Abstract
Background
Isoflurane releases renal tubular transforming growth factor-beta 1 (TGF-β1) and protects against ischemic acute kidney injury (AKI). Recent studies suggest that TGF-β1 can induce a cytoprotective cytokine interleukin (IL)-11. Here, we tested the hypothesis that isoflurane protects against ischemic AKI by direct induction of renal tubular IL-11 synthesis.
Methods
Human kidney proximal tubule (HK-2) cells were treated with 1.25-2.5% isoflurane or carrier gas (room air+5% carbon dioxide) for 0-16 h. We also anesthetized C57BL/6 mice with 1.2% isoflurane or with equi-anesthetic dose of pentobarbital for 4 h. In addition, we subjected IL-11 receptor (IL-11R) wild type, IL-11R deficient or IL-11 neutralized mice to 30-min renal ischemia followed by reperfusion under 4 h of pentobarbital or isoflurane (1.2%) anesthesia.
Results
Isoflurane increased IL-11 synthesis in human (~300-500% increase, N = 6) and mouse (23 ± 4 (mean ± SD) fold over carrier gas group, N = 4) proximal tubule cells that were attenuated by a TGF-β1 neutralizing antibody. Mice anesthetized with isoflurane showed significantly increased kidney IL-11 messenger RNA (13.8 ± 2 fold over carrier gas group, N = 4) and protein (31 ± 9 vs. 18±2 pg/mg protein or ~80% increase, N = 4) expression compared to pentobarbital anesthetized mice and this increase was also attenuated by a TGF-β1 neutralizing antibody. Furthermore, isoflurane-mediated renal protection in IL-11R wild-type mice were absent in IL-11R deficient mice or in IL-11R wild-type mice treated with IL-11 neutralizing antibody (N = 4-6).
Conclusions
Our studies suggest that isoflurane induces renal tubular IL-11 via TGF-β1 signaling to protect against ischemic AKI.
Introduction
Acute kidney injury (AKI) remains a major perioperative complication and results in extremely high mortality and morbidity costing more than 10 billion dollars per year in the United States.1,2 Furthermore, AKI is frequently associated with other life-threatening complications including remote multiorgan injury and sepsis.2-5 Renal ischemia-reperfusion (I/R) injury or ischemic AKI is a frequent complication for patients subjected to major cardiac, hepatobiliary or transplant surgery.3,6 Unfortunately, there is no effective clinically proven therapy for ischemic AKI. Furthermore, patients who survive initial renal injury frequently suffer from long term chronic kidney disease.
Volatile halogenated anesthetic are one of the most widely used drugs during the perioperative period.7 Our previous studies demonstrated that clinically utilized volatile halogenated anesthetics including isoflurane at clinically relevant concentrations (~1-2 minimum alveolar concentration) protect against ischemic AKI by attenuating renal tubular necrosis and by retarding renal tubular inflammation with reduction in influx of proinflammatory leukocytes.8,9 We also demonstrated that volatile halogenated anesthetics produce direct anti-inflammatory and anti-necrotic effects in cultured human kidney proximal tubule (HK-2) cells.10,11 Subsequently, we discovered that volatile halogenated anesthetics protect against renal tubular necrosis and inflammation by direct renal tubular production of transforming growth factor-beta 1 (TGF-β1).11-13 However, the downstream signaling mechanisms of volatile halogenated anesthetic-mediated renal protection generated by TGF-β1 remain incompletely understood. Moreover, isoflurane therapy for critically ill patients may be limited by its anesthetic and cardiovascular effects. One way to mitigate this is to utilize the distal signaling molecules synthesized with isoflurane treatment devoid of systemic hemodynamic and anesthetic effects.
Interleukin (IL)-11 is a 20 kDa member of the IL-6-type cytokine family. IL-11 promotes megakaryocyte maturation and is already clinically approved to increase platelet counts in patients receiving chemotherapy.14 In addition to its hematopoietic effects, IL-11 protects against intestinal, cardiomyocyte and endothelial cell death.15 We recently showed that recombinant human IL-11 treatment before or after renal ischemia attenuated ischemic AKI in mice.16 Specifically, IL-11 administration significantly attenuated necrosis, inflammation and apoptosis after ischemic AKI closely mimicking the renal protective effects of volatile halogenated anesthetics. This IL-11-mediated protection against ischemic AKI requires the downstream induction of another cytoprotective protein sphingosine kinase-1. Interestingly, we also showed that isoflurane-mediated protection against ischemic AKI also requires sphingosine kinase-1 induction.17 Finally, previous studies suggest that TGF-β1 induces IL-11 in lung epithelial cells and fibroblasts.18,19 Therefore, in this study we tested the hypothesis that isoflurane induces TGF-β1 mediated renal proximal tubular IL-11 synthesis. We also tested whether IL-11 plays a critical role in isoflurane-mediated renal protection.
Materials and Methods
Human and mouse proximal tubule cell culture and exposure to isoflurane
Immortalized human renal proximal tubule (HK-2) cells (American Type Culture Collection, Manassas, VA) were grown and passaged with 50:50 mixture of Dulbecco’s Modified Eagle Media/F12 with 10% fetal bovine serum (Invitrogen, Carlsbad, CA) and antibiotics (100 U/ml penicillin G, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B (Invitrogen) at 37°C in a 100% humidified atmosphere of 5% carbon dioxide-95% air. This cell line has been characterized extensively and retains the phenotypic and functional characteristics of proximal tubule cells in culture.20 We also cultured mouse kidney proximal tubule cells. Mouse kidneys were removed, minced and digested in collagenase A (1 mg/mL, Sigma, St. Louis, MO) at 37°C for 45 min with occasional agitation. The cellular digest was filtered through a nylon mesh, centrifuged at 600g for 10 min, and washed twice. Mouse kidney proximal tubules were isolated according to the method of Vinay et al. using Percoll density gradient separation.21 Cells were used in the experiments described below when confluent after 24-h serum deprivation.
HK-2 cells or mouse proximal tubules in culture were placed in an air tight, 37°C, humidified modular incubator chamber (Billups-Rothenberg, Inc., Del Mar, CA) with inflow and outflow ports. The inlet port was connected to a vaporizer (Datex-Ohmeda, GE Healthcare, Oklahoma City, OK) to deliver isoflurane (Abbott Laboratories, North Chicago, IL) mixed with 95% air and 5% carbon dioxide (carrier gas) at 10 L/min. The outlet port was connected to a Datex-Ohmeda 5250 RGM gas analyzer that measured isoflurane concentrations. Exposure to isoflurane (1.25-2.5%) lasted 0-16 h. Control cells were exposed to carrier gas in an identical modular incubator chamber. To block the effects of TGF-β1 generated by isoflurane, some HK-2 cells were pretreated with neutralizing TGF-β1 antibody (10μg/ml, R&D Systems, Minneapolis, MN) 30 min. before isoflurane treatment, respectively. We also used non-neutralizing control isotype antibody to test the specificity of the neutralizing TGF-β1 antibody (BD Biosciences, San Jose, CA).
Reverse transcription polymerase chain reaction for IL-11
With reverse transcription polymerase chain reaction, we measured messenger RNA (mRNAs) encoding human (HK-2 cells) or mouse IL-11 as described.22 Amplification of the human IL-11 complementary deoxyribonucleic acid was performed using the following primers: forward primer, 5′-CTG AAG ACT CGG CTG TGA CC-3′ and reverse primer, 5′-CAG GGC AGA AGT CTG TGG AC-3′ with an annealing temperature of 66°C resulting in a 300 bp product. Amplification of the mouse IL-11 complementary deoxyribonucleic acid was performed using the following primers: forward primer, 5′-AAC TGT GTT TGT CGC CTG GT-3′ and reverse primer, 5′-AAG CTG CAA AGA TCC CAA TG -3′ with an annealing temperature of 68°C resulting in a 267 bp product. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) complementary deoxyribonucleic acid amplification was performed to control for lane loading: forward primer, 5′-ACC ACA GTC CAT GCC ATC AC-3′ and reverse primer, 5′-CAC CAC CCT GTT GCT GTA GCC-3′ with an annealing temperature of 65°C resulting in a 450 bp product.
IL-11 Enzyme-linked immunosorbent assay (ELISA)
HK-2 cell supernatant or mouse kidney cortex lysate IL-11 protein expression was measured using human or mouse-specific sandwich IL-11 ELISA kit (R&D Systems), respectively.
Mouse anesthesia and induction of renal I/R injury
After Columbia University Institutional Animal Care and Use Committee (New York, New York) approval, we used adult male IL-11 receptor deficient (IL-11R knockout) mice or wild-type (WT) litter mates (IL-11R WT) on a C57BL/6 background (B6.129S1-Il11ra1tm1Wehi/J, Jackson Labs, Bar Harbor, ME). Mice were initially anesthetized with intraperitoneal pentobarbital (Henry Schein Veterinary Co., Indianapolis, IN; 50 mg/kg body weight, or to effect) and subjected to right nephrectomy and 30 min of left renal ischemia or to sham-operation (laparotomy, right nephrectomy without renal ischemia).8,9 After closure of the abdomen in two layers, the mice were then exposed to an additional 4 h of equipotent doses of either pentobarbital or 1.2% isoflurane as described previously.23 The mice were placed on a heating pad under a warming light to maintain body temperature ~36-38°C. To neutralize IL-11 in vivo, some IL-11R WT mice were injected intravenously with 1 mg/kg monoclonal anti-IL-11 (MAB418, R&D Systems) 20 min before reperfusion of ischemic kidney or sham-operation. We collected kidney (cortex and cortico-medullary junction) and plasma 6-24 h after I/R injury to examine the severity of renal dysfunction (plasma creatinine, renal histology, apoptosis and neutrophil infiltration) and IL-11 mRNA and protein detection. Plasma creatinine was measured as described with an enzymatic creatinine reagent kit according to the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA).24 Unlike the Jaffe method, this method of creatinine measurement largely eliminates the interferences from mouse plasma chromagens.
Some IL-11R WT mice were anesthetized with pentobarbital or with 1.2% isoflurane without being subjected to renal I/R injury. To test the critical role of TGF-β1 signaling in isoflurane-mediated IL-11 induction in vivo, some IL-11 WT mice were injected intravenously with 5 mg/kg monoclonal anti-TGF-β1 (MAB240, R&D Systems) or control isotype antibody 30 min. before isoflurane or pentobarbital anesthesia.
Histological detection of kidney necrosis, apoptosis and neutrophil infiltration
Morphological assessment of kidney hematoxylin and eosin stain staining was performed by renal pathologist (V.DA.) who was unaware of the treatment that each animal had received. An established grading scale of necrotic injury (0-4, Renal injury score) to the proximal tubules was used for the histopathological assessment of I/R-induced damage as outlined by Jablonski et al.25 and as described previously in our studies.26,27 We detected kidney apoptosis with terminal deoxynucleotidyl transferase 2′-deoxyuridine-5′-triphosphate nick end labeling staining as described elsewhere28 using a commercially available in situ cell death detection kit (Roche, Indianapolis, IN) according to the instructions provided by the manufacturer. Apoptotic terminal deoxynucleotidyl transferase 2′-deoxyuridine-5′-triphosphate nick end labeling positive cells were quantified in 5-7 randomly chosen 100× microscope images fields in the corticomedullary junction and results were expressed as apoptotic cells counted per 100× field. Immunohistochemistry for neutrophils was performed as described previously29 with a rat anti-mouse Ly6B monoclonal antibody against polymorphonuclear leukocytes (clone 7/4, AbD Serotec, Raleigh, NC). A primary antibody that recognized IgG2a (MCA1212, AbD Serotec) was used as a negative isotype control in all experiments. Neutrophils infiltrating the kidney were quantified in 5-7 randomly chosen 200× microscope image fields in the cortico-medullary junction and results were expressed as neutrophils counted per 200× field.
IL-11 immunohistochemistry
Immunohistochemistry detected mouse kidney IL-11 protein expression and localization 4 hr after anesthesia under pentobarbital or 1.2% isoflurane with rat anti-IL-11 antibody (MAB418, 1:50 dilution; R&D Systems) and biotin conjugated anti-rat IgG (1:100 dilution, Vector Laboratories, Burlingame, CA). Normal rat IgG2a (Vector Laboratories) was used at the same concentration as the primary antibody as a negative isotype control. Kidney IL-11 immunohistochemistry was quantified as described by Kristina et al. with some modifications.30 Integrated image densities of five to seven randomly selected renal tubule areas from each slide were averaged and background measured from isotype control slides were subtracted. Renal tubular IL-11 intensity was expressed as fold increase over pentobarbital anesthetized mice.
Statistical analysis
The data were analyzed with two-tailed Student’s t-test when comparing means between two groups or one-way or two-way analysis of variance plus Tukey’s post hoc multiple comparison test when comparing multiple groups. The ordinal values of the renal injury scores were analyzed by the Mann-Whitney nonparametric test. We used Graphad InStat and Graphad Prism for statistical analyses (GraphPad Software, Inc., La Jolla, CA). In all cases, a probability statistic P < 0.05 was taken to indicate significance. All data are expressed throughout the text as means ± SD.
Results
Isoflurane induces IL-11 in human and mouse proximal tubule cells via TGF-β1 signaling
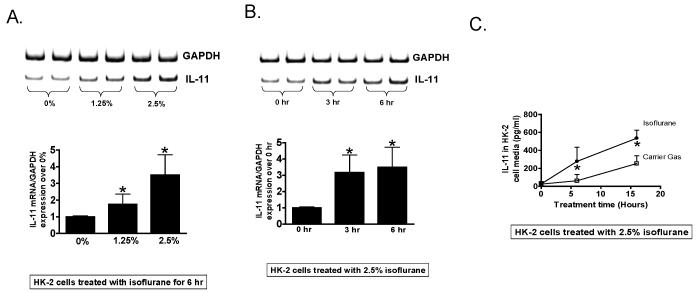
Figure 1A shows a concentration-dependent (0-2.5%) induction of IL-11 mRNA in HK-2 cells after isoflurane treatment (N = 6). Figure 1B shows that 2.5% isoflurane significantly increased IL-11 mRNA after 3- or 6-h treatment. Isoflurane treatment (2.5%) in HK-2 cells for 6-16 hr also induced IL-11 protein (released into cell culture media, fig. 1C) in HK-2 cells compared to carrier gas-treated cells (N = 6).
Figure 1. Isoflurane induces interleukin (IL)-11 messenger ribonucleic acid (mRNA) and protein synthesis in human proximal tubule (HK-2) cells.
A and B. IL-11 mRNA measured with reverse transcription polymerase chain reaction (RTPCR) in HK-2 cells treated with 0-2.5% isoflurane for 6 h (A. N = 6) or 2.5% isoflurane for 0-6 h (B, N = 6). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression was quantified to normalize lane loading. Data are presented as means ± SD. * P < 0.05 vs. IL-11 mRNA measured after 0% isoflurane-treatment (A) or at 0 h (B). C. Isoflurane increases IL-11 protein (pg/ml) in cell culture media from HK-2 cells (N = 6). HK-2 cells were treated with 2.5% isoflurane or with carrier gas for 6 h or 16 h. * P < 0.05 vs. carrier gas treated group.
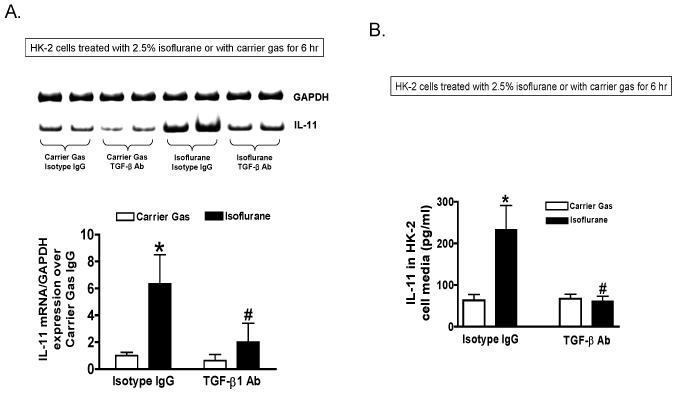
HK-2 cells pretreated with control isotype antibody (mouse IgG) demonstrated significant induction of IL-11 mRNA (fig. 2A) and protein (fig. 2B) expression (N = 6) after isoflurane exposure (2.5% for 6 h). We determined that TGF-β1 neutralizing antibody (10 μg/ml) significantly attenuated the upregulation of IL-11 mRNA as well as protein expression after isoflurane treatment.
Figure 2. Isoflurane induces interleukin (IL)-11 in human proximal tubule (HK-2) cells via transforming growth factor-beta 1 (TGF-β1).
A. IL-11 messenger ribonucleic acid (mRNA) (detected with reverse transcription polymerase chain reaction (RTPCR)) expression in HK-2 cells treated with 2.5% isoflurane for 6 h (N = 6). Representative images (top) and band intensity quantifications (bottom) expressed as fold increases in IL-11 expression over carrier gas plus immunoglobulin G (IgG) isotype antibody treated controls. B. IL-11 protein (detected with enzyme-linked immunosorbent assay (ELISA)) expression in HK-2 cells treated with 2.5% isoflurane for 6 h (N = 6). * P < 0.05 vs. carrier gas group treated with IgG isotype antibody. # P < 0.05 vs. isoflurane group treated with IgG isotype antibody. Error bars represent 1 SD. TGF-β1 antibody (10 μg/ml) prevents isoflurane-mediated induction of IL-11 mRNA and protein expression in human proximal tubule cells.
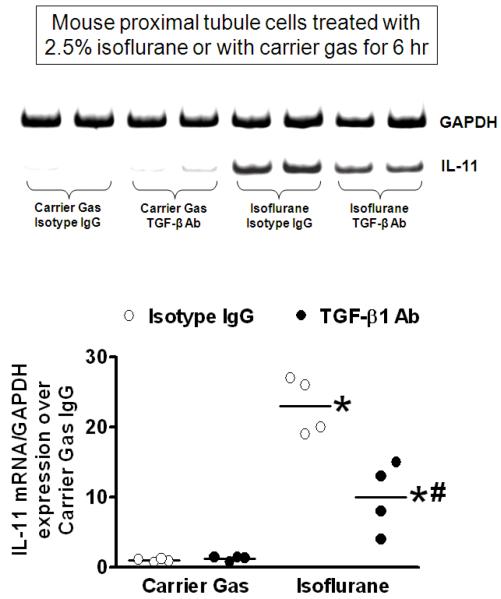
Figure 3 shows that primary cultures of mouse kidney proximal tubule cells pretreated with control isotype mouse IgG had significant IL-11 mRNA induction after 6-h treatment with 2.5% isoflurane (N= 4). TGF-β1 neutralization significantly attenuated the upregulation of IL-11 mRNA in isoflurane-treated mouse proximal tubule cells. Therefore, our studies show that isoflurane-induced TGF-β1 directly promotes the synthesis of IL-11 in both immortalized and primary cultures of renal proximal tubule cells.
Figure 3. Isoflurane induces interleukin (IL)-11 messenger ribonucleic acid (mRNA) expression in primary culture of mouse proximal tubule cells via transforming growth factor-beta 1 (TGF-β1).
IL-11 mRNA (reverse transcription polymerase chain reaction (RTPCR)) expression in primary culture of mouse proximal tubule cells treated with 2.5% isoflurane for 6 h (N = 4). Representative images (top) and band intensity quantifications (bottom) expressed as fold increases in IL-11 expression over carrier gas and immunoglobulin G (IgG) isotype antibody treated controls. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression was also quantified to normalize lane loading. * P < 0.05 vs. carrier gas group treated with IgG isotype antibody. # P < 0.05 vs. isoflurane group treated with IgG isotype antibody. Error bars represent 1 SD. TGF-β1 antibody (10 μg/ml) prevents isoflurane-mediated induction of IL-11 mRNA and protein expression in mouse proximal tubule cells.
Isoflurane-mediated induction of kidney IL-11 in vivo via TGF-β1 signaling
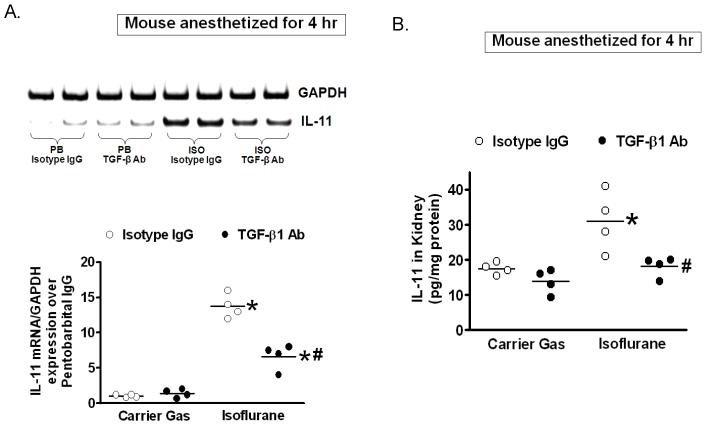
Figure 4 shows that isoflurane anesthesia (1.2% for 4 h) significantly induced IL-11 mRNA (fig. 4A) and protein expression (fig. 4B) measured in mouse kidneys compared to mice anesthetized with pentobarbital for 4 h (N = 4). We also determined that TGF-β1 neutralizing antibody (5 mg/kg monoclonal anti-TGF-β1, MAB240) prevented the induction of kidney IL-11 mRNA and protein expression after isoflurane anesthesia in mice (fig. 4, N = 4).
Figure 4. Isoflurane increases interleukin (IL)-11 messenger ribonucleic acid (mRNA) and protein synthesis in mouse kidney via transforming growth factor-beta 1 (TGF-β1) signaling.
Naïve IL-11 wild-type (WT) mice were exposed to pentobarbital (PB) or 1.2% isoflurane (ISO) for 4 h. A. Representative bands for IL-11 mRNA (reverse transcription polymerase chain reaction (RTPCR)) expression in mouse kidney (N = 4). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as an internal loading control. B. Kidney lysate IL-11 protein (detected by enzyme-linked immunosorbent assay (ELISA)) in IL-11 naïve WT mice exposed to pentobarbital (PB) or 1.2% isoflurane (ISO) for 4 h (N = 4). To neutralize TGF-β1 in vivo, some IL-11 WT mice were injected with 5 mg/kg monoclonal anti-TGF-β1 (MAB240) antibody intravenous injection. TGF-β1 neutralization prevented the induction of IL-11 after isoflurane anesthesia. * P < 0.05 vs. pentobarbital anesthetized mice treated with immunoglobulin G (IgG) isotype antibody. # P < 0.05 vs. isoflurane anesthetized mice treated with IgG isotype antibody. Error bars represent 1 SD. Isoflurane anesthesia significantly increased kidney IL-11 mRNA and protein expression in mice.
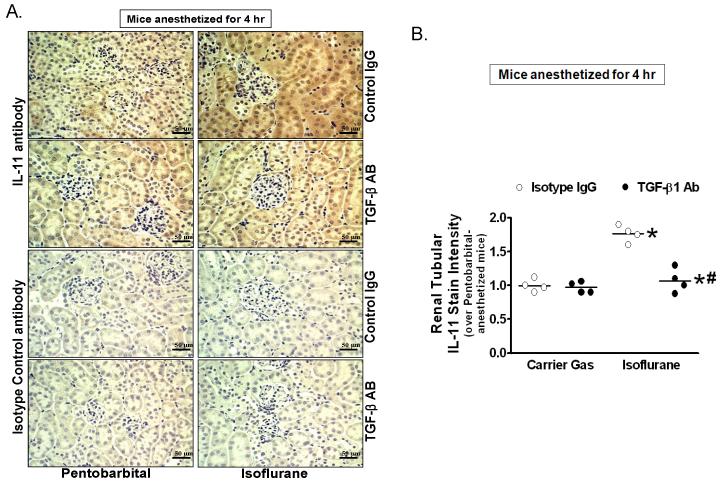
Figure 5A shows diffuse renal tubular IL-11 staining in mice anesthetized with pentobarbital and increased IL-11 staining in the kidneys of mice anesthetized with 1.2% isoflurane for 4 h (representative of 4 experiments, 100× and 400× images shown). Quantification of immunohistochemical staining confirmed significant increase in IL-11 immunoreactivity in mice anesthetized with isoflurane (fig. 5B, N= 4). This increase in IL-11 immunoreactivity with isoflurane anesthesia was again attenuated by treating mice with TGF-β1 neutralizing antibody before isoflurane anesthesia. Therefore, from these experiments we conclude that isoflurane induces IL-11 and synthesis in vivo via TGF-β1 signaling. IL-11 staining was not visible in the kidneys stained with negative isotype control antibody.
Figure 5. Isoflurane increases interleukin (IL)-11 immunoreactivity in mouse kidney via transforming growth factor-beta 1 (TGF-β1).
A. IL-11 immunohistochemistry (400× shown, N = 4) in kidneys from IL-11 receptor wild type (IL-11R WT) mice anesthetized with pentobarbital or with 1.2% isoflurane for 4 h. IL-11 staining was darker in kidneys of mice anesthetized with isoflurane. TGF-β1 neutralizing antibody attenuated isoflurane-mediated increase in IL-11 immunoreactivity. Finally, IL-11 staining was not visible in kidneys from mice stained with negative isotype control antibodies (representative of four experiments). B. Quantifications of renal tubular IL-11 staining in mice anesthetized with pentobarbital or with 1.2% isoflurane for 4 h. Kidney IL-11 immunoreactivity significantly increased in mice anesthetized with isoflurane and attenuated with TGF-β1 neutralizing antibody. # P < 0.05 vs. isoflurane anesthetized mice treated with immunoglobulin G (IgG) isotype antibody. * P < 0.05 vs. pentobarbital-anesthetized mice. Error bars represent 1 SD.
Critical role of IL-11 in isoflurane-mediated protection against ischemic AKI in vivo
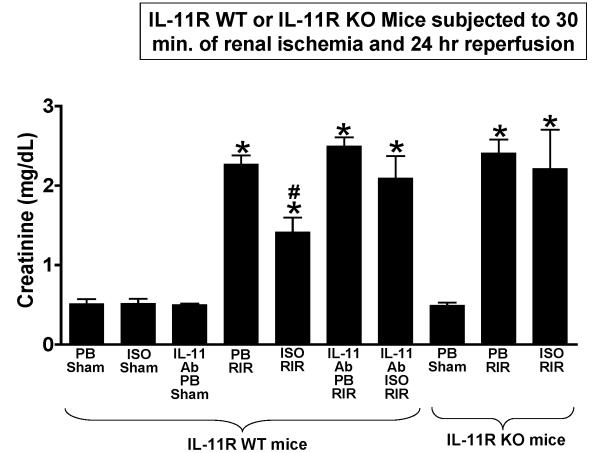
IL-11R WT mice anesthetized with pentobarbital or with 1.2% isoflurane for 4 hr had similar plasma creatinine values after sham-operation (fig. 6). Similarly, IL-11R WT mice pretreated with IL-11 neutralizing antibody or IL-11R knockout mice had similar plasma creatinine values after sham operation under pentobarbital anesthesia. Plasma creatinine significantly increased in IL-11R WT mice subjected to 30 min of renal ischemia and 2-h reperfusion compared to sham-operated mice (fig. 6, N = 6). However, IL-11R WT mice anesthetized with 1.2% isoflurane for 4 h after renal ischemia (isoflurane postconditioning) had significantly decreased plasma creatinine 24 h after injury compared to mice anesthetized with pentobarbital after renal ischemia. Supporting a critical role for IL-11 in isoflurane-mediated protection against ischemic AKI, IL-11R knockout or IL-11R WT mice pretreated with IL-11 neutralizing antibody before renal ischemia were not protected against ischemic AKI with isoflurane anesthesia after renal I/R (fig. 6). IL-11R knockout mice and IL-11R WT mice pretreated with IL-11 neutralizing antibody had similar degree of renal injury after I/R under pentobarbital anesthesia. Collectively, these studies suggest that IL-11 induction by isoflurane is required to trigger in vivo renal protection.
Figure 6. Interleukin (IL)-11 is critical for isoflurane-mediated renal protection against ischemic acute kidney injury.
Plasma creatinine levels from IL-11 wild-type (WT) or IL-11 deficient (KO) mice subjected to 30 min renal ischemia and 24 h reperfusion (N = 4-6 per group). After renal ischemia reperfusion (RIR), mice were further anesthetized with 1.2% isoflurane (ISO) or with equi-anesthetic dose of pentobarbital (PB). Some IL-11 receptor (IL-11R) WT mice were pretreated with an IL-11 neutralizing antibody (1 mg/kg, intravenous injection) 20 min before reperfusion or sham-operation. Isoflurane postconditioning significantly reduced plasma creatinine after RIR injury in IL-11R WT mice. However, IL-11R deficiency or IL-11 neutralizing antibody prevented the renal protective effects of isoflurane post-conditioning. * P < 0.05 vs. respective sham-operated mice. # P < 0.05 vs. pentobarbital anesthetized mice subjected to RIR. Data are from 6 mice per group and represented as mean ± SD.
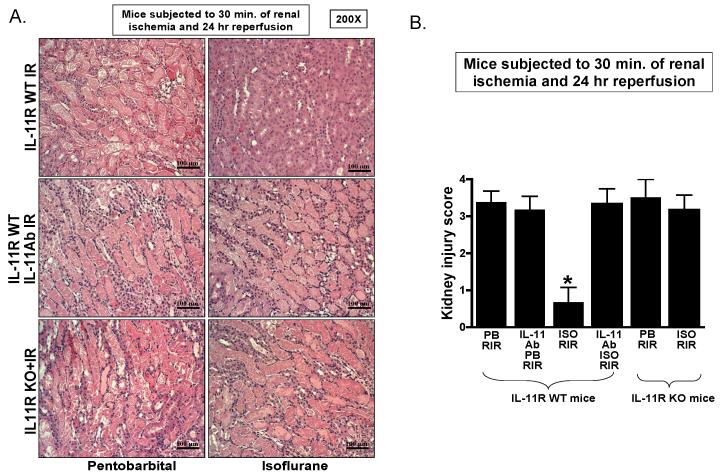
Figure 7A demonstrates severe necrotic renal injury in IL-11R WT mice subjected to renal I/R under pentobarbital anesthesia. Compared to sham-operated mice (not shown), the kidneys of mice subjected to renal I/R showed significant tubular necrosis, cast formation and congestion. In contrast, consistent with the plasma creatinine data, isoflurane anesthesia reduced renal tubular necrosis 24 h after I/R injury (fig. 7A). Supporting a critical role of IL-11 in isoflurane-mediated renal protection against I/R, isoflurane failed to reduce renal tubular necrosis in IL-11R WT mice pretreated with a neutralizing IL-11 antibody or in IL-11R knockout mice. The Jablonski scale25 renal injury score was used to grade renal tubular necrosis 24 h after renal I/R (fig. 7B). Thirty min. of renal ischemia and 24 h of reperfusion resulted in severe acute tubular necrosis in IL-11R WT mice anesthetized with pentobarbital after renal I/R injury. Consistent with the renal histology data, isoflurane significantly reduced the renal injury score in IL-11R WT mice but not in IL-11R knockout mice or in IL11R WT mice pretreated with IL-11 neutralizing antibody.
Figure 7. Interleukin (IL)-11 is critical for isoflurane-mediated reduction in renal tubular necrosis after ischemia and reperfusion (IR).
A. Representative photomicrographs of five to six experiments for hematoxylin and eosin staining (magnification 200×) of kidneys of IL-11 receptor wild-type (IL-11R WT) mice, IL-11 receptor deficient (IL-11R KO) mice and IL-11R WT mice pretreated with IL-11 neutralizing antibody and subjected to 30 min renal ischemia and 24-h reperfusion (I/R). B. Summary of Jablonski scale renal injury scores (N = 4, graded from hematoxylin and eosin staining, scale 0-4) for mice subjected to renal I/R. * P < 0.05 vs. pentobarbital-anesthetized IL-11R WT mice subjected to renal I/R. Error bars represent 1 SD. IL-11R WT mice anesthetized with pentobarbital after renal ischemia showed severe renal tubular necrosis. Isoflurane post-conditioning significantly attenuated renal tubular necrosis and renal injury scores after renal IR. IL-11R deficiency (IL-11R KO) or IL-11 neutralization prevented renal protection with isoflurane postconditioning in mice.
IL-11 is critical for isoflurane-mediated reduction in kidney neutrophil infiltration and apoptosis
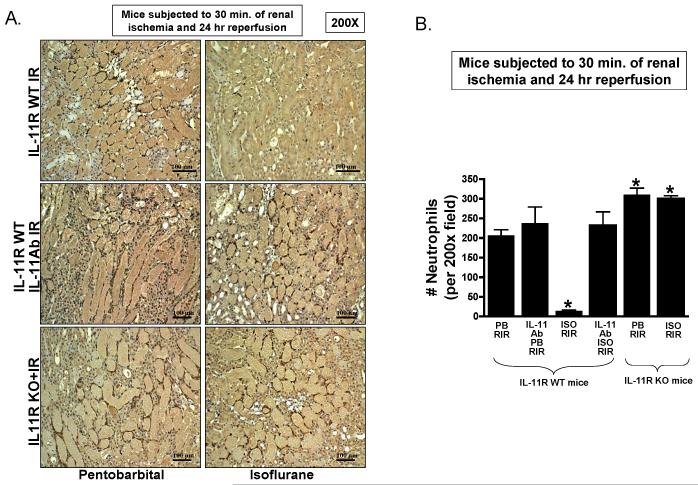
Figure 8A shows representative images (from 4-6 experiments) of neutrophil immunohistochemistry in kidneys (magnification 200×) of mice subjected to 30 min. of renal ischemia and 24-h reperfusion. In sham-operated mice, we were unable to detect any neutrophils in the kidney (data not shown). There was heavy neutrophil infiltration (dark brown) in the kidneys of pentobarbital anesthetized IL-11R WT mice subjected to renal I/R. In contrast, IL-11R WT mice anesthetized with isoflurane for 4 h after renal ischemia had significantly reduced neutrophil infiltration in the kidney 24 h after I/R (fig. 8B). Again, isoflurane failed to reduce kidney neutrophil infiltration in IL-11R WT mice treated with IL-11 neutralizing antibody and in IL-11R knockout mice.
Figure 8. Interleukin (IL)-11 is critical for isoflurane post-conditioning mediated reduction in renal neutrophil infiltration after ischemia and reperfusion.
A. Representative photomicrographs of four to six experiments for immunohistochemistry (brown staining) for neutrophil infiltration (200×) from kidneys IL-11 receptor wild-type (IL-11R WT) mice, IL-11 receptor deficient (IL-11R KO) mice and IL-11R WT mice pretreated with IL-11 neutralizing antibody and subjected to 30 min. renal ischemia and 24-h reperfusion. B. Quantifications of infiltrated neutrophils per 200× field in the kidneys of mice after renal ischemia reperfusion (RIR). * P < 0.05 vs. vehicle-treated pentobarbital anesthetized mice subjected to RIR. Error bars represent 1 SD. IL-11R WT mice anesthetized with pentobarbital after renal ischemia showed heavy neutrophil infiltration. Isoflurane postconditioning significantly attenuated renal tubular neutrophil infiltration after RIR. IL-11 deficiency (IL-11R KO) or IL-11 neutralization attenuated these reductions in renal neutrophil infiltration with isoflurane post-conditioning in mice.
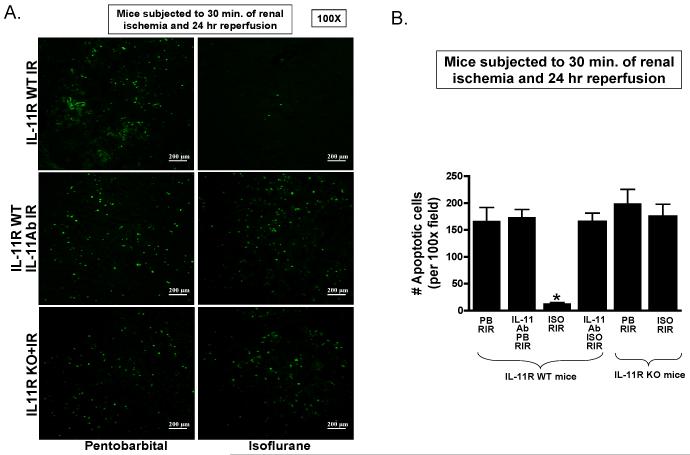
Terminal deoxynucleotidyl transferase 2′-deoxyuridine-5′-triphosphate nick end labeling staining (from 4-5 experiments) detected apoptotic renal cells in the kidneys of mice subjected to renal I/R resulting in severe proximal tubule cell apoptosis (fig. 9A, 100×). Renal ischemia and 24 h of reperfusion resulted in significant apoptosis in the kidneys of pentobarbital anesthetized IL-11R WT mice. However, IL-11R WT mice anesthetized with isoflurane for 4 h after renal ischemia had significantly reduced number of apoptotic terminal deoxynucleotidyl transferase 2′-deoxyuridine-5′-triphosphate nick end labeling-positive cells in the kidney 24 h after I/R (fig. 9B). Again, isoflurane failed to reduce renal tubular apoptosis in IL-11R WT mice treated with IL-11 neutralizing antibody and in IL-11R knockout mice.
Figure 9. Interleukin (IL)-11 is critical for isoflurane postconditioning mediated reduction in renal tubular apoptosis after ischemia and reperfusion.
A. Representative photomicrographs of four to six experiments for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining (representing apoptotic nuclei, magnification 100×) from kidneys IL-11 receptor wild-type (IL-11R WT) mice, IL-11 receptor deficient (IL-11R KO [knockout]) mice and IL-11R WT mice pretreated with IL-11 neutralizing antibody and subjected to 30 min renal ischemia and 24-h reperfusion. B. Quantifications of apoptotic cells per 100× field in the kidneys of mice after renal ischemia reperfusion (RIR). * P < 0.05 vs. vehicle-treated pentobarbital anesthetized mice subjected to RIR. Error bars represent 1 SD. IL-11R WT mice anesthetized with pentobarbital after renal ischemia showed numerous TUNEL positive cells. Isoflurane postconditioning significantly attenuated renal tubular apoptosis after RIR. IL-11 deficiency (IL-11R KO) or IL-11 neutralization attenuated these reductions in renal tubular apoptosis with isoflurane postconditioning in mice.
Discussion
We show in this study that isoflurane increases renal tubular IL-11 mRNA and protein synthesis via TGF-β1 dependent signaling in vivo as well as in vitro. TGF-β1 is a powerful anti-inflammatory cytokine that regulates multiple cellular processes including immune modulation, cellular differentiation and proliferation and oncogenesis.31,32 We previously demonstrated that volatile halogenated anesthetics including isoflurane cause translocation of phosphatidylserine from the inner leaflet to the outer leaflet of the plasma membrane (membrane externalization) resulting in the release of antiinflammatory TGF-β1.11,12 Furthermore, volatile halogenated anesthetics-mediated reduction in renal tubular necrosis and inflammation was dependent on the release of renal tubular TGF-β1. Finally, volatile halogenated anesthetic-mediated renal protection was abolished in mice deficient in TGF-β1 or WT mice treated with neutralizing TGF-β1 antibody.33
We also recently demonstrated that exogenous human recombinant IL-11 attenuated ischemic AKI in mice and reduced necrosis and apoptosis in human kidney (HK-2) proximal tubule cells.16 In mice, recombinant IL-11 treatment attenuated renal tubular necrosis and apoptosis as well as the influx of proinflammatory neutrophils after renal I/R. We further demonstrate in this study that isoflurane-mediated protection against renal tubular necrosis (Jablonski score), inflammation (neutrophil infiltration) and apoptosis (terminal deoxynucleotidyl transferase 2′-deoxyuridine-5′-triphosphate nick end labeling staining) is directly mediated by the induction of IL-11 as isoflurane failed to reduce these critical indices of renal injury in IL-11R deficient mice or mice treated with IL-11 neutralized antibody. Taken together, as blockade or genetic deletion of either TGF-β1 or IL-11 abolished renal protective effects of isoflurane, our current and previous studies suggest that isoflurane induces non-redundant proximal (TGF-β1) and distal (IL-11) cytoprotective signaling molecules to protect against ischemic AKI.
The most exciting aspect of isoflurane-mediated induction of IL-11 leading to renal protection is that recombinant IL-11 (Oprelvekin, Wyeth Pharmaceuticals, Philadelphia, PA) is already clinically approved to treat chemotherapy-induced thrombocytopenia. IL-11 is a member of the IL-6-type cytokine family isolated from bone marrow-derived stromal cells.34 IL-11 receptor activation potently regulates hematopoiesis by promoting megakaryocyte maturation.35 However, in addition to its effects on platelets, recent studies show a cytoprotective role for IL-11 as exogenous IL-11 administration attenuates cell death in several cell types and organs.16,36-38 In the heart, intestine and endothelial cells, IL-11 directly reduces necrotic as well as apoptotic cell death via mitogen-activated protein kinase and the Janus kinase/signal transducers and activators of transcription signaling.15 In addition to its anti-apoptotic and anti-necrotic properties, IL-11 receptor activation attenuates lipopolysaccharide-induced systemic inflammation in mice, toxic nephritis and leukocyte-mediated liver injury.39-42 Therefore, we propose that isoflurane-mediated induction of IL-11 and renal tubular IL-11 receptor activation produces powerful protection against ischemic AKI by targeting all three pathways of cell death: necrosis, apoptosis and inflammation. Furthermore, as IL-11 as well as IL-11 receptors are expressed in many tissues and cell types15, it remains to be determined whether isoflurane can induce IL-11 in nonrenal cells (e.g., hepatocytes, intestinal epithelial cells and endothelial cells) and confer protection in these organs against AKI induced remote organ injury.
The signal transduction mechanisms of IL-11 induced cytoprotection have been investigated in other cell types. IL-11 ligand and receptor complex interacts with a common receptor subunit, glycoprotein 130 (gp130), leading to gp130-associated kinase-mediated tyrosine phosphorylation.43 In cardiac myocytes, IL-11 reduces injury and fibrosis by the Janus kinase/signal transducers and activators of transcription 3 activation.38,43,44 In vascular endothelial and intestinal epithelial cells, IL-11 protects against oxidant induced necrosis and apoptosis via mechanisms involving extracellular signal-regulated kinase mitogen-activated protein kinase, protein kinase B and/or induction of heat shock protein 25.37,45,46
Isolfurane induced IL-11 generation may also produce antiinflammatory effects after renal I/R via modulating the nuclear factor-kappa B activity. Indeed, nuclear factor-kappa B is one of the most important proinflammatory transcription factors.47 IL-11 has been shown to attenuate transcription factor NF-kB in several cell lines and in mouse models of kidney inflammation.40,48,49 Furthermore, IL-11 treatment in vivo decreases glomerular nuclear factor-κB activity and reduces renal injury in experimental glomerulonephritis.50
We previously showed that IL-11 produces renal protection by direct induction of sphingosine kinase-1 via nuclear translocation of hypoxia-inducible factor-1α.16 Sphingosine kinase-1 produces sphingosine 1-phosphate – a well known antiinflammatory immune modulator.51 Based on our previous9,52 and current experimental data, we propose that isoflurane may directly induce several antiinflammatory signaling molecules (e.g., IL-11, sphingosine 1-phosphate) to protect against hyper-inflammatory response after ischemic AKI. Interestingly, several of the IL-11-mediated cytoprotective signal transduction proteins (e.g., extracellular signal-regulated kinase mitogen-activated protein kinase, protein kinase B, sphingosine kinase) are also activated with volatile halogenated anesthetics as we demonstrated previously.12,13,17
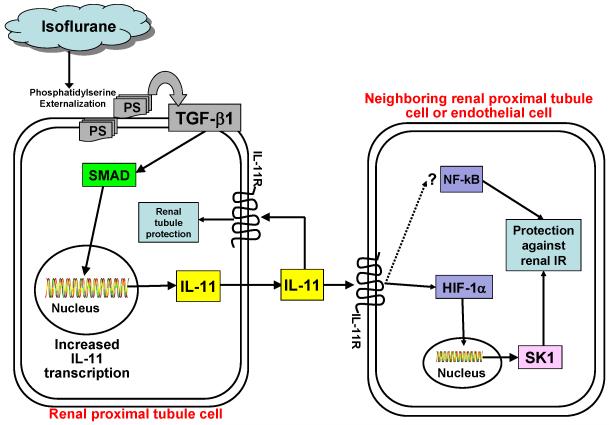
We show here that isoflurane post-conditioning produces potent renal protection against ischemic AKI via IL-11 mediated reduction in kidney tubule necrosis, apoptosis and renal inflammation. We propose that after renal I/R injury, necrotic tubules cells release proinflammatory cytokines (e.g., monocyte chemotactic protein-1 release) that can further aggravate inflammation by stimulating resident kidney macrophages and dendritic cells.4 These cells in turn release additional pro-inflammatory cytokines (e.g., macrophage inflammatory protein 2α, keratinocyte derived cytokine) to promote cytotoxic T-lymphocyte, neutrophil and macrophage infiltration into the kidney interstitial space. Infiltrating proinflammatory leukocytes release additional cytotoxic proinflammatory and proapoptotic cytokines (e.g., tumor necrosis factor-α) which will exacerbate renal tubular cell necrosis as well as apoptosis. Figure 10 summarizes the potential mechanisms of isoflurane-mediated renal protection involving renal tubular IL-11 synthesis via TGF-β1 signaling.
Figure 10. Proposed summary of cellular mechanisms of renal protection with post-ischemic isoflurane treatment.
Collectively, our data suggest that isoflurane anesthesia increases interleukin (IL)-11 messenger RNA (mRNA) and protein synthesis via TGF-β1 signaling. We propose that IL-11 synthesized then subsequently activates IL-11R in neighboring renal tubules, or endothelial cells to induce cytoprotective signaling. Since previous studies have shown that IL-11 reduces the activity of a well-known proinflammatory transcription factor NF-kB49;50, it is highly possible that IL-11 generated with isoflurane treatment may also attenuate NF-kB activity to protect against renal inflammation and injury after acute kidney injury. SMADs are intracellular proteins that transduce extracellular signals from TGF-β1 to the nucleus to initiate downstream gene transcription. Hypothetical pathways (e.g., NF-kB inhibition) leading to cytoprotection are shown in dashed lines. We previous showed that IL-11 produces renal protection by direct induction of sphingosine kinase-1 via nuclear translocation of HIF-1α.14
HIF = hypoxia-inducible factor; IL-11R = interleukin 11 receptor; IR = ischemia reperfusion; NF-kB = nuclear factor kappa-light-chain-enhancer of activated B cells; PS = phosphatidylserine; SMAD = SMA (from Caenorhabditis elegans protein sma for small body size) and MAD (from Drosophila protein mothers against decapentaplegic) related family of transduction proteins; TGF-β1 = transforming growth factor-beta 1.
We believe that potential for clinical use of recombinant IL-11 therapy would be far superior to therapy against ischemic AKI with recombinant TGF-β1. Active form of TGF-β1 has a very short plasma half-life (2-3 min) as nonlatent TGF-β1 gets rapidly taken up by the liver, kidneys, lungs, and spleen and degraded.53 This is in contrast to the more prolonged half-life of recombinant IL-11 (~7 h).34 Furthermore, prolonged exposure to TGF-β1 may promote kidney fibrosis.54 Therefore, prolonged TGF-β1 therapy may transiently reduce inflammation and necrosis but may cause increased renal tubular fibrosis that may paradoxically prolong renal dysfunction after I/R. Unlike TGF-β1, prolonged and high dose IL-11 therapy does not induce tissue fibrosis.
We recently showed that isoflurane via TGF-β1 induces ecto-5′-nucleotidase (CD73) to generate cytoprotective adenosine in renal proximal tubule cells.55 CD73 induction and CD73-mediated adenosine generation was critical for isoflurane-mediated renal protection against ischemic AKI. However, the downstream target of isoflurane-mediated adenosine generation as well as the specific adenosine receptor subtype(s) responsible for renal protection against ischemic AKI remains unclear. We hypothesize that isoflurane-mediated induction of CD73 activity and adenosine generation directly stimulates renal tubular IL-11 synthesis to protect against ischemic AKI. Consistent with this hypothesis, we recently also showed that a specific A1 adenosine receptor agonist CCPA also induces IL-11 in proximal tubule cells.56 It remains to be tested in future studies whether isoflurane-mediated adenosine generation results in the activation of renal tubular A1 adenosine receptors to protect against renal ischemia and reperfusion injury.
There are several limitations to our study. Our results may not completely translate to clinical setting as differences in pathophysiology between human and mouse ischemic AKI exists. Furthermore, our studies focused on renal tubular synthesis of IL-11 and protection whereas clinical AKI results in both impairment of glomerular filtration and renal tubular dysfunction. In addition, our in vitro studies have limitation as HK-2 cells used in this study are immortalized and primary culture of proximal tubule cells may undergo rapid phenotypic changes ex vivo. Finally, complete isoflurane concentration-response curves were not generated in most of our experiments.
In summary, we demonstrated that a widely utilized volatile halogenated anesthetic isoflurane protects against renal tubular necrosis and inflammation after renal I/R by inducing cytoprotective IL-11 generation.
MS #201304178 – Final Boxed Summary Statement.
What we already know about this topic:
Halogenated anesthetics protects against acute kidney injury (AKI) by renal tubular production of transforming growth factor-beta 1 (TGF-β1)
What this article tells us that is new:
Isoflurane increased interleukin-11 synthetis in human and mouse proximal tubular cells via TGF-β1 signaling to protect against ischemic AKI
Acknowledgments
We appreciate the surgical, analytical and technical assistance provided by Sang Won Park, Ph.D. (Current Position: Assistant Professor of Pharmacology, Gyeongnam National University, Jin Ju, South Korea)
Funding: This work was supported in part by Department of Anesthesiology, Columbia University New York, New york and R01 DK-058547 and R01 GM-067081 (to H.T.L.) from the National Institutes of Health, Bethesda, Maryland.
Footnotes
Disclosure: The authors declare no competing interests.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–70. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 2.Yap SC, Lee HT. Acute kidney injury and extrarenal organ dysfunction: New concepts and experimental evidence. Anesthesiology. 2012;116:1139–48. doi: 10.1097/ALN.0b013e31824f951b. [DOI] [PubMed] [Google Scholar]
- 3.Elapavaluru S, Kellum JA. Why do patients die of acute kidney injury? Acta Clin Belg Suppl. 2007:326–31. doi: 10.1179/acb.2007.074. [DOI] [PubMed] [Google Scholar]
- 4.Okusa MD. The changing pattern of acute kidney injury: From one to multiple organ failure. Contrib Nephrol. 2010;165:153–8. doi: 10.1159/000313754. [DOI] [PubMed] [Google Scholar]
- 5.Faubel S. Acute kidney injury and multiple organ dysfunction sindrome. Minerva Urol Nefrol. 2009;61:171–88. [PubMed] [Google Scholar]
- 6.Legrand M, Payen D. Case scenario: Hemodynamic management of postoperative acute kidney injury. Anesthesiology. 2013;118:1446–54. doi: 10.1097/ALN.0b013e3182923e8a. [DOI] [PubMed] [Google Scholar]
- 7.Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Report. 2009:1–25. [PubMed] [Google Scholar]
- 8.Lee HT, Ota-Setlik A, Fu Y, Nasr SH, Emala CW. Differential protective effects of volatile anesthetics against renal ischemia-reperfusion injury in vivo. Anesthesiology. 2004;101:1313–24. doi: 10.1097/00000542-200412000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Lee HT, Kim M, Kim M, Kim N, Billings FT, 4th, D’Agati VD, Emala CW., Sr Isoflurane protects against renal ischemia and reperfusion injury and modulates leukocyte infiltration in mice. Am J Physiol Renal Physiol. 2007;293:F713–22. doi: 10.1152/ajprenal.00161.2007. [DOI] [PubMed] [Google Scholar]
- 10.Lee HT, Kim M, Jan M, Emala CW. Anti-inflammatory and anti-necrotic effects of the volatile anesthetic sevoflurane in kidney proximal tubule cells. Am J Physiol Renal Physiol. 2006;291:F67–78. doi: 10.1152/ajprenal.00412.2005. [DOI] [PubMed] [Google Scholar]
- 11.Lee HT, Kim M, Song JH, Chen SWC, Gubitosa G, Emala CW. Sevoflurane mediated TGF-beta1 signaling in renal proximal tubule cells. Am J Physiol Renal Physiology. 2008;294:F371–8. doi: 10.1152/ajprenal.00277.2007. [DOI] [PubMed] [Google Scholar]
- 12.Lee HT, Kim M, Kim J, Kim N, Emala CW. TGF-beta1 release by volatile anesthetics mediates protection against renal proximal tubule cell necrosis. Am J Nephrol. 2007;27:416–24. doi: 10.1159/000105124. [DOI] [PubMed] [Google Scholar]
- 13.Song JH, Kim M, Park SW, Chen SW, Pitson SM, Lee HT. Isoflurane via TGF-beta1 release increases caveolae formation and organizes sphingosine kinase signaling in renal proximal tubules. Am J Physiol Renal Physiol. 2010;298:F1041–F1050. doi: 10.1152/ajprenal.00115.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldman SC, Bracho F, Davenport V, Slack R, Areman E, Shen V, Lenarsky C, Weinthal J, Hughes R, Cairo MS. Feasibility study of IL-11 and granulocyte colony-stimulating factor after myelosuppressive chemotherapy to mobilize peripheral blood stem cells from heavily pretreated patients. J Pediatr Hematol Oncol. 2001;23:300–5. doi: 10.1097/00043426-200106000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Du X, Williams DA. Interleukin-11: Review of molecular, cell biology, and clinical use. Blood. 1997;89:3897–908. [PubMed] [Google Scholar]
- 16.Lee HT, Park SW, Kim M, Ham A, Anderson LJ, Brown KM, D’Agati VD, Cox GN. Interleukin-11 protects against renal ischemia and reperfusion injury. Am J Physiol Renal Physiol. 2012;303:F1216–24. doi: 10.1152/ajprenal.00220.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim M, Kim M, Kim N, D’Agati VD, Emala CW, Sr, Lee HT. Isoflurane mediates protection from renal ischemia-reperfusion injury via sphingosine kinase and sphingosine-1-phosphate-dependent pathways. Am J Physiol Renal Physiol. 2007;293:F1827–35. doi: 10.1152/ajprenal.00290.2007. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Zhu Z, Nolfo R, Elias JA. Dexamethasone regulation of lung epithelial cell and fibroblast interleukin-11 production. Am J Physiol. 1999;276:L175–85. doi: 10.1152/ajplung.1999.276.1.L175. [DOI] [PubMed] [Google Scholar]
- 19.Tang W, Yang L, Yang YC, Leng SX, Elias JA. Transforming growth factor-beta stimulates interleukin-11 transcription via complex activating protein-1-dependent pathways. J Biol Chem. 1998;273:5506–13. doi: 10.1074/jbc.273.10.5506. [DOI] [PubMed] [Google Scholar]
- 20.Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B. HK-2: An immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994;45:48–57. doi: 10.1038/ki.1994.6. [DOI] [PubMed] [Google Scholar]
- 21.Vinay P, Gougoux A, Lemieux G. Isolation of a pure suspension of rat proximal tubules. Am J Physiol. 1981;241:F403–11. doi: 10.1152/ajprenal.1981.241.4.F403. [DOI] [PubMed] [Google Scholar]
- 22.Kim M, Kim M, Park SW, Pitson SM, Lee HT. Isoflurane protects human kidney proximal tubule cells against necrosis via sphingosine kinase and sphingosine-1-phosphate generation. Am J Nephrol. 2010;31:353–62. doi: 10.1159/000298339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redel A, Stumpner J, Tischer-Zeitz T, Lange M, Smul TM, Lotz C, Roewer N, Kehl F. Comparison of isoflurane-, sevoflurane-, and desflurane-induced pre- and postconditioning against myocardial infarction in mice in vivo. Exp Biol Med (Maywood.) 2009;234:1186–91. doi: 10.3181/0902-RM-58. [DOI] [PubMed] [Google Scholar]
- 24.Slot C. Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest. 1965;17:381–7. doi: 10.3109/00365516509077065. [DOI] [PubMed] [Google Scholar]
- 25.Jablonski P, Howden BO, Rae DA, Birrel CS, Marshall VC, Tange J. An experimental model for assessment of renal recovery from warm ischemia. Transplantation. 1983;35:198–204. doi: 10.1097/00007890-198303000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Lee HT, Gallos G, Nasr SH, Emala CW. A1 adenosine receptor activation inhibits inflammation, necrosis, and apoptosis after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2004;15:102–11. doi: 10.1097/01.asn.0000102474.68613.ae. [DOI] [PubMed] [Google Scholar]
- 27.Lee HT, Xu H, Nasr SH, Schnermann J, Emala CW. A1 adenosine receptor knockout mice exhibit increased renal injury following ischemia and reperfusion. Am J Physiol Renal Physiol. 2004;286:F298–F306. doi: 10.1152/ajprenal.00185.2003. [DOI] [PubMed] [Google Scholar]
- 28.Park SW, Kim M, Chen SW, Brown KM, D’Agati VD, Lee HT. Sphinganine-1-phosphate protects kidney and liver after hepatic ischemia and reperfusion in mice through S1P(1) receptor activation. Lab Invest. 2010;90:1209–24. doi: 10.1038/labinvest.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen SW, Kim M, Kim M, Song JH, Park SW, Wells D, Brown K, Belleroche JD, D’Agati VD, Lee HT. Mice that overexpress human heat shock protein 27 have increased renal injury following ischemia reperfusion. Kidney Int. 2008;75:499–510. doi: 10.1038/ki.2008.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matkowskyj KA, Schonfeld D, Benya RV. Quantitative immunohistochemistry by measuring cumulative signal strength using commercially available software photoshop and matlab. J Histochem Cytochem. 2000;48:303–12. doi: 10.1177/002215540004800216. [DOI] [PubMed] [Google Scholar]
- 31.Wang XJ, Han G, Owens P, Siddiqui Y, Li AG. Role of TGF beta-mediated inflammation in cutaneous wound healing. J Investig Dermatol Symp Proc. 2006;11:112–7. doi: 10.1038/sj.jidsymp.5650004. [DOI] [PubMed] [Google Scholar]
- 32.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee HT, Chen SW, Doetschman TC, Deng C, D’Agati VD, Kim M. Sevoflurane protects against renal ischemia and reperfusion injury in mice via the transforming growth factor-beta1 pathway. Am J Physiol Renal Physiol. 2008;295:F128–36. doi: 10.1152/ajprenal.00577.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaye JA. The clinical development of recombinant human interleukin 11 (NEUMEGA rhIL-11 growth factor) Stem Cells. 1996;14(Suppl 1):256–60. doi: 10.1002/stem.5530140733. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds CH. Clinical efficacy of rhIL-11. Oncology (Williston.Park) 2000;14:32–40. [PubMed] [Google Scholar]
- 36.Kuenzler KA, Pearson PY, Schwartz MZ. IL-11 pretreatment reduces cell death after intestinal ischemia-reperfusion. J Surg Res. 2002;108:268–72. doi: 10.1006/jsre.2002.6542. [DOI] [PubMed] [Google Scholar]
- 37.Waxman AB, Mahboubi K, Knickelbein RG, Mantell LL, Manzo N, Pober JS, Elias JA. Interleukin-11 and interleukin-6 protect cultured human endothelial cells from H2O2-induced cell death. Am J Respir Cell Mol Biol. 2003;29:513–22. doi: 10.1165/rcmb.2002-0044OC. [DOI] [PubMed] [Google Scholar]
- 38.Kimura R, Maeda M, Arita A, Oshima Y, Obana M, Ito T, Yamamoto Y, Mohri T, Kishimoto T, Kawase I, Fujio Y, Azuma J. Identification of cardiac myocytes as the target of interleukin 11, a cardioprotective cytokine. Cytokine. 2007;38:107–15. doi: 10.1016/j.cyto.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 39.Bozza M, Bliss JL, Maylor R, Erickson J, Donnelly L, Bouchard P, Dorner AJ, Trepicchio WL. Interleukin-11 reduces T-cell-dependent experimental liver injury in mice. Hepatology. 1999;30:1441–7. doi: 10.1002/hep.510300616. [DOI] [PubMed] [Google Scholar]
- 40.Trepicchio WL, Bozza M, Pedneault G, Dorner AJ. Recombinant human IL-11 attenuates the inflammatory response through down-regulation of proinflammatory cytokine release and nitric oxide production. J Immunol. 1996;157:3627–34. [PubMed] [Google Scholar]
- 41.Sheridan BC, Dinarello CA, Meldrum DR, Fullerton DA, Selzman CH, McIntyre RC., Jr Interleukin-11 attenuates pulmonary inflammation and vasomotor dysfunction in endotoxin-induced lung injury. Am J Physiol. 1999;277:L861–7. doi: 10.1152/ajplung.1999.277.5.L861. [DOI] [PubMed] [Google Scholar]
- 42.Lai PC, Cook HT, Smith J, Keith JC, Jr, Pusey CD, Tam FW. Interleukin-11 attenuates nephrotoxic nephritis in Wistar Kyoto rats. J Am Soc Nephrol. 2001;12:2310–20. doi: 10.1681/ASN.V12112310. [DOI] [PubMed] [Google Scholar]
- 43.Fujio Y, Maeda M, Mohri T, Obana M, Iwakura T, Hayama A, Yamashita T, Nakayama H, Azuma J. Glycoprotein 130 cytokine signal as a therapeutic target against cardiovascular diseases. J Pharmacol Sci. 2011;117:213–22. doi: 10.1254/jphs.11r05cr. [DOI] [PubMed] [Google Scholar]
- 44.Obana M, Maeda M, Takeda K, Hayama A, Mohri T, Yamashita T, Nakaoka Y, Komuro I, Takeda K, Matsumiya G, Azuma J, Fujio Y. Therapeutic activation of signal transducer and activator of transcription 3 by interleukin-11 ameliorates cardiac fibrosis after myocardial infarction. Circulation. 2010;121:684–91. doi: 10.1161/CIRCULATIONAHA.109.893677. [DOI] [PubMed] [Google Scholar]
- 45.Ropeleski MJ, Tang J, Walsh-Reitz MM, Musch MW, Chang EB. Interleukin-11-induced heat shock protein 25 confers intestinal epithelial-specific cytoprotection from oxidant stress. Gastroenterology. 2003;124:1358–68. doi: 10.1016/s0016-5085(03)00282-8. [DOI] [PubMed] [Google Scholar]
- 46.Naugler KM, Baer KA, Ropeleski MJ. Interleukin-11 antagonizes Fas ligand-mediated apoptosis in IEC-18 intestinal epithelial crypt cells: Role of MEK and Akt-dependent signaling. Am J Physiol Gastrointest Liver Physiol. 2008;294:G728–37. doi: 10.1152/ajpgi.00002.2007. [DOI] [PubMed] [Google Scholar]
- 47.Barnes PJ, Karin M. Nuclear factor-kappaB: A pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–71. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 48.Bamba S, Andoh A, Yasui H, Makino J, Kim S, Fujiyama Y. Regulation of IL-11 expression in intestinal myofibroblasts: role of c-Jun AP-1- and MAPK-dependent pathways. Am J Physiol Gastrointest Liver Physiol. 2003;285:G529–38. doi: 10.1152/ajpgi.00050.2003. [DOI] [PubMed] [Google Scholar]
- 49.Trepicchio WL, Wang L, Bozza M, Dorner AJ. IL-11 regulates macrophage effector function through the inhibition of nuclear factor-kappaB. J Immunol. 1997;159:5661–70. [PubMed] [Google Scholar]
- 50.Stangou M, Bhangal G, Lai PC, Smith J, Keith JC, Jr, Boyle JJ, Pusey CD, Cook T, Tam FW. Effect of IL-11 on glomerular expression of TGF-beta and extracellular matrix in nephrotoxic nephritis in Wistar Kyoto rats. J Nephrol. 2011;24:106–11. doi: 10.5301/jn.2010.5094. [DOI] [PubMed] [Google Scholar]
- 51.Jo SK, Bajwa A, Awad AS, Lynch KR, Okusa MD. Sphingosine-1-phosphate receptors: Biology and therapeutic potential in kidney disease. Kidney Int. 2008;73:1220–30. doi: 10.1038/ki.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim M, Park SW, Kim M, D’Agati VD, Lee HT. Isoflurane activates intestinal sphingosine kinase to protect against renal ischemia-reperfusion-induced liver and intestine injury. Anesthesiology. 2011;114:363–73. doi: 10.1097/ALN.0b013e3182070c3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wakefield LM, Winokur TS, Hollands RS, Christopherson K, Levinson AD, Sporn MB. Recombinant latent transforming growth factor beta 1 has a longer plasma half-life in rats than active transforming growth factor beta 1, and a different tissue distribution. J Clin Invest. 1990;86:1976–84. doi: 10.1172/JCI114932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roberts AB, Tian F, Byfield SD, Stuelten C, Ooshima A, Saika S, Flanders KC. Smad3 is key to TGF-beta-mediated epithelial-to-mesenchymal transition, fibrosis, tumor suppression and metastasis. Cytokine Growth Factor Rev. 2006;17:19–27. doi: 10.1016/j.cytogfr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 55.Kim M, Ham A, Kim JY, Brown KM, D’Agati VD, Lee HT. The volatile anesthetic isoflurane induces ecto-5′-nucleotidase (CD73) to protect against renal ischemia and reperfusion injury. Kidney Int. 2013;84:90–103. doi: 10.1038/ki.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim JY, Kim M, Ham A, Brown KM, Greene RW, D’Agati VD, Lee HT. IL-11 is required for A1 adenosine receptor-mediated protection against ischemic AKI. J Am Soc Nephrol. 2013 doi: 10.1681/ASN.2013010114. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]