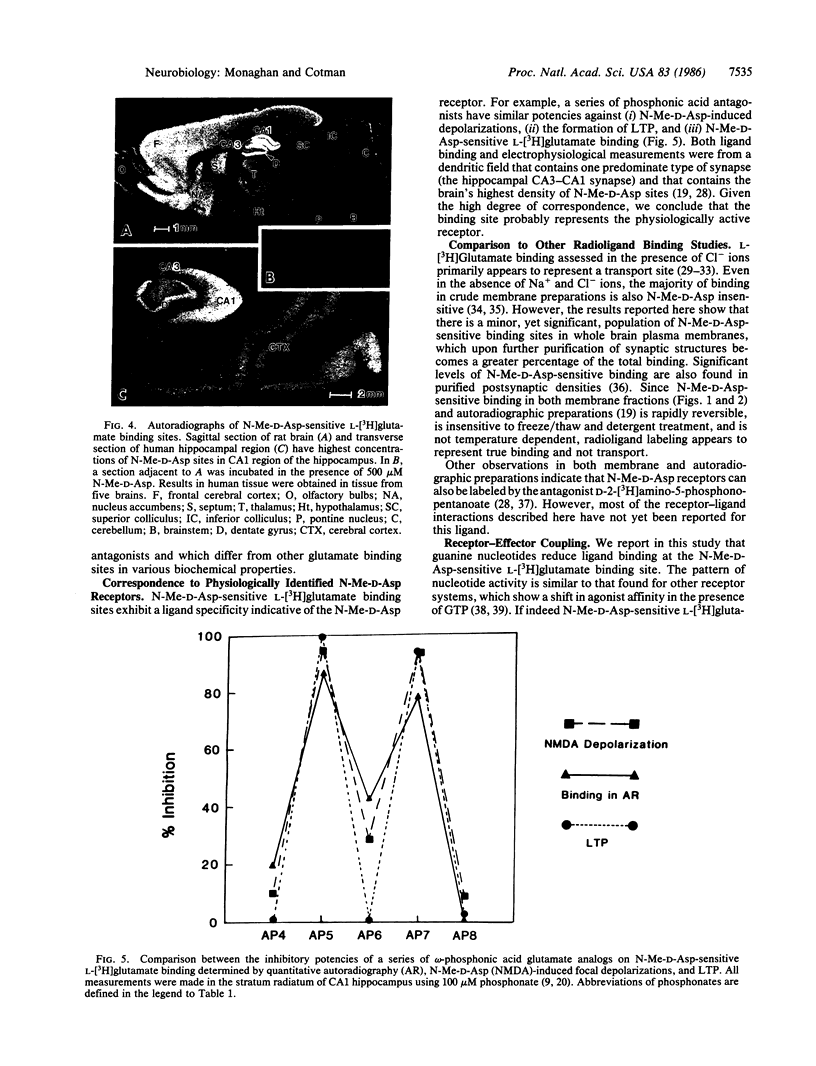
Abstract
The excitatory amino acid receptors selectively activated by N-methyl-D-aspartate (N-Me-D-Asp) (also known as NMDA) are a major determinant of central nervous system neuronal excitability. We report here that rat brain synaptic plasma membranes contain a distinct population of L-[3H]glutamate binding sites with pharmacological properties indicative of the N-Me-D-Asp receptor. The N-Me-D-Asp sites are readily distinguished from other L-[3H]glutamate binding and uptake sites by their sharp pH optimum, more rapid association rate, preferential localization in synaptic structures, and lack of dependence on temperature and inorganic ions. As with other receptor systems, ligand binding at the N-Me-D-Asp site is reduced by guanine nucleotides but not by adenosine nucleotides. Binding is insensitive to ketamine and cyclazocine, indicating that sigma opiates inhibit N-Me-D-Asp excitation at a site different from that of the N-Me-D-Asp binding site. The quantitative pharmacological properties of N-Me-D-Asp-sensitive L-[3H]glutamate binding sites determined in a well-defined dendritic field (stratum radiatum of CA1) by quantitative autoradiography closely correlate to those of both the electrophysiologically identified N-Me-D-Asp receptors in the same dendritic field and the N-Me-D-Asp sites studied in membrane preparations. Under conditions that selectively reveal N-Me-D-Asp receptors, these sites are found to exhibit considerable anatomical specificity as evidenced by variations within cortical, striatal, and thalamic regions. Autoradiography also showed that regions in rodent and primate brain that are especially sensitive to anoxic and excitotoxic neuronal damage (e.g., Sommer's sector or CA1) have a high level of N-Me-D-Asp sites. Since N-Me-D-Asp receptors are known to contribute to these causes of neuronal loss, their selective distribution partially accounts for the pattern of selective damage seen in these pathological conditions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baudry M., Evans J., Lynch G. Excitatory amino acids inhibit stimulation of phosphatidylinositol metabolism by aminergic agonists in hippocampus. Nature. 1986 Jan 23;319(6051):329–331. doi: 10.1038/319329a0. [DOI] [PubMed] [Google Scholar]
- Berrie C. P., Birdsall N. J., Burgen A. S., Hulme E. C. Guanine nucleotides modulate muscarinic receptor binding in the heart. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1000–1005. doi: 10.1016/s0006-291x(79)80006-6. [DOI] [PubMed] [Google Scholar]
- Biziere K., Thompson H., Coyle J. T. Characterization of specific, high-affinity binding sites for L-[3H]glutamic acid in rat brain membranes. Brain Res. 1980 Feb 10;183(2):421–433. doi: 10.1016/0006-8993(80)90476-x. [DOI] [PubMed] [Google Scholar]
- Bridges R. J., Hearn T. J., Monaghan D. T., Cotman C. W. A comparison of 2-amino-4-phosphonobutyric acid (AP4) receptors and [3H]AP4 binding sites in the rat brain. Brain Res. 1986 Jun 4;375(1):204–209. doi: 10.1016/0006-8993(86)90977-7. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman C. W., Taylor D. Isolation and structural studies on synaptic complexes from rat brain. J Cell Biol. 1972 Dec;55(3):696–711. doi: 10.1083/jcb.55.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagg G. E., Lanthorn T. H. Cl-/Ca2+-dependent L-glutamate binding sites do not correspond to 2-amino-4-phosphonobutanoate-sensitive excitatory amino acid receptors. Br J Pharmacol. 1985 Nov;86(3):743–751. doi: 10.1111/j.1476-5381.1985.tb08954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagg G. E., Matus A. Selective association of N-methyl aspartate and quisqualate types of L-glutamate receptor with brain postsynaptic densities. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6876–6880. doi: 10.1073/pnas.81.21.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster A. C., Mena E. E., Fagg G. E., Cotman C. W. Glutamate and aspartate binding sites are enriched in synaptic junctions isolated from rat brain. J Neurosci. 1981 Jun;1(6):620–625. doi: 10.1523/JNEUROSCI.01-06-00620.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes J. W., Monaghan D. T., Cotman C. W., Lott I. T., Kim R. C., Chui H. C. Plasticity of hippocampal circuitry in Alzheimer's disease. Science. 1985 Dec 6;230(4730):1179–1181. doi: 10.1126/science.4071042. [DOI] [PubMed] [Google Scholar]
- Greenamyre J. T., Penney J. B., Young A. B., D'Amato C. J., Hicks S. P., Shoulson I. Alterations in L-glutamate binding in Alzheimer's and Huntington's diseases. Science. 1985 Mar 22;227(4693):1496–1499. doi: 10.1126/science.2858129. [DOI] [PubMed] [Google Scholar]
- Harris E. W., Ganong A. H., Cotman C. W. Long-term potentiation in the hippocampus involves activation of N-methyl-D-aspartate receptors. Brain Res. 1984 Dec 3;323(1):132–137. doi: 10.1016/0006-8993(84)90275-0. [DOI] [PubMed] [Google Scholar]
- Harrison N. L., Simmonds M. A. Quantitative studies on some antagonists of N-methyl D-aspartate in slices of rat cerebral cortex. Br J Pharmacol. 1985 Feb;84(2):381–391. doi: 10.1111/j.1476-5381.1985.tb12922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré T., Lauridsen J., Krogsgaard-Larsen P. The binding of [3H]AMPA, a structural analogue of glutamic acid, to rat brain membranes. J Neurochem. 1982 Jan;38(1):173–178. doi: 10.1111/j.1471-4159.1982.tb10868.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larder A. P., McLennan H. Binding sites for L-glutamate in the central nervous system of the rat. Neurochem Res. 1984 Mar;9(3):393–403. doi: 10.1007/BF00963986. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Mullikin D., Caron M. G. Regulation of beta-adrenergic receptors by guanyl-5'-yl imidodiphosphate and other purine nucleotides. J Biol Chem. 1976 Aug 10;251(15):4686–4692. [PubMed] [Google Scholar]
- Lodge D., Johnston G. A. Effect of ketamine on amino acid-evoked release of acetylcholine from rat cerebral cortex in vitro. Neurosci Lett. 1985 May 23;56(3):371–375. doi: 10.1016/0304-3940(85)90271-x. [DOI] [PubMed] [Google Scholar]
- London E. D., Coyle J. T. Specific binding of [3H]kainic acid to receptor sites in rat brain. Mol Pharmacol. 1979 May;15(3):492–505. [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Meldrum B. Possible therapeutic applications of antagonists of excitatory amino acid neurotransmitters. Clin Sci (Lond) 1985 Feb;68(2):113–122. doi: 10.1042/cs0680113. [DOI] [PubMed] [Google Scholar]
- Mena E. E., Whittemore S. R., Monaghan D. T., Cotman C. W. Ionic regulation of glutamate binding sites. Life Sci. 1984 Dec 10;35(24):2427–2433. doi: 10.1016/0024-3205(84)90451-x. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Cotman C. W. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985 Nov;5(11):2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan D. T., Holets V. R., Toy D. W., Cotman C. W. Anatomical distributions of four pharmacologically distinct 3H-L-glutamate binding sites. Nature. 1983 Nov 10;306(5939):176–179. doi: 10.1038/306176a0. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Yao D., Cotman C. W. L-[3H]Glutamate binds to kainate-, NMDA- and AMPA-sensitive binding sites: an autoradiographic analysis. Brain Res. 1985 Aug 12;340(2):378–383. doi: 10.1016/0006-8993(85)90936-9. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Yao D., Olverman H. J., Watkins J. C., Cotman C. W. Autoradiography of D-2-[3H]amino-5-phosphonopentanoate binding sites in rat brain. Neurosci Lett. 1984 Dec 21;52(3):253–258. doi: 10.1016/0304-3940(84)90170-8. [DOI] [PubMed] [Google Scholar]
- Morris R. G., Anderson E., Lynch G. S., Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. 1986 Feb 27-Mar 5Nature. 319(6056):774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Nicoletti F., Meek J. L., Iadarola M. J., Chuang D. M., Roth B. L., Costa E. Coupling of inositol phospholipid metabolism with excitatory amino acid recognition sites in rat hippocampus. J Neurochem. 1986 Jan;46(1):40–46. doi: 10.1111/j.1471-4159.1986.tb12922.x. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Olverman H. J., Jones A. W., Watkins J. C. L-glutamate has higher affinity than other amino acids for [3H]-D-AP5 binding sites in rat brain membranes. Nature. 1984 Feb 2;307(5950):460–462. doi: 10.1038/307460a0. [DOI] [PubMed] [Google Scholar]
- Pin J. P., Bockaert J., Recasesn M. The Ca2+/C1- dependent L-[3H]glutamate binding: a new receptor or a particular transport process? FEBS Lett. 1984 Sep 17;175(1):31–36. doi: 10.1016/0014-5793(84)80563-3. [DOI] [PubMed] [Google Scholar]
- Rothman S. M., Olney J. W. Glutamate and the pathophysiology of hypoxic--ischemic brain damage. Ann Neurol. 1986 Feb;19(2):105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- Simon R. P., Swan J. H., Griffiths T., Meldrum B. S. Blockade of N-methyl-D-aspartate receptors may protect against ischemic damage in the brain. Science. 1984 Nov 16;226(4676):850–852. doi: 10.1126/science.6093256. [DOI] [PubMed] [Google Scholar]
- Sircar R., Zukin S. R. Quantitative localization of [3H]TCP binding in rat brain by light microscopy autoradiography. Brain Res. 1985 Sep 30;344(1):142–145. doi: 10.1016/0006-8993(85)91198-9. [DOI] [PubMed] [Google Scholar]
- Sladeczek F., Pin J. P., Récasens M., Bockaert J., Weiss S. Glutamate stimulates inositol phosphate formation in striatal neurones. Nature. 1985 Oct 24;317(6039):717–719. doi: 10.1038/317717a0. [DOI] [PubMed] [Google Scholar]
- Sloviter R. S., Dempster D. W. "Epileptic" brain damage is replicated qualitatively in the rat hippocampus by central injection of glutamate or aspartate but not by GABA or acetylcholine. Brain Res Bull. 1985 Jul;15(1):39–60. doi: 10.1016/0361-9230(85)90059-0. [DOI] [PubMed] [Google Scholar]
- Stringer J. L., Greenfield L. J., Hackett J. T., Guyenet P. G. Blockade of long-term potentiation by phencyclidine and sigma opiates in the hippocampus in vivo and in vitro. Brain Res. 1983 Nov 28;280(1):127–138. doi: 10.1016/0006-8993(83)91180-0. [DOI] [PubMed] [Google Scholar]
- Waniewski R. A., Martin D. L. Characterization of L-glutamic acid transport by glioma cells in culture: evidence for sodium-independent, chloride-dependent high affinity influx. J Neurosci. 1984 Sep;4(9):2237–2246. doi: 10.1523/JNEUROSCI.04-09-02237.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- Wieloch T. Hypoglycemia-induced neuronal damage prevented by an N-methyl-D-aspartate antagonist. Science. 1985 Nov 8;230(4726):681–683. doi: 10.1126/science.2996146. [DOI] [PubMed] [Google Scholar]
- Wieloch T., Lindvall O., Blomqvist P., Gage F. H. Evidence for amelioration of ischaemic neuronal damage in the hippocampal formation by lesions of the perforant path. Neurol Res. 1985 Mar;7(1):24–26. doi: 10.1080/01616412.1985.11739695. [DOI] [PubMed] [Google Scholar]
- Wieloch T. Neurochemical correlates to selective neuronal vulnerability. Prog Brain Res. 1985;63:69–85. doi: 10.1016/S0079-6123(08)61976-7. [DOI] [PubMed] [Google Scholar]
- Wigström H., Gustafsson B. A possible correlate of the postsynaptic condition for long-lasting potentiation in the guinea pig hippocampus in vitro. Neurosci Lett. 1984 Feb 24;44(3):327–332. doi: 10.1016/0304-3940(84)90044-2. [DOI] [PubMed] [Google Scholar]