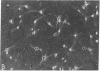
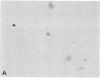
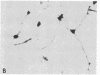
Abstract
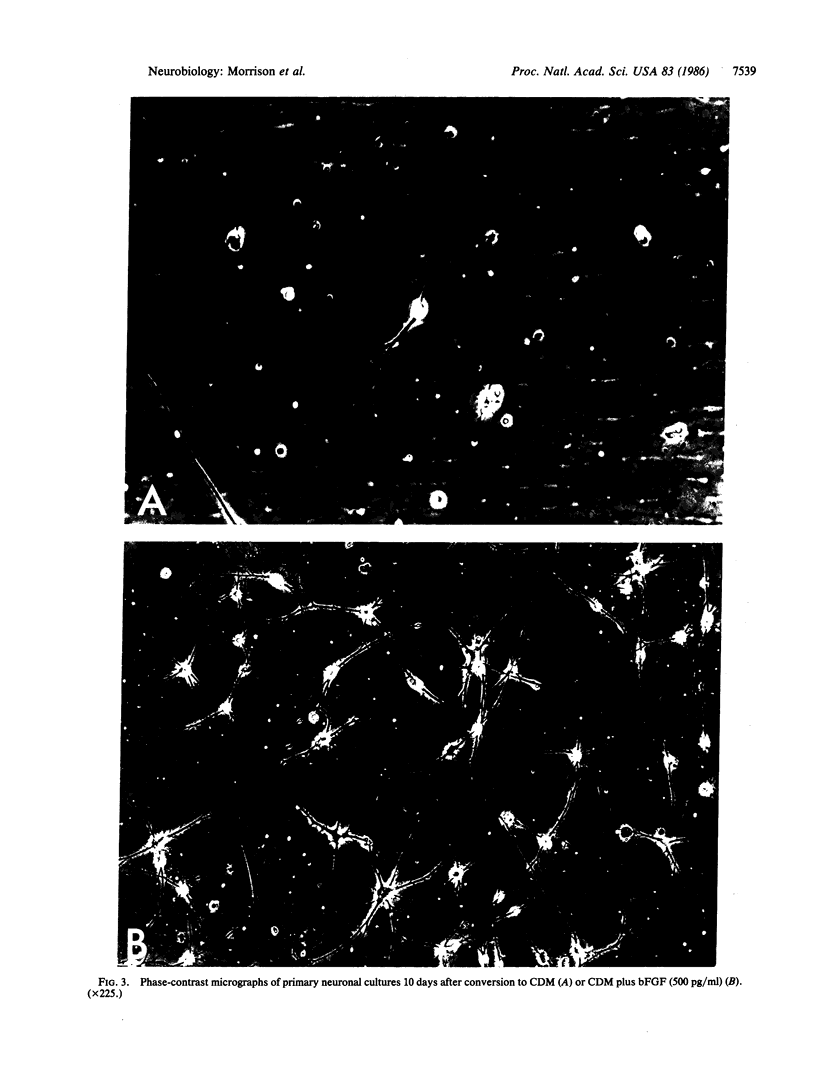
Bovine basic fibroblast growth factor (bFGF) is a potent mitogen isolated from bovine pituitary glands and brain. The addition of homogeneous bFGF to primary cultures of rat cerebral cortical neurons markedly enhances cell survival and elaboration of neurites. These effects are dose-dependent, with optimal stimulation occurring at a concentration of 500 pg/ml. Maintenance of survival and neurite outgrowth require the continuous presence of bFGF. Other growth factors, such as thrombin, platelet-derived growth factor, beta nerve growth factor, and interleukin 2, have no effect on neuronal survival or process formation. Although the cellular site(s) of bFGF synthesis has not yet been established, these results suggest that bFGF may function as a neurotrophic agent in the central nervous system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird A., Böhlen P., Ling N., Guillemin R. Radioimmunoassay for fibroblast growth factor (FGF): release by the bovine anterior pituitary in vitro. Regul Pept. 1985 Apr;10(4):309–317. doi: 10.1016/0167-0115(85)90043-6. [DOI] [PubMed] [Google Scholar]
- Banker G. A. Trophic interactions between astroglial cells and hippocampal neurons in culture. Science. 1980 Aug 15;209(4458):809–810. doi: 10.1126/science.7403847. [DOI] [PubMed] [Google Scholar]
- Barbin G., Manthorpe M., Varon S. Purification of the chick eye ciliary neuronotrophic factor. J Neurochem. 1984 Nov;43(5):1468–1478. doi: 10.1111/j.1471-4159.1984.tb05410.x. [DOI] [PubMed] [Google Scholar]
- Barde Y. A., Edgar D., Thoenen H. New neurotrophic factors. Annu Rev Physiol. 1983;45:601–612. doi: 10.1146/annurev.ph.45.030183.003125. [DOI] [PubMed] [Google Scholar]
- Barde Y. A., Edgar D., Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1(5):549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. K. New neuronal growth factors. Annu Rev Neurosci. 1984;7:149–170. doi: 10.1146/annurev.ne.07.030184.001053. [DOI] [PubMed] [Google Scholar]
- Bradshaw R. A. Nerve growth factor. Annu Rev Biochem. 1978;47:191–216. doi: 10.1146/annurev.bi.47.070178.001203. [DOI] [PubMed] [Google Scholar]
- Böhlen P., Baird A., Esch F., Ling N., Gospodarowicz D. Isolation and partial molecular characterization of pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5364–5368. doi: 10.1073/pnas.81.17.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. Induction of neurite outgrowth by a conditioned-medium factor bound to the culture substratum. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5210–5213. doi: 10.1073/pnas.75.10.5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch F., Baird A., Ling N., Ueno N., Hill F., Denoroy L., Klepper R., Gospodarowicz D., Böhlen P., Guillemin R. Primary structure of bovine pituitary basic fibroblast growth factor (FGF) and comparison with the amino-terminal sequence of bovine brain acidic FGF. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6507–6511. doi: 10.1073/pnas.82.19.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Bialecki H., Thakral T. K. The angiogenic activity of the fibroblast and epidermal growth factor. Exp Eye Res. 1979 May;28(5):501–514. doi: 10.1016/0014-4835(79)90038-1. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J., Lui G. M., Baird A., Böhlent P. Isolation of brain fibroblast growth factor by heparin-Sepharose affinity chromatography: identity with pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6963–6967. doi: 10.1073/pnas.81.22.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D. Localisation of a fibroblast growth factor and its effect alone and with hydrocortisone on 3T3 cell growth. Nature. 1974 May 10;249(453):123–127. doi: 10.1038/249123a0. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Massoglia S., Cheng J., Lui G. M., Böhlen P. Isolation of pituitary fibroblast growth factor by fast protein liquid chromatography (FPLC): partial chemical and biological characterization. J Cell Physiol. 1985 Feb;122(2):323–332. doi: 10.1002/jcp.1041220223. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D. Purification of a fibroblast growth factor from bovine pituitary. J Biol Chem. 1975 Apr 10;250(7):2515–2520. [PubMed] [Google Scholar]
- Kligman D. Isolation of a protein from bovine brain which promotes neurite extension from chick embryo cerebral cortex neurons in defined medium. Brain Res. 1982 Oct 28;250(1):93–100. doi: 10.1016/0006-8993(82)90955-6. [DOI] [PubMed] [Google Scholar]
- Lemmon S. K., Bradshaw R. A. Purification and partial characterization of bovine pituitary fibroblast growth factor. J Cell Biochem. 1983;21(3):195–208. doi: 10.1002/jcb.240210302. [DOI] [PubMed] [Google Scholar]
- Lemmon S. K., Riley M. C., Thomas K. A., Hoover G. A., Maciag T., Bradshaw R. A. Bovine fibroblast growth factor: comparison of brain and pituitary preparations. J Cell Biol. 1982 Oct;95(1):162–169. doi: 10.1083/jcb.95.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R. Developmental neurobiology and the natural history of nerve growth factor. Annu Rev Neurosci. 1982;5:341–362. doi: 10.1146/annurev.ne.05.030182.002013. [DOI] [PubMed] [Google Scholar]
- Lindsay R. M. Adult rat brain astrocytes support survival of both NGF-dependent and NGF-insensitive neurones. Nature. 1979 Nov 1;282(5734):80–82. doi: 10.1038/282080a0. [DOI] [PubMed] [Google Scholar]
- Lindsay R. M., Tarbit J. Developmentally regulated induction of neurite outgrowth from immature chick sensory neurons (DRG) by homogenates of avian or mammalian heart, liver and brain. Neurosci Lett. 1979 May;12(2-3):195–200. doi: 10.1016/0304-3940(79)96061-0. [DOI] [PubMed] [Google Scholar]
- Martínez H. J., Dreyfus C. F., Jonakait G. M., Black I. B. Nerve growth factor promotes cholinergic development in brain striatal cultures. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7777–7781. doi: 10.1073/pnas.82.22.7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy K. D., de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980 Jun;85(3):890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill J. F., Chao M. V., Ishii D. N. Insulin, insulin-like growth factor II, and nerve growth factor effects on tubulin mRNA levels and neurite formation. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7126–7130. doi: 10.1073/pnas.82.20.7126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monard D., Solomon F., Rentsch M., Gysin R. Glia-induced morphological differentiation in neuroblastoma cells. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1894–1897. doi: 10.1073/pnas.70.6.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. S., De Vellis J., Lee Y. L., Bradshaw R. A., Eng L. F. Hormones and growth factors induce the synthesis of glial fibrillary acidic protein in rat brain astrocytes. J Neurosci Res. 1985;14(2):167–176. doi: 10.1002/jnr.490140202. [DOI] [PubMed] [Google Scholar]
- Morrison R. S., de Vellis J. Growth of purified astrocytes in a chemically defined medium. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7205–7209. doi: 10.1073/pnas.78.11.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld G., Gospodarowicz D. The identification and partial characterization of the fibroblast growth factor receptor of baby hamster kidney cells. J Biol Chem. 1985 Nov 5;260(25):13860–13868. [PubMed] [Google Scholar]
- Pettmann B., Weibel M., Sensenbrenner M., Labourdette G. Purification of two astroglial growth factors from bovine brain. FEBS Lett. 1985 Sep 9;189(1):102–108. doi: 10.1016/0014-5793(85)80851-6. [DOI] [PubMed] [Google Scholar]
- Recio-Pinto E., Ishii D. N. Effects of insulin, insulin-like growth factor-II and nerve growth factor on neurite outgrowth in cultured human neuroblastoma cells. Brain Res. 1984 Jun 8;302(2):323–334. doi: 10.1016/0006-8993(84)90246-4. [DOI] [PubMed] [Google Scholar]
- Slack J. R., Pockett S. Motor neurotrophic factor in denervated adult skeletal muscle. Brain Res. 1982 Sep 9;247(1):138–140. doi: 10.1016/0006-8993(82)91037-x. [DOI] [PubMed] [Google Scholar]
- Soares M. B., Ishii D. N., Efstratiadis A. Developmental and tissue-specific expression of a family of transcripts related to rat insulin-like growth factor II mRNA. Nucleic Acids Res. 1985 Feb 25;13(4):1119–1134. doi: 10.1093/nar/13.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H., Sakai M., Obata K. Effects of serum, tissue extract, conditioned medium, and culture substrata on neurite appearance from spinal cord explants of chick embryo. Brain Res. 1982 Jul;256(3):303–312. doi: 10.1016/0165-3806(82)90142-0. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Barde Y. A. Physiology of nerve growth factor. Physiol Rev. 1980 Oct;60(4):1284–1335. doi: 10.1152/physrev.1980.60.4.1284. [DOI] [PubMed] [Google Scholar]