Abstract
Hepatocellular carcinoma is the 3rd most common cancer worldwide. It is an inflammation-associated cancer. Multiple investigators have demonstrated that analysis of the tumor microenvironment may be used to predict patient outcome indicating the importance of local immune responses in this disease. In contrast to other types of cancer, in which surgery, radiation and systemic cytotoxic chemotherapies dominate the treatment options, in HCC loco-regional treatments are widely applied. Such treatments induce rapid tumor cell death and anti-tumor immune responses, which may favor or impair patients’ outcome. Recent immunotherapy studies demonstrating promising results include trials evaluating intra-tumoral injection of an oncolytic virus expressing GM-CSF, glypican-3 targeting treatments and anti-CTLA4 treatment. While some of these novel approaches may provide benefit as single agents, there is a clear opportunity in HCC to evaluate these in combination with the standard modalities to more effectively harness the immune response.
Keywords: Cancer vaccines, glypican 3, antibody treatment, vaccinia, oncolytic virus
Introduction
According to the International Agency for Research on Cancer hepatocellular carcinoma is the 3rd. most common cause of cancer-related death worldwide with an estimated 692,000 cases/year. HCC typically occurs in the setting of chronic inflammation such as viral hepatitis. While patients with early disease have a relatively good prognosis with a 5-year survival of more than 70%, the majority of patients with HCC are diagnosed with late stage disease resulting in an overall 5-year survival rate of less than 16% (1). Impaired metabolism due to liver cirrhosis limits the use of cytotoxic chemotherapy and a number of studies indicate intrinsic resistance of tumor cells to commonly used chemotherapeutic reagents in HCC (2). Sorafenib treatment has shown modest improvement in survival for patients with advanced HCC (3), but no other systemic treatment has demonstrated efficacy at the phase III level in the past five years. With the recent approval of ipilimumab for patients with melanoma and Sipuleucel-T for patients with prostate cancer, immunotherapy has gained the wider attention of both basic scientists and clinicians interested in solid tumors in general, including HCC. There are several characteristics relating to both the treatment and biology of HCC, which make it amenable to immunotherapy. In this review we will discuss some of these HCC-specific aspects and why we believe immunotherapy is an attractive research option for patients with this type of cancer either as an alternative modality or complementary to already existing treatments. We will summarize the data currently available relating to how the human immune system potentially promotes hepatocarcinogenesis, and how it responds to HCC progression. We also review recent promising results from clinical trials using immune-based approaches to treat patients with HCC and discuss the future of immunotherapy for the treatment of HCC.
HCC – an inflammation induced cancer
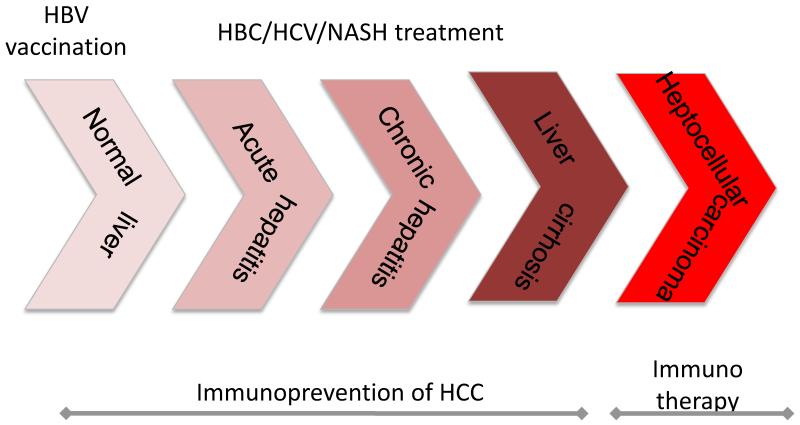
HCC can be considered a classical inflammation-induced cancer. Hepatitis B and Hepatitis C infection are known risk factors for the development of HCC. In most cases patients with chronic viral hepatitis will first develop liver cirrhosis and than HCC, however select patients - with chronic HBV infection for example – are at high risk of developing HCC even in the absence of liver cirrhosis. Based on the pivotal vaccination studies performed in Taiwan, which clearly demonstrated that HBV vaccination decrease the number of children diagnosed with HCC (4), global childhood vaccination against HBV has been introduced and may even be considered the first prophylactic cancer vaccines. New risk factors are emerging however. Obesity, and especially visceral adiposity, can result in non-alcoholic fatty liver disease and non-alcoholic steatohepatitis (NASH). Based on murine studies, local intra-hepatic chronic inflammatory processes promote hepatocarcinogenesis in mice with NASH (5) and accumulating human data indicate an increasing role for NASH as a risk factor for HCC development (6). With a dramatic increase of obesity in the Western World (7), treating NASH (and thereby inflammatory processes) may move more into focus as a way to prevent HCC for this new and rapidly increasing patient population (Figure 1).
Figure 1.
HCC a typical inflammation associated carcinoma
Acute viral infection is followed by chronic infection, development of liver cirrhosis and HCC. Immune based therapies are used to prevent HCC development at various stages and potentially to treat HCC.
Spontaneous immune responses and immune suppression in HCC
Spontaneous immune responses including T-cell responses (8) as well as humoral responses to different tumor-associated antigens (9) have been described in HCC. Different immune cell subsets, cytokines and chemokines have been studied in HCC with respect to their relevance for patient outcome. A number of studies have described that tumor infiltrating CD4+ regulatory T-cells correlate with poor outcome in patients who undergo surgical resection (10,11). Myeloid derived suppressor cells (MDSC) represent a different subset of immune suppressor cells. These cells are not only increased in frequency in patients with HCC and suppress both T and NK cells (12), but have also been shown to induce CD4+ regulatory T cells suggesting a dense interactive network of different immune mechanisms within the tumor microenvironment. A recent study demonstrated an inverse correlation between MDSC frequencies and patient outcome after RFA treatment (13). Relevant markers and cells, which either correlate with outcome or are different from healthy controls are summarized in Tables 1 and 2.
Table 1. Prognostic factors*.
| Good prognosis | Poor prognosis | |
|---|---|---|
| Intratumoral | CCL2 | B7H3 |
| CCL22 | CD3+CD56+ (↓) | |
| CD3+ | IDO (↓) | |
| CD4+ | iNKT | |
| IL-6 | NKG2D (↓) | |
| LTA | PDL1 | |
| NCR3 | Tim3+ | |
| TNF-α | IFN-γ | |
| TLR3 | CD15+ | |
| TLR4 | Tc17 | |
| PD-L1+CD68+ | ||
| Peripheral blood | CD14+HLA-DRlo (↑) | |
| IL-10 (↑) |
prognostic markers, which are increased (↑) or decreased (↓) are marked accordingly
Markers, which have been shown to correlate with better or poor prognosis in patients with HCC.
Table 2. Immune markers changed in HCC.
| Elevated | Reduced | |
|---|---|---|
| Intra-tumoral | Treg Th17 PD1+CD8+ |
CD8+ CD8+FoxP3+ γδ T cell CD56dimCD16+ NK MHC I CD80/CD86 |
| Peripheral blood | IL-1α,IL-3, IL-6 IL-8,IL-12p40 CCL27, CXCL1,CXCL10 CXCL12, IFN-α2,M-CSF GM-CSF,CXCL9,β-NGF SCF,SCGF-β,TNF-β sCD25,TGF-ß CD11b+CD14−CD33+ Treg, Th17 |
CDlc+Lin− |
Markers, which have shown to be elevated or reduced in patients with HCC in comparison to healthy controls, without known correlation to outcome.
Systemic inflammatory responses are routinely described by a combination of serum C-reactive protein levels and albumin concentration (Glasgow prognostic score (GPS)). Inflammation-associated indices have been shown to predict patient outcome both in patients with surgically resectable disease (14) as well in patients with more advanced HCC (15). One difficulty with these indices however is the confounding fact of co-existent liver dysfunction, which is present in the majority of patients with HCC. The GPS may be influenced by liver function and future prospective studies are clearly needed to verify these results. Gene signature studies have been widely conducted to identify HCC subgroups according to their prognosis and even more importantly to identify those patients who may response to specific treatments (16). It is important to note that not only the tumor tissue may be critical to analyze in such studies but also the non-tumoral liver tissue, which can also provide gene signatures correlating with patient outcome (17). A gene signature consisting of 17 immune related genes changing the tumor microenvironment from a Th1 into a Th2 type milieu has been described to predict development of venous metastasis in HCC and impaired outcome (18).
Disease-specific considerations: why HCC is an attractive target for immune-based approaches
The clinical management of HCC has been summarized elsewhere but arguably it differs from that of most other solid tumors in that a wider array of modalities have more common application compared to other tumors whose management is largely confined to (19) surgical resection, radiation and chemotherapy. In HCC, orthotopic liver transplantation is an alternative to surgical resection and requires lifetime immunosuppression. Local ablative approaches are performed to a much larger extent than in other solid tumors and these loco-regional approaches are used with both curative and palliative intent in HCC. Radiofrequency ablation (RFA) is the most commonly applied ablative procedure but alternatives therapies - cryoablation, photodynamic therapy, percutaneous ethanol or acetic acid injection, laser ablation and high intensity frequency ultrasound – are also commonly used depending on local expertise. Transarterial chemoembolization (TACE) is a palliative treatment option for those with liver-confined disease not amenable to potentially curative ablation or surgery. The basic principle of all local ablative therapies is to induce tumor cell death through different mechanistic approaches such as physical destruction, radiation or elimination of vascular supply in combination with chemotherapy. From an immunological point of view these type of procedures result in the release of tumor antigens which are taken up by antigen-presenting cells (mainly dendritic cells) and which have been shown to activate a tumor-specific immune response (20). Ablated tumor tissue has been shown to promote dendritic cell maturation (and resultant T-cell stimulatory properties) (21,22). This antigen release is potentially significant because, although ablative procedures are very effective in eradicating visible lesions, a tumor-specific immune response may prevent recurrent disease in addition to treating distant metastases. In other words, RFA and TACE have the potential to turn a patient’s tumor into an endogenous vaccine. Indeed several studies have documented an increase in peripheral antitumor immunity following interventional radiological procedures and these are summarized below.
Immune responses following RFA and TACE
Activation and increased cytolytic activity of tumor specific CD8+ T cell responses after radio-frequency ablation have been demonstrated in patients with HCC (Figure 2) and colorectal liver metastases (23). Mizokushi et al. evaluated T cell responses in HCC patients undergoing RFA. These investigators observed immune responses to antigens for which no T cell response was detected at baseline prior to RFA and the number of tumor-specific T cells after RFA correlated with the prevention of HCC recurrence in patients treated with curative intent (24). Zerbini et al. found that RFA treatment was followed by a significant increase of patients responsive to tumor antigens derived from both the untreated and the necrotic tumor tissue. Here, T-cell responses to recall antigens were also significantly augmented and phenotypic analysis of circulating T and natural killer cells showed an increased expression of activation and cytotoxic surface markers. (21,25). Ayaru et al. evaluated the immune response in 10 HCC patients undergoing TACE and described an expansion of AFP-specific CD4 T cell responses upon TACE. Patients with increased frequencies of AFP-specific CD4 T cells after treatment also demonstrated more tumor necrosis and an improved clinical outcome (26). In a different study AFP-specific responses were evaluated in patients after TACE in which patients also received a DC infusion. An increase in frequency of AFP-specific T cells was observed in some patients after ablation. However, tumor recurrence was not completely prevented in patients albeit displayed enhanced immune responses (24). Two other small studies investigated tumor specific immune responses in response to combined TACE and RFA treatment or each individual treatment and confirmed the finding that ablative therapies induce tumor-specific T cell responses in individual patients upon ablative therapies (27,28). Recent studies demonstrated that both cryoablation as well as high intensity focused ultrasound (HiFU) might induce immune responses. A change in CD4:CD8 T cell ration in peripheral blood was observed in cancer patients treated with HiFU (29). In a different study the association between circulating PD-L1/ PD-1 levels and prognosis after cryoablation in patients with HBV-related HCC was studied. In this study upregulation of circulating PD-L1/PD-1 was associated with poor post-cryoablation prognosis (30). While many studies suggest that tumor ablation induced anti-tumor immune responses, no direct comparison of different ablative approaches has been performed in patients with HCC to identify the most immunogenic ablation technique (31).
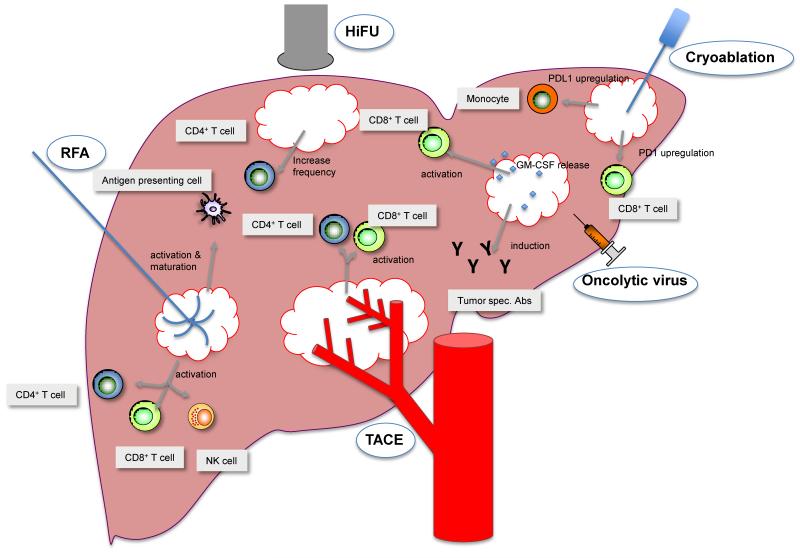
Figure 2.
Effect of ablative therapies
This figure summarizes immunological effects of different ablative treatments (RFA, HiFU, TACE, cryoablation, oncolytic viruses) on CD4+ T cells, CD8+ T cells, dendritic cells, NK cells, monocytes, PD1/PDL1 expression and generation of antibody responses.
Sorafenib treatment
Many chemotherapeutics and molecular targeting agents have been shown to effect anti-tumor immune responses (32). Sorafenib is the only approved and effective drug for the treatment of HCC. In mice, sorafenib has been shown to have limited effects on anti-tumor immunity (33). In vitro studies suggest that low dose sorafenib may promote effector CD4+ T cell function by eliminating Treg suppressor function in PBMC obtained from patients with HCC (34). Furthermore, it has been reported that sorafenib treatment decreases Th2 and regulatory T cells in peripheral blood from patients with HCC (35).
Immunotherapy in HCC
Immunotherapy has been tested in HCC for many years. Most studies in the past have either used cytokine based or antigen-based approaches (36,37). While most of these studies have proven to be safe and able to induce tumor-specific immune responses, most of them have failed to demonstrate clinical efficacy. Here we would like to summarize recent results from different immune-based approaches in HCC. These studies differ from previous immunotherapy studies in a number of respects: the approach taken to activate patients immune system; the target; the quality of the immune response being activated; and finally the more promising results seen.
Oncolytic viruses
The use of oncolytic virus for the treatment of HCC is a very recent development and promising preliminary data have been presented using this approach in early clinical trials. JX-594 is an oncolytic poxvirus modified by insertion of β-galactosidase, a surrogate marker for detection of viral gene expression and human GM-CSF to stimulate anti-tumor immune responses into the thymidine kinase region. The vaccinia virus replicates in cancer cells harboring activation of the epidermal growth factor receptor (EGFR)/Ras pathway and depends on cellular TK, the concentration of which is increased by cell-cycle abnormalities in cancer cells. JX-595 induces cell lysis in infected cells followed by the induction of a GM-CSF-enhanced anticancer immunity (38). While initial studies using a JX-594 prototype were done in patients with melanoma (39) intra-tumoral application of JX-594 was shown to be safe in a phase I study in HCC (40). Results from a second study in 30 patients with advanced HCC were recently reported (41). In this trial, patients were randomized for treatment with low-dose (108 PFU) or high dose (109 PFU) JX-594 and safety, patient outcome and induction of immunity both against cancer and vaccinia were studied. JX-594 was administered by imaging-guided intra-tumoral injection on days 1, 15 and 29. Objective responses rates were 15% and an intrahepatic disease control of 50% was achieved. It should be noted that disease control rates were equivalent in injected and distant untreated tumors suggesting the presence of a systemic immune response. In contrast to tumor response rates and immune endpoints, there was a clear dose relationship with regard to overall survival (6.7 months in the low and 14.1 months in the high dose).
Immune monitoring studies demonstrated that at least 11 of 16 patients developed HCC-specific antibody responses. Cellular T cell responses were noted and a significant decrease in HBV DNA concentrations were noted in patients treated with JX-594 previously (42).
Glypican 3 targeted therapies
Glypican-3 (GPC3) has emerged as an interesting target for immunotherapy in HCC. GPC3 is a member of the glypican family of heparan sulfate (HS) proteoglycans that are attached to the cell surface. GPC3 is specifically overexpressed in approximately 80% of HCC and correlates with poor prognosis (43,44). Different studies suggest that it may be used as a serum marker in patients with HCC. Immunostaining for GPC3 is recommended by international guidelines for pathological diagnosis of HCC especially since it can be used to distinguish high grade dysplastic nodules from early HCC (45). GPC3 is an attractive target as a tumor antigen because it is not only tumor-specific but also important for cell proliferation (46). Both a peptide based vaccine approach as well as anti-GPC3 antibodies are currently in clinical development. Thirty-three patients with advanced HCC were vaccinated with two different GPC3 derived peptides depending on their HLA haplotype. While safety was the primary endpoint of this study and as expected vaccination was well tolerated, secondary endpoint analysis demonstrated a partial response in one patient and 19 patients with stable disease 2 months after initiation of treatment. GPC-3 specific T cell responses were observed in 30 patients and GPC-3 specific CTL frequency after vaccination correlated with OS (47). In one case, an autopsy was performed on one of the patients, which revealed central necrosis in most of the intrahepatic tumor following peptide vaccination with an infiltration of GPC-3 reactive CD8-positive T cells in the residual carcinoma, but not within the cirrhotic area (48). A phase II study using this approach in the adjuvant setting after surgical resection is currently ongoing.
In an alternative approach anti-glypican 3 antibodies have been developed and are currently in preclinical and clinical evaluation for the treatment of patients with HCC. GC33 is a novel recombinant fully humanized monoclonal antibody that binds to human glypican-3 (GPC3). Twenty patients were enrolled and treated with GC33 in a pilot trial. Patients tolerated treatment well and no dose-limiting toxicities (DLT) were observed. Potential antitumor activity that was associated with the target GPC3 expression was observed in this study. Stable disease was seen in 4 patients, all of whom had high GPC3 expression. In a second study the combination of sorafenib with anti-glypican-3 is tested. Using phage display technology, anti-glypican 3 antibody HN3 was identified. This antibody binds to a unique conformational epitope in the core protein of GPC3 with high affinity (Kd = 0.6 nM). In preclinical studies it was shown that HN3 inhibits proliferation of GPC3-positive HCC tumor cells implementing a second and immune independent mode of action by direct inhibition of cell proliferation by targeting cell-cycle arrest through inactivating yap (49).
Immune checkpoint inhibitors
Monoclonal antibodies that target the immunoregulatory dampening mechanisms of host responses to tumor-associated antigens have recently gained a lot of interest with the approval of anti-CTLA-4 (ipilimumab) for the treatment of patients with advanced melanoma. CTLA-4 is induced upon antigen stimulation and blocks further T cell activation. The most important molecular function of CTLA-4 seems to be the inhibition of CD28 co-stimulation, as evidenced by the CD28-dependent uncontrolled lymphoproliferative and autoimmune syndrome observed in CTLA-4 null mice. Ipilimumab blocks the interaction of CTLA4 with its ligands CD80 and CD86 and thereby promoting T cell activation (50). In patients with previously treated advanced melanoma ipilimumab improved patient’s median overall survival by almost 4 months and resulted in long term disease control in a significant minority (51). Based on these encouraging results tremelimumab a different anti-CTLA4 antibody was tested in a pilot clinical trial in twenty patients with advanced HCC and HCV infection. Tremelimumab was given at a dose of 15 mg/kg every 90 days i.v. Treatment was tolerated well. Three out of 17 evaluable patients developed confirmed partial responses and 10 patients had stable disease as the best response to treatment. HCV-specific T cell responses were studied in these patients as a surrogate marker for the anti-CTLA4-induced immune responses. HCV-specific T cell responses were noted in a number of patients and a decline in viral load was observed in most patients followed for at least 3 months. Three patients had a complete viral response (52). Other immune checkpoint inhibitors such as anti-PD1 are currently under clinical investigation (NCT01658878).
Outlook and future
While this report has focused on human studies and it is clearly beyond the scope of this review to summarize data from murine studies, it is obvious that good animal models mimicking the patients’ disease are needed to develop future immune based approaches for the treatment of HCC. The fact that 90% of patients with HCC also suffer from underlying liver disease, which impairs metabolism of cytotoxic and targeted therapies, in conjunction with recent advances in immunotherapy clearly calls for the development of new immune-based treatment approached in HCC. The observation of tumor regression at site distant from the primary site of radiotherapy in a melanoma patient treated with ipilimumab (53) suggests that combination of local tumor treatments with checkpoint inhibitors may induce strong anti-tumor immune responses leading to clinical responses. Recent observations using checkpoint inhibitors for the treatment of patients with other types of solid cancer clearly call for studies also in HCC. Since local tumor ablation is routinely used in patients with HCC and has been shown to induce immune responses, a rational extension would be to boost this immune response by combination with, for example, a checkpoint inhibitor. These as well as all other immune-based treatment approaches and concepts including but not restricted to dendritic cell based vaccines, antibody based and cytokine treatments have the potential to induce delayed but long lasting anti-tumor effects in a patient population currently poorly served by systemic therapies (Figure 3). Choosing the best response evaluation criteria will be a true challenge. Two different response evaluation tools already exist for HCC, RECIST and modified RECIST (19). Changes induced by inflammation inside the tumors and frequently observed delayed responses in other indications have formed the rationale to develop “immune-related response criteria” (54), which will need to be used for HCC patients undergoing immunotherapy trials. Underlying liver cirrhosis with impaired liver function as well as chronic hepatitis infection commonly found in patients with HCC will require considerable expertise when designing new study concepts. Only trials with adequate immune-monitoring will advance the field of immunotherapy in HCC. Most importantly, investigators need to keep in mind that HCC is vastly different from other types of cancer and may require disease specific considerations when developing new treatment approaches for patients with HCC.
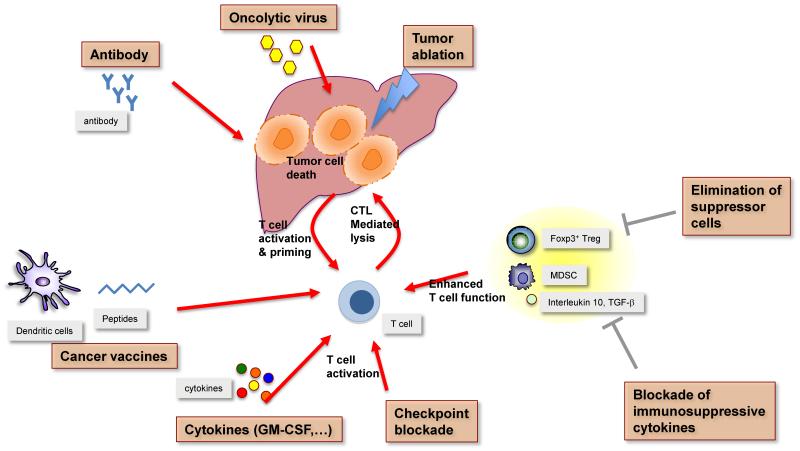
Figure 3.
Immune based combination approaches in HCC.
Tumor cells are targeted through different mechanisms such as tumor ablation (TACE, RFA), antibody mediated cytotoxicity or oncolytic viruses, which all lead to antigen release and T cell activation and priming. Tumor specific T cells are activated by different mechanisms (vaccines, cytokines, checkpoint blockade) or by blockade of inhibitory cells (Tregs and MDSC) or inhibitory cytokines and ultimately can kill tumor cells.
Acknowledgement
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. Our research is supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research to T.F.G.
Financial Support: Intramural Research Program of the NIH, National Cancer Institute
Footnotes
Conflict of interest: The authors report no potential conflicts of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: A Cancer Journal for Clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Asghar U, Meyer T. Are there opportunities for chemotherapy in the treatment of hepatocellular cancer? Journal of Hepatology. 2012;56:686–95. doi: 10.1016/j.jhep.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J-F, et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 4.Chang MH, Chen CJ, Lai MS, Hsu HM, Wu T-C, Kong MS, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N. Engl. J. Med. 1997;336:1855–9. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 5.Park EJ, Lee JH, Yu G-Y, He G, Ali SR, Holzer RG, et al. Dietary and Genetic Obesity Promote Liver Inflammation and Tumorigenesis by Enhancing IL-6 and TNF Expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–32. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Overweight and Obesity [Internet] 2013 May 20; Available from: http://www.cdc.gov/obesity/data/adult.html.
- 8.Mizukoshi E, Nakamoto Y, Arai K, Yamashita T, Sakai A, Sakai Y, et al. Comparative analysis of various tumor-associated antigen-specific t-cell responses in patients with hepatocellular carcinoma. Hepatology. 2011;53:1206–16. doi: 10.1002/hep.24149. [DOI] [PubMed] [Google Scholar]
- 9.Korangy F, Ormandy LA, Bleck JS, Klempnauer J, Wilkens L, Manns MP, et al. Spontaneous tumor-specific humoral and cellular immune responses to NY-ESO-1 in hepatocellular carcinoma. Clin. Cancer Res. 2004;10:4332–41. doi: 10.1158/1078-0432.CCR-04-0181. [DOI] [PubMed] [Google Scholar]
- 10.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–39. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 11.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J. Clin. Oncol. 2007;25:2586–93. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 12.Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50:799–807. doi: 10.1002/hep.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arihara F, Mizukoshi E, Kitahara M, Takata Y, Arai K, Yamashita T, et al. Increase in CD14(+)HLA-DR (-/low) myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol Immunother. 2013;62:1421–30. doi: 10.1007/s00262-013-1447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishizuka M, Kubota K, Kita J, Shimoda M, Kato M, Sawada T. Impact of an inflammation-based prognostic system on patients undergoing surgery for hepatocellular carcinoma: a retrospective study of 398 Japanese patients. Am. J. Surg. 2012;203:101–6. doi: 10.1016/j.amjsurg.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 15.Pinato DJ, Stebbing J, Ishizuka M, Khan SA, Wasan HS, North BV, et al. A novel and validated prognostic index in hepatocellular carcinoma: The inflammation based index (IBI) Journal of Hepatology. 2012;57:1013–20. doi: 10.1016/j.jhep.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 16.Hoshida Y, Moeini A, Alsinet C, Kojima K, Villanueva A. Gene signatures in the management of hepatocellular carcinoma. Seminars in Oncology. 2012;39:473–85. doi: 10.1053/j.seminoncol.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N. Engl. J. Med. (2008 ed.) 2008;359:1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Budhu A, Forgues M, Ye Q-H, Jia H-L, He P, Zanetti KA, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 19.European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer EASL-EORTC Clinical Practice Guidelines: Management of hepatocellular carcinoma. Journal of Hepatology. 2012;56:908–43. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Brok den MH, Sutmuller RP, Nierkens S, Bennink EJ, Frielink C, Toonen LW, et al. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer. (2006 ed.) 2006;95:896–905. doi: 10.1038/sj.bjc.6603341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zerbini A, Pilli M, Penna A, Pelosi G, Schianchi C, Molinari A, et al. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res. 2006;66:1139–46. doi: 10.1158/0008-5472.CAN-05-2244. [DOI] [PubMed] [Google Scholar]
- 22.Zerbini A, Pilli M, Fagnoni F, Pelosi G, Pizzi MG, Schivazappa S, et al. Increased immunostimulatory activity conferred to antigen-presenting cells by exposure to antigen extract from hepatocellular carcinoma after radiofrequency thermal ablation. J. Immunother. 2008;31:271–82. doi: 10.1097/CJI.0b013e318160ff1c. [DOI] [PubMed] [Google Scholar]
- 23.Hansler J, Wissniowski TT, Schuppan D, Witte A, Bernatik T, Hahn EG, et al. Activation and dramatically increased cytolytic activity of tumor specific T lymphocytes after radio-frequency ablation in patients with hepatocellular carcinoma and colorectal liver metastases. World J. Gastroenterol. (2006 ed.) 2006;12:3716–21. doi: 10.3748/wjg.v12.i23.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizukoshi E, Yamashita T, Arai K, Sunagozaka H, Ueda T, Arihara F, et al. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2013;57:1448–57. doi: 10.1002/hep.26153. [DOI] [PubMed] [Google Scholar]
- 25.Zerbini A, Pilli M, Laccabue D, Pelosi G, Molinari A, Negri E, et al. Radiofrequency thermal ablation for hepatocellular carcinoma stimulates autologous NK-cell response. Gastroenterology. 2010;138:1931–42. doi: 10.1053/j.gastro.2009.12.051. [DOI] [PubMed] [Google Scholar]
- 26.Ayaru L, Pereira SP, Alisa A, Pathan AA, Williams R, Davidson B, et al. Unmasking of alpha-fetoprotein-specific CD4(+) T cell responses in hepatocellular carcinoma patients undergoing embolization. J. Immunol. 2007;178:1914–22. doi: 10.4049/jimmunol.178.3.1914. [DOI] [PubMed] [Google Scholar]
- 27.Hiroishi K, Eguchi J, Baba T, Shimazaki T, Ishii S, Hiraide A, et al. Strong CD8(+) T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J. Gastroenterol. 2010;45:451–8. doi: 10.1007/s00535-009-0155-2. [DOI] [PubMed] [Google Scholar]
- 28.Nobuoka D, Motomura Y, Shirakawa H, Yoshikawa T, Kuronuma T, Takahashi M, et al. Radiofrequency ablation for hepatocellular carcinoma induces glypican-3 peptide-specific cytotoxic T lymphocytes. Int. J. Oncol. 2012;40:63–70. doi: 10.3892/ijo.2011.1202. [DOI] [PubMed] [Google Scholar]
- 29.Wu F, Wang Z-B, Lu P, Xu Z-L, Chen W-Z, Zhu H, et al. Activated anti-tumor immunity in cancer patients after high intensity focused ultrasound ablation. Ultrasound Med Biol. 2004;30:1217–22. doi: 10.1016/j.ultrasmedbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Zeng Z, Shi F, Zhou L, Zhang M-N, Chen Y, Chang X-J, et al. Luk J, editor. Upregulation of Circulating PD-L1/PD-1 Is Associated with Poor Post-Cryoablation Prognosis in Patients with HBV-Related Hepatocellular Carcinoma. PLoS ONE. 2011;6:e23621. doi: 10.1371/journal.pone.0023621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greten TF, Korangy F. Radiofrequency ablation for the treatment of HCC--maybe much more than simple tumor destruction? Journal of Hepatology. 2010;53:775–6. doi: 10.1016/j.jhep.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov. 2012;11:215–33. doi: 10.1038/nrd3626. [DOI] [PubMed] [Google Scholar]
- 33.Kapanadze T, Gamrekelashvili J, Ma C, Chan C, Zhao F, Hewitt S, et al. Regulation of accumulation and function of myeloid derived suppressor cells in different murine models of hepatocellular carcinoma. Journal of Hepatology. 2013 doi: 10.1016/j.jhep.2013.06.010. inpress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cabrera R, Ararat M, Xu Y, Brusko T, Wasserfall C, Atkinson MA, et al. Immune modulation of effector CD4+ and regulatory T cell function by sorafenib in patients with hepatocellular carcinoma. Cancer Immunol Immunother. 2013;62:737–46. doi: 10.1007/s00262-012-1380-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagai H, Mukozu T, Matsui D, Kanekawa T, Kanayama M, Wakui N, et al. Hindawi Publishing Corporation Sorafenib Prevents Escape from Host Immunity in Liver Cirrhosis Patients with Advanced Hepatocellular Carcinoma. Clin. Dev. Immunol. (9 ed.) 2012;2012:1–8. doi: 10.1155/2012/607851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greten TF, Manns MP, Korangy F. Immunotherapy of HCC. Rev Recent Clin Trials. 2008;3:31–9. doi: 10.2174/157488708783330549. [DOI] [PubMed] [Google Scholar]
- 37.Greten TF, Manns MP, Korangy F. Immunotherapy of hepatocellular carcinoma. Journal of Hepatology. 2006;45:868–78. doi: 10.1016/j.jhep.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Kirn DH, Thorne SH. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer. 2009;9:64–71. doi: 10.1038/nrc2545. [DOI] [PubMed] [Google Scholar]
- 39.Mastrangelo MJ, Maguire HC, Eisenlohr LC, Laughlin CE, Monken CE, McCue PA, et al. Intratumoral recombinant GM-CSF-encoding virus as gene therapy in patients with cutaneous melanoma. Cancer Gene Ther. 1999;6:409–22. doi: 10.1038/sj.cgt.7700066. [DOI] [PubMed] [Google Scholar]
- 40.Park B-H, Hwang T, Liu T-C, Sze DY, Kim J-S, Kwon H-C, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. The Lancet Oncology. 2008;9:533–42. doi: 10.1016/S1470-2045(08)70107-4. [DOI] [PubMed] [Google Scholar]
- 41.Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nature Medicine. 2013;19:329–36. doi: 10.1038/nm.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu T-C, Hwang T, Park B-H, Bell J, Kirn DH. The targeted oncolytic poxvirus JX-594 demonstrates antitumoral, antivascular, and anti-HBV activities in patients with hepatocellular carcinoma. Mol. Ther. 2008;16:1637–42. doi: 10.1038/mt.2008.143. [DOI] [PubMed] [Google Scholar]
- 43.Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, et al. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89–97. doi: 10.1016/s0016-5085(03)00689-9. [DOI] [PubMed] [Google Scholar]
- 44.Filmus J, Capurro M. Glypican-3: a marker and a therapeutic target in hepatocellular carcinoma. FEBS J. 2013 doi: 10.1111/febs.12126. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 45.International Consensus Group for Hepatocellular NeoplasiaThe International Consensus Group for Hepatocellular Neoplasia Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49:658–64. doi: 10.1002/hep.22709. [DOI] [PubMed] [Google Scholar]
- 46.Capurro MI, Xiang Y-Y, Lobe C, Filmus J. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res. 2005;65:6245–54. doi: 10.1158/0008-5472.CAN-04-4244. [DOI] [PubMed] [Google Scholar]
- 47.Sawada Y, Yoshikawa T, Nobuoka D, Shirakawa H, Kuronuma T, Motomura Y, et al. Phase I Trial of a Glypican-3-Derived Peptide Vaccine for Advanced Hepatocellular Carcinoma: Immunologic Evidence and Potential for Improving Overall Survival. Clinical Cancer Research. 2012;18:3686–96. doi: 10.1158/1078-0432.CCR-11-3044. [DOI] [PubMed] [Google Scholar]
- 48.Sawada Y, Yoshikawa T, Fujii S, Mitsunaga S, Nobuoka D, Mizuno S, et al. Remarkable tumor lysis in a hepatocellular carcinoma patient immediately following glypican-3-derived peptide vaccination: An autopsy case. Hum Vaccin Immunother. 2013;9:1228–1233. doi: 10.4161/hv.24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feng M, Gao W, Wang R, Chen W, Man YG, Figg WD, et al. Therapeutically targeting glypican-3 via a conformation-specific single-domain antibody in hepatocellular carcinoma. Proceedings of the National Academy of Sciences. 2013;110:E1083–91. doi: 10.1073/pnas.1217868110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Melero I, Hervás-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7:95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- 51.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sangro B, Gomez-Martin C, la Mata de M, Iñarrairaegui M, Garralda E, Barrera P, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. Journal of Hepatology. 2013;59:81–8. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 53.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012;366:925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]