Abstract
Leptin signaling has received considerable attention in the Alzheimer disease (AD) field. Within the past decade, the peptide hormone has been demonstrated to attenuate tau hyperphosphorylation in neuronal cells and to be modulated by amyloid-β. Moreover, a role in neuroprotection and neurogenesis within the hippocampus has been shown in animal models. To further characterize the association between leptin signaling and vulnerable regions in AD, we assessed the profile of leptin and the leptin receptor in AD and control patients. We analyzed leptin levels in cerebrospinal fluid (CSF), and the concentration and localization of leptin and leptin receptor in the hippocampus. Significant elevations in leptin levels in both CSF and hippocampal tissue of AD patients, compared to age-matched control cases, indicate a physiological upregulation of leptin in AD. However, the level of leptin receptor mRNA decreased in AD brain and the leptin receptor protein was localized to neurofibrillary tangles, suggesting a severe discontinuity in the leptin signaling pathway. Collectively, our results suggest that leptin resistance in the hippocampus may play a role in the characteristic changes associated with the disease. These findings are the first to demonstrate such dysregulated leptin-signaling circuitry and provide novel insights into the possible role of aberrant leptin signaling in AD.
Keywords: Alzheimer disease, tau, leptin, leptin receptor, neurofibrillary tangles
Introduction
The biological actions of leptin are typically associated with hypothalamic modulation of feeding behavior and energy expenditure. Leptin is an anorexigenic peptide hormone synthesized in and secreted from peripheral adipocytes; its receptor binding within the hypothalamus induces a biochemical cascade that ultimately reduces food intake and increases energy usage (Morris & Rui 2009). While the molecular and biochemical mechanisms of leptin signaling in the hypothalamus are relatively well characterized, those involved in other brain regions such as hippocampus are less clear. Interestingly, the long form of the leptin receptor (Ob-Rb), as well as the five alternatively spliced leptin receptors (Ob-Ra, Ob-Rc, Ob-Rd, Ob-Re, and Ob-Rf), are distributed throughout the hippocampus (Elmquist et al. 1998, Morash et al. 1999), and increasing evidence is establishing the role of leptin in this region (Garza et al. 2008, Harvey 2007, Harvey 2003, Shanley et al. 2002), particularly so in Alzheimer disease (AD) (Power et al. 2001, Olsson et al. 1998, Greco et al. 2008, Greco et al. 2009b).
AD is a pathologically complex disease characterized by multiple etiological and biochemical aberrations including oxidative stress, cell cycle aberration, transition metal dyshomeostasis, neurofibrillary tangle (NFT) formation, and amyloid-β (Aβ) oligomerization/fibrillation (Castellani et al. 2010). In longitudinal studies, several biomarkers are also changed and predictive for the development of AD (Bateman et al. 2012). Notably, the serum levels of leptin has been shown to be correlated with the development of AD (Holden et al. 2009), suggesting a potential pathogenic role of leptin. Indeed, leptin has previously been shown to modulate tau hyperphosphorylation, the principal prerequisite to the accumulation of NFTs in the brain (Greco et al. 2009a, Greco et al. 2009b, Greco et al. 2008), and to be affected by Aβ-related signaling (Erol 2008, Fewlass et al. 2004). Moreover, the upregulation of leptin has been proposed as a method of therapeutic intervention for AD (Tezapsidis et al. 2009).
However, the status of the leptin signaling pathway in hippocampus and cerebral cortex, the vulnerable regions of AD, has not been determined. To this end, in this study, using multiple biochemical and molecular biological methods, we analyzed the levels of leptin, its major receptor Ob-Rb, and the activated Ob-Rb (phosphorylated on tyrosine 985, pOb-RbT985) in the hippocampus of AD and control patients, as well as in patients exhibiting mild cognitive impairment (MCI), a prodromal dementia associated with AD (Petersen et al. 1999).
Materials and Methods
Tissue
Using an approved IRB protocol, human AD and non-demented control hippocampal or cortical tissue samples were obtained at autopsy (AD: n = 33, age 63–95 y, mean 77.1 ± 8.7 y; young controls: n = 10, age 19–46 y, mean 29.5 ± 11.7 y; aged controls: n = 16, age 62–86 y, mean 71.9 ± 8.3 y) from the Case Medical Center at Case Western Reserve University. Brain samples were fixed in either routine formalin or methacarn (methanol; chloroform; acetic acid; 6:3:1 v/v/v) at 4°C overnight. Following fixation, tissue was dehydrated through ascending concentrations of ethanol, embedded in paraffin, and 5 μm sections were placed on coated slides.
Immunohistochemistry
Tissue sections were deparaffinized in xylene and hydrated through descending ethanol concentrations, and endogenous peroxidase activity was quenched by 30 min incubation in 3% hydrogen peroxide in methanol. For some experiments, antigen retrieval through pressure cooking was performed using manufacturer’s recommendations (Biocare Medical, Concord, MA). Subsequently, in all cases, non-specific binding sites were blocked with 30 min incubation in 10% normal goat serum (NGS) in Tris buffered saline, and then treated overnight at 4°C with primary antibodies. Primary antibodies utilized for various detections included rabbit polyclonal antibodies to leptin (Abcam 2125, Cambridge, MA), leptin receptor (Pierce Thermo Scientific PA1-053. Rockford, IL), phosphorylated leptin receptor at Tyr-985 (Millipore 07-097, Billerica, MA), and mouse monoclonal antibody to hyperphosphorylated tau (Pierce-Endogen AT8, Rockford, IL). Following incubation with species-specific secondary antibodies and PAP complexes, the antibodies were detected with 3, 3′-diaminobenzidine, as the chromogen (Dako, Carpinteria, CA). Verification of antibody specificity for anti-Ob-Rb was done via absorption of the antibody with the Ob-Rb peptide (Thermo-Pierce PEP-014). Specifically, diluted antibody in 1% NGS was incubated with its specific peptide antigen and then applied onto an adjacent serial section stained with the antibody alone.
Double label fluorescent microscopy was used to confirm the localization of Ob-Rb and AT8. Sections from 3 cases of AD were rehydrated as above and after incubation with both mouse monoclonal AT8 and rabbit antisera to leptin receptor, AlexaFluor 468 and 588 labeled secondary antibodies were applied. Images were obtained using Zeiss Axiophot and axiocam.
Quantification of the levels of immunoreactivity in the same neurons for both Ob-Rb and pOb-RbT985 was performed. Serial adjacent sections from 3 AD cases were immunostained. 3 of the exact same fields (20X magnification) were imaged for each antibody using a Zeiss Axiocam. Using landmark vessels, identical field orientation was determined and the same neurons located in each set. Using the Axiovision software the relative staining intensity was measured with the background subtracted for neurons with and without NFT. Similar methodology was applied to compare neurons stained with Ob-Rb and AT8. Images were obtained from the same fields from adjacent serial sections from 8 cases of AD and the numbers of NFT stained with AT8 only, Ob-Rb only, or both AT8 and Ob-Rb were counted.
Leptin RIA
To determine the leptin levels in CSF, samples obtained at autopsy from a series of well-characterized cases were obtained from the University of Kentucky Sanders-Brown Center on Aging. For these cases, age, postmortem interval, gender, Braak stage, Mini-Mental Status Exam, body mass index (BMI), and protein levels were available. All samples were collected with a low postmortem interval (mean 3 h). AD (n= 21; age 57–95 y, mean 78.5 ± 10.2 y, Braak VI), MCI (n= 8; age 87–99 y, mean 91.8 ± 4.5 y, Braak III, IV, V), and control (n= 13; age 72–95 y, mean 83.7 ± 8.1 y, Braak 0, I, II) cases were used. Table 1 details the case information. Samples were sent blinded for the sensitive human leptin RIA (Linco, Millipore, St. Louis, MO) and were performed in quadruplicates.
Table 1.
Information on the cases used for CSF leptin analysis
| Diagnosis | Age (yrs) | Braak Stage | PMI (hrs) | BMI | Gender |
|---|---|---|---|---|---|
| Control | 90 | 0 | 3.5 | 24.4 | Male |
| Control | 92 | 0 | 3.25 | 25.8 | Male |
| Control | 95 | 0 | 3.5 | 23.0 | Female |
| Control | 72 | 1 | 3.75 | 28.7 | Female |
| Control | 74 | 1 | 4 | 25.8 | Male |
| Control | 75 | 1 | 3.5 | 26.5 | Female |
| Control | 76 | 1 | 2 | 20.9 | Female |
| Control | 86 | 1 | 1.75 | 21.8 | Female |
| Control | 79 | 2 | 2.25 | 28.5 | Male |
| Control | 79 | 2 | 1.75 | 27.5 | Male |
| Control | 87 | 2 | 2 | 25.8 | Male |
| Control | 90 | 2 | 4 | 24.0 | Female |
| Control | 93 | 2 | 2.75 | NA | Female |
| MCI | 87 | 3 | 2.25 | 26.6 | Male |
| MCI | 91 | 3 | 5 | NA | Female |
| MCI | 93 | 3 | 2.75 | 20.0 | Female |
| MCI | 97 | 3 | 2.75 | 25.7 | Female |
| MCI | 87 | 4 | 3.5 | 24.0 | Male |
| MCI | 92 | 4 | 2 | 26.4 | Male |
| MCI | 88 | 5 | 2.25 | 34.1 | Female |
| MCI | 99 | 5 | 2 | 24.9 | Female |
| Late AD | 57 | 6 | 3 | NA | Female |
| Late AD | 65 | 6 | 4 | 18.7 | Male |
| Late AD | 67 | 6 | 2.25 | NA | Female |
| Late AD | 68 | 6 | 3.25 | 31.8 | Female |
| Late AD | 69 | 6 | 4 | NA | Male |
| Late AD | 70 | 6 | 3.25 | 21.1 | Female |
| Late AD | 72 | 6 | 2.25 | 20.7 | Female |
| Late AD | 73 | 6 | 2 | 30.4 | Male |
| Late AD | 75 | 6 | 2.33 | 21.8 | Female |
| Late AD | 78 | 6 | 3.75 | 21.2 | Male |
| Late AD | 78 | 6 | 3.5 | 25.0 | Male |
| Late AD | 84 | 6 | 4.5 | 21.6 | Male |
| Late AD | 84 | 6 | 2.75 | 25.4 | Male |
| Late AD | 85 | 6 | 2.75 | 21.8 | Male |
| Late AD | 86 | 6 | 4.25 | 31.7 | Female |
| Late AD | 86 | 6 | 3.25 | NA | Female |
| Late AD | 86 | 6 | 2 | NA | Female |
| Late AD | 90 | 6 | 2.8 | 21.0 | Female |
| Late AD | 90 | 6 | 2.75 | 21.6 | Female |
| Late AD | 90 | 6 | 3.25 | 23.0 | Male |
| Late AD | 95 | 6 | 4 | 25.8 | Male |
Quantitative RT-PCR
Frozen hippocampal tissues from clinically and pathologically confirmed cases of AD (n=16, ages 65–85, mean 78.1) and non-AD age-matched controls (n=11, ages 61–91, mean 79.8) with National Institute of Aging and Consortium to Establish a Registry for Alzheimer’s Disease criteria were obtained at autopsy by the Case Western Reserve University Brain Bank under an IRB-approved protocol. Frozen white adipose tissue was used for verification of PCR technique (provided by the Human Tissue Procurement Facility of Case Western Reserve University and UH Case Medical Center).
Frozen tissue was homogenized in Tri reagent (Ambion, Carlsbad, CA) following chloroform extraction. RNA was further purified with the RNeasy Mini Kit (Qiagen, Germantown, MD), following the instructions of the manufacturer. RNA was treated with DNase (Turbo DNase, Ambion) to remove trace DNA. RNA was converted to cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA) according to the manufacturer’s instructions. Briefly, the purified total RNA (100 ng) was reverse transcribed with cloned Moloney murine leukemia virus reverse transcriptase (5 U) by incubating at 37°C for 120 min, followed by heating at 85°C for 5 min. Real time RT-PCR was used to quantify relative leptin and Ob-Rb expression. TaqMan PCR assay for leptin (Hs00174877_m1) and Ob-Rb (Hs00174497_m1) was performed on cDNA samples in 96-well optical plates on an ABI StepOne Plus Thermocycler with GAPDH used as an endogenous control (Taqman, Applied Biosystems). For each 20 μl TaqMan reaction, 20 ng cDNA was mixed with 10 μl 2× TaqMan Fast Universal PCR Master Mix (Applied Biosystems) and 1 μl of 20× TaqMan assay mix. Standard fast PCR parameters were used. Data is expressed as relative quantity (RQ value).
Western blot
To assess if leptin is degraded over time, as a potential result of increased PMI, in vitro analysis was performed by incubating 1 μg purified leptin in either TBS or human CSF (n=2) for 4 hours on ice or at 37° C to mimic the longest PMI of the samples used in the RIA analysis, and then resolved on 15% SDS-PAGE and analyzed by Western blot.
Frozen hippocampus samples were homogenized in lysis buffer (Cell signaling) with protease inhibitors added (Roche). After determining protein concentration with BCA assay (Pierce), 30 ug of protein were resolved using SDS-PAGE. For leptin analysis, 15% gels were used, for Ob-Rb 10% gels were used. Proteins were transferred to Immobilon PVDF membrane (Millipore), blocked with 10% mile or 5 % BSA. Primary antibodies were incubated overnight, then the blots rinsed in TBST and HRP-labeled secondary antibodies applied. After rinsing in TBST, blots were developed using Millipore ECL detection reagent and blots were exposed to X-ray film.
Statistical Analysis
Data are expressed as means +/− SEM. Differences between groups were determined by ANOVA followed by Bonferroni’s post hoc test using Origin 8 program (OriginLab, Northampton, MA). For Figure 1A, a two-way ANOVA was used for the analysis. p < 0.05 was considered statistically significant.
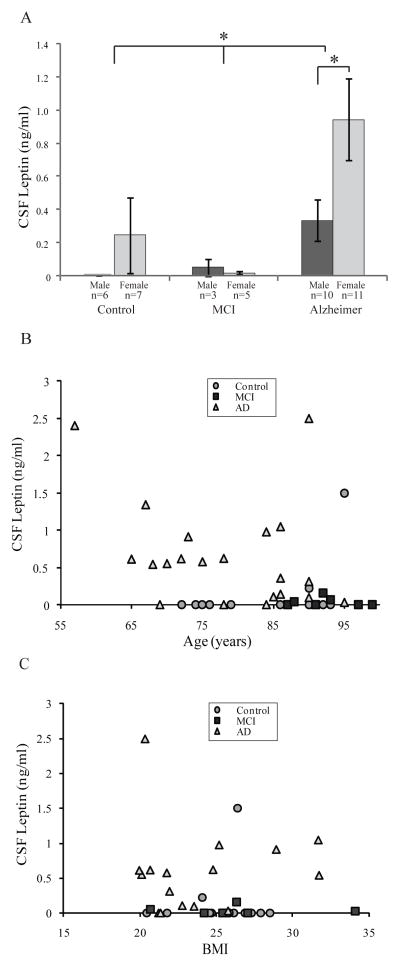
Figure 1.
CSF leptin levels in control, mild cognitive impairment (MCI), and AD. A) CSF leptin is significantly higher in AD than MCI and control cases (*p < 0.05). CSF leptin was higher in females compared to males within the AD group (*p < 0.05). B) CSF leptin level did not correlate with age in control MCI or AD suggesting higher level of CSF leptin in AD cases is disease related rather than age relate. C) Nor did the leptin levels correlate with BMI in any of the groups.
Results
Leptin Levels in CSF and Brain
Leptin concentration in CSF obtained at the time of autopsy was significantly higher in AD compared to both MCI (**p = 0.02) and control (**p = 0.02) cases (Fig 1A). CSF leptin was higher in females compared to males only within the AD group (*p < 0.05) (Fig 1A), in agreement with previous findings demonstrating a gender difference in circulating leptin levels (Fulda et al. 2010, Lieb et al. 2009). Leptin level was lower in MCI than controls; however, this trend did not reach statistical significance (Fig 1A). Moreover, CSF leptin concentration was significantly higher in late severe stage AD cases than early mild stage AD and MCI cases when correlated with Braak staging of pathological NFT burden. Specifically, statistical comparison of Braak stage VI cases and Braak stage 0–V cases found the mean CSF leptin in stage VI was more than six times greater (‡p < 0.01). However, CSF leptin concentration did not correlate with age in control (R = 0.43), MCI (R = −0.02), or AD groups (R = −0.31) (Fig 1B), indicating the observed trend of higher CSF leptin in late stage AD is more likely a disease-related rather than an age-related phenomenon.
There were no significant differences in BMI between experimental groups, ages, and genders (data not shown). Furthermore, no correlation was found between CSF leptin levels and BMI in the control (R = 0.10), MCI (R = −0.02), or even the AD group (R = 0.05) (Fig 1C). Regarding the stability of leptin in our CSF samples, we found no correlation between CSF leptin levels and PMI in any of the groups, or even across all samples together. To further confirm the stability of leptin over time, in vitro analysis was performed. Purified leptin was incubated in either TBS or in aliquots of CSF and kept on ice or placed at 37°C to mimic the in vivo post-mortem environment. No degradation of leptin was found in either condition even after 4 hours, the longest PMI of the CSF samples used in the RIA analysis (Suppl Fig 1A). Consistent with this finding, in multiple previous studies, leptin was shown to be relatively stable in biological samples (Ma et al. 1996, Flower et al. 2000, Miller et al. 2006).
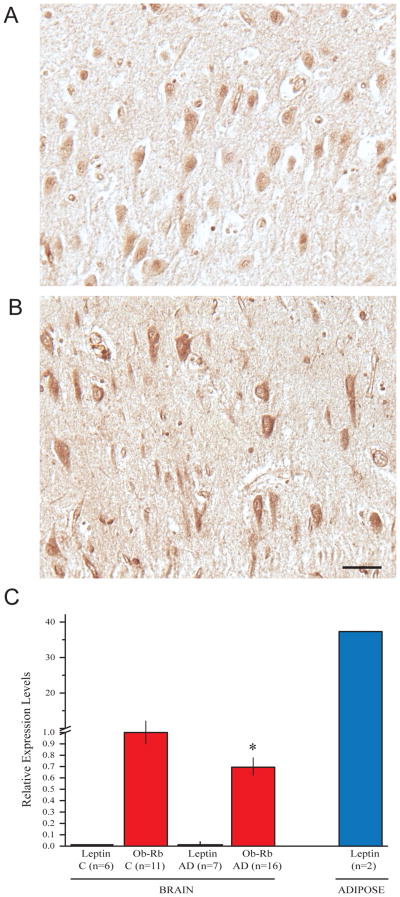
Immunohistochemical analysis of hippocampal tissue sections revealed leptin localized to pyramidal neurons in both control (Fig 2A) and AD cases (Fig 2B), a subpopulation of neurons in the AD cases demonstrated relatively higher levels of leptin. Quantitative RT-PCR was performed on leptin mRNA purified from hippocampus from cases of AD and control to confirm the source of leptin. No leptin mRNA was found in any case, confirming leptin is not synthesized in the hippocampus, while the positive control adipose tissue showed high levels of leptin mRNA (Fig 2C).
Figure 2.
Immunohistochemical staining finds the antibody to leptin stains neuronal cytoplasm in the hippocampus from both control (A) and AD cases (B). Many neurons in the AD cases demonstrated relatively higher levels of leptin. Scale bar = 50 μm. Quantitative RT-PCR found leptin mRNA isolated from brain tissue of AD and control absent in all cases, confirming leptin is not synthesized in these brain tissues, while the positive control adipose tissue showed high levels of leptin mRNA (C). qRT-PCR analysis found the level of Ob-Rb mRNA was significantly low in AD hippocampal tissue (*p<0.05, C) compared to control.
Leptin Receptor Levels in the Brain
In quantitative RT-PCR analysis with total RNA isolated from AD and control brain, the expression level of Ob-Rb mRNA was significantly low in AD hippocampal tissue (p<0.05) (Fig 2C) compared to control. Interestingly, there was no significant change in Ob-Rb mRNA levels as a function of age in either AD or control group (data not shown).
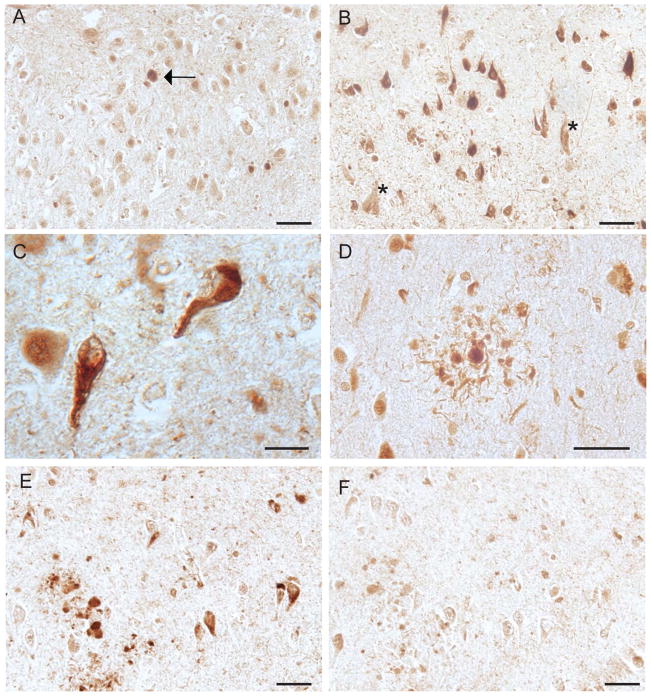
Immunohistochemical staining further confirmed the presence of Ob-Rb in hippocampal, and cortical neurons. In age-matched controls, a weak cytoplasmic localization was prominent with occasional nuclear staining (Fig 3A). Notably, AD cases showed a robust association of Ob-Rb with both intracellular and extracellular NFTs (Fig 3B, C) as well as neuritic plaques (Fig 3B). In older controls, age-related neurofibrillary pathology was also labeled by Ob-Rb antibody (Fig 3A, arrow). The specificity of the antibody for Ob-Rb was confirmed by the absorption assay with its specific peptide which greatly reduced the antibody (Fig 3G,H) and no difference in immunoreactivity was observed between methacarn and formalin fixed sections (data not shown). Western blot analysis of soluble fraction of hippocampal homogenates from both AD and control cases revealed the antibody used recognized the full length leptin receptor (approximately 125kDa) in both AD and control patients (Suppl Fig 1B). It is not unexpected that no striking increase in Ob-Rb is seen in the AD cases by Western blot, since it is likely that the Ob-Rb in NFT could be insoluble and difficult to detect with this method.
Figure 3.
Immunohistochemistry reveals weak Ob-Rb localization to neuronal cytoplasm in pyramidal neurons of control individuals (A) as well as the occasional age-related neurofibrillary tangles (A, arrow). In the AD cases examined, however, NFT are prominently labeled (B) both intracellullar and extracellular NFT (marked by * in B). The NFT exhibits typical fibrillar morphology (C). Ob-Rb is also colocalized with the neuritic pathology associated with senile plaques (D). Adsorption with the peptide antigen nearly abolishes the NFT and neuritic plaque localization (E) compared to the non-absorbed adjacent sections (F). Scale bars = 50 μm (A,B,D-F) and 20 μm (C).
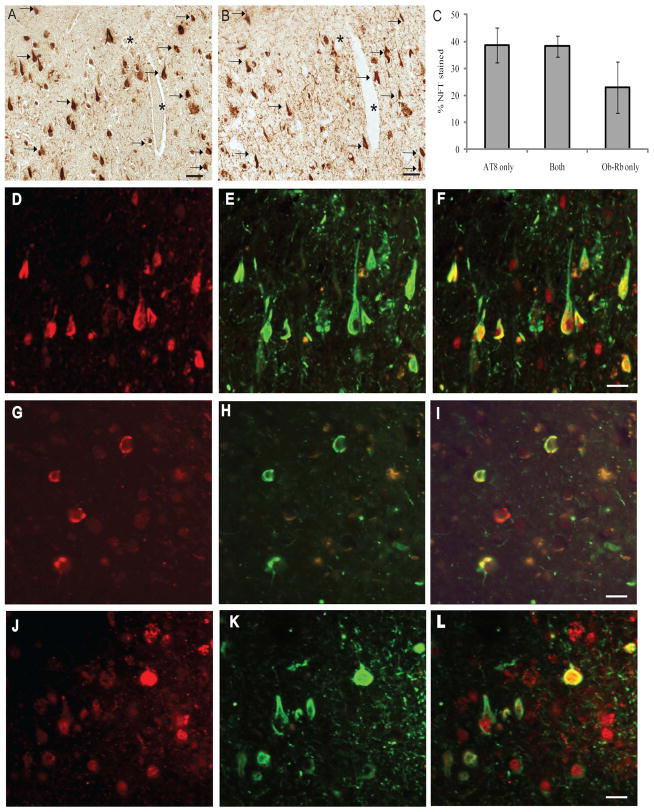
To confirm the co-localization of Ob-Rb with NFTs, we performed a comparative analysis of adjacent serial sections from 8 cases of AD and found that on average 40% of neurons stained with Ob-Rb (Fig 4A) also contained the pathological form of tau, labeled with the monoclonal antibody AT8 (Fig 4B). Another 40% of NFT contained only AT8, and about 20% of NFT were only positive for Ob-Rb (Fig 4C). To substantiate the quantification obtained using serial adjacent sections stained using anti-Ob-Rb and AT8, double fluorescent microscopy on a single section was also used to confirm that many of the neurons strongly stained for Ob-Rb (red) also contained AT8 (green), a marker for NFT (Fig 4D–F). Indeed, some NFT only demonstrated Ob-Rb (Fig 4G–I) and some only displayed AT8 (Fig 4J–L), yet Ob-Rb was still seen as a weak nuclear localization, but not accumulated in the cytoplasmic NFT formation in these instances.
Figure 4.
Staining of adjacent serial sections reveals many of the neurons containing Ob-Rb (A) also contain phosphorylated tau, labeled with the monoclonal antibody AT8 (B), marked by arrows. * denotes landmark vessel. Quantification of 8 cases of AD found that on average 40% of neurons stained with Ob-Rb also contained the pathological form of tau, while another 40% of NFT contained only AT8, and about 20% of NFT were only positive for Ob-Rb (C). Double fluorescent microscopy on a single section was also used to confirm that many of the neurons strongly stained for Ob-Rb (left panels, red, D, G, J) also contained AT8, a marker for NFT (middle panels, green, E, H, K). Right panels show the merged images. Indeed, some NFT only demonstrated Ob-Rb (G-I) and some only displayed AT8 (J-L), yet Ob-Rb was still seen as a weak nuclear localization, but not accumulated in the cytoplasmic NFT formation in these instances.
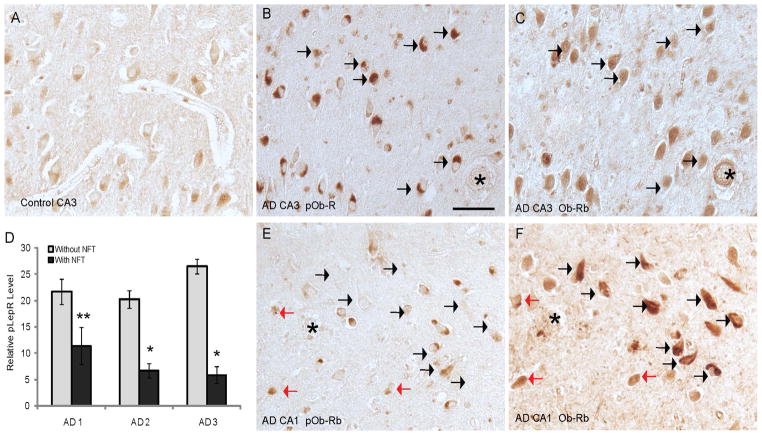
When leptin binds to its receptor, Ob-Rb becomes phosphorylated at T985 for its activation. Using an antibody specific for the activated form, we found that pOb-RbT985 localized to neuronal cytoplasm at qualitatively higher levels in the hippocampus in AD (Fig 5B) compared to controls (Fig 5A). However, importantly, the neurons positive for pOb-RbT985 did not appear to be associated with NFTs in any cases tested in this study. To confirm this finding further, we carefully examined the cornus ammonus (CA) regions of the hippocampus within individual AD cases. Neurons within the CA3 region, which contains relatively little NFT pathology, exhibited strong pOb-RbT985 staining (Fig 5F) and Ob-Rb was also present in many of these same neurons containing the activated form (Fig 5E, F, black arrows). Conversely, neurons in the CA1 region on the same sections from the same patients visually had less pOb-RbT985 immunoreactivity (Fig 5E). Compared to the CA3 region, many NFT showed robust Ob-Rb staining (Fig 5C), yet most of these cells had little or no pOb-RbT985 immunoreactivity (black arrows in Fig 5C, D). Many of the non-NFT bearing neurons in the CA1 region contained both Ob-Rb and pOb-RbT985 (red arrows in Fig 5C,D).
Figure 5.
The activated form of the leptin receptor, pOb-RbT985, is detected at higher levels in the hippocampus of the AD cases (B) compared to controls (A). However, within individual AD cases, the pOb-RbT985 immunoreactivity is strongest in the CA4/CA3 regions, and is much reduced in the CA2/CA1 regions. In the CA3 region of an AD case, many of the neurons with high levels of pOb-RbT985 (B), also demonstrate a diffuse cytoplasmic localization of Ob-Rb (C; same neurons denoted by black arrows). On the same section, pyramidal neurons in the CA1 region contain lower levels of pOb-RbT985 (E). Red arrows mark those cells containing pOb-RbT985 and diffuse cytoplasmic Ob-Rb pattern (D). Conversely, the NFT-bearing neurons immunostained for Ob-Rb (black arrows, F), often do not contain pOb-RbT985 (E).) * denotes landmark vessels. Scale bar = 50 μm. Quantification (D) reveals a significantly lower relative intensity of pOb-RbT985 in those cells with NFT compared to cells without NFT (*=p<0.001, **=p<0.05)
Quantification of the pOb-RbT985 immunoreactivity in the population of neurons with and without Ob-Rb-positive NFT revealed a significant decrease in the activated form in NFT. Using adjacent serial sections from 3 cases of AD, neurons that were present in both sections stained for either Ob-Rb or pOb-RbT985 were densitometrically analyzed. pOb-RbT985 immunoreactivity was found to be lower in NFT-bearing neurons in all 3 cases analyzed (p<0.05 and p<0.001, Fig 5D).
Discussion
Our findings indicate a marked alteration in leptin signaling in the hippocampus of AD patients. We found elevations in leptin concentrations in CSF samples (Fig 1A) from AD patients when compared to controls. These differences were not correlated with variations in age or BMI within or between groups (Fig 1B, C); they are therefore likely associated with the disease itself. While the women AD end stage patients were found to have higher leptin levels in the CSF, women have previously been shown to have higher ciruculating levels of leptin in the serum than men (Bennett et al. 1997). This study may have implications for understanding the gender differences associated with AD incidence. Interestingly, our analyses of leptin CSF levels along the Braak staging continuum demonstrated a striking leptin increase in the most severe cases. Specifically, Braak VI patients exhibited a six-fold increase in leptin CSF concentrations compared to Braak 0–V patients. The Braak scoring system provides an assessment of neuritic pathology in postmortem brain tissue that is independent of, though complementary to, antemortem diagnosis, with Braak 0 or I representing minimal tau pathology and Braak VI representing severe NFT pathology (Braak & Braak 1994). Braak stages I–II are typically manifest as clinically silent, stages III–IV as incipient, mild AD, and stages V–VI as fully developed, severe AD (Braak & Braak 1995). These results, taken together with prospective studies in which increased levels of baseline plasma leptin concentrations correlated with a reduced risk of dementia or AD onset (Lieb et al. 2009, Holden et al. 2009), strongly suggest a correlation between the leptin level in brains and CSF with the progression of AD. These studies did not monitor leptin levels following the initial measurement and, thus did not collect end-stage data for patients in the Braak VI category of AD, for which we found striking increases of leptin. Nonetheless, our study also found a trend toward decreased levels of CSF leptin in MCI compared to control, which supports the attributed benefits associated with leptin signaling in the brain, and may be important in elucidating the etiological occurrence of AD.
Leptin is synthesized in the periphery and actively transported across the blood-brain barrier (BBB) or blood-CSF barrier (Kurrimbux et al. 2004), thus our finding that leptin mRNA is not present in the brain while its protein is localized in neurons is consistent with previous reports. The precise dynamics of leptin transport are incompletely understood; it is not yet clear whether CSF leptin is exiting the brain via the choroid plexus epithelial cells (Uotani et al. 1999) or whether it is entering the brain in a manner independent of leptin BBB entry. Furthermore, leptin transport across the BBB is believed to be a saturable system whereby brain entry is strictly limited by transport dynamics (Banks et al. 1996). Given that BBB has been demonstrated to be compromised in AD brains (Deane & Zlokovic 2007), increased level of leptin in severe AD cases may be reflective of this disease-associated pathology. Along with the marked increase in leptin in AD CSF, we found a significant decrease in Ob-Rb mRNA levels in AD patients compared to controls. The expression of Ob-Rb is regulated by a number of factors, including circulating leptin levels, insulin levels, and diet, and the precise dynamics and conditions of leptin receptor regulation are incompletely understood (Hikita et al. 2000, Mitchell et al. 2009). Therefore, the alteration found in our study (i.e., the increased levels of CSF leptin, the high levels of Ob-Rb sequestered in NFT, the upregulation of pOb-RbT985 in non-tangle-bearing neurons) may all contribute to the negative regulation of Ob-Rb mRNA expression and this mechanism need to be determined in future study.
Leptin plays a major role in the regulation of appetite and body energy metabolism. It has been shown that biomarkers for AD such as Aβ and tau are associated with lower BMI in both MCI and cognitively normal individuals (Vidoni et al. 2011). For example, patients with moderate AD were shown to have lower lean body mass, and the male patients energy homeostasis, measured by serum ghrelin and leptin levels, is not maintained (Theodoropoulou et al. 2012). Other appetite and body energy metabolism associated neuropeptides, such as galanin and MSH in the CSF, are also correlated with AD progression and specifically NFT related biomarkers tau (Costa et al. 2011). Many studies have found relationships to midlife obesity and increased risk of AD and dementia. However, weight loss is also a factor in AD patients, though it may not be directly related to loss of fat and instead may be a result of loss of muscle (Lee 2011). Other AD related factors may also play a role in the dysregulation of leptin signaling reported here. Mice lacking APOE show decreased circulating leptin, linking appetite, leptin, cholesterol levels with APOE, of which the APOE4 allele is less effective at clearing away cholesterol, and which poses the strongest genetic risk factor for AD (Raber et al. 2000). In any case, there seems to be a severe disconnect in the leptin signaling pathway in AD.
Our data demonstrate a striking colocalization of the leptin receptor to NFTs in the hippocampus (Fig 3) with a weak association between the active form of Ob-Rb (pOb-RbT985) and NFTs (Fig 4) in AD. Within the same tissue sections of the same AD cases, we noted discrepancies in the regional immunoreactivity of pOb-RbT985 and resident NFT, such that the NFT-positive region (i.e., CA1) contained drastically reduced pOb-RbT985 staining (Fig 4) while the pathology-free regions (CA3/4) contained elevated pOb-RbT985 staining (Fig 4E, F). These incongruities continue despite the elevated presence of leptin in the AD brains and CSF mentioned above. Thus, there seems to be an NFT-induced blockade of Ob-Rb accessibility and an effective leptin resistance. That is, due to pathological sequestration of Ob-Rb by NFT, regions containing high concentrations of NFT lose the ability to communicate leptin signal transmission and therefore do not demonstrate immunohistochemical presence of pOb-RbT985 and fail to receive the aforementioned benefits of leptin signaling in the hippocampus by effectively preventing leptin from gaining access to its receptor in the affected region. Consequently, these pathological alterations in leptin signaling result in leptin resistance in the vulnerable neurons in AD (Fig 6) and this mechanism may be critical to the cognitive dwindling phenotype characteristic of AD. Supporting this hypothesis, it is interesting to note that, throughout the cycle from expression to degradation, Ob-Rb follows an elaborate trafficking pathway that relies chiefly on microtubules (Wilcke & Walum 2000). Since tau is a microtubule binding protein (Castellani et al. 2008) and becomes hyperphosphorylated and aggregates into fibrillized NFTs in AD (Mondragon-Rodriguez et al. 2008), it is likely that Ob-Rb becomes sequestered within the pathological NFTs during is processing and movement along the cytoskeleton. Indeed, aberration in cytosolic transport machinery has been previously identified in AD, affecting the functioning of signaling cascades associated with Smad2 (Lee et al. 2006b) and importin α1 (Lee et al. 2006a), and the data presented here corresponds nicely to these observations. Additionally, reduction in downstream signaling molecules of the leptin pathway in AD has been described, indicating a lack of effective leptin-based communication in the disease (Chiba et al. 2009).
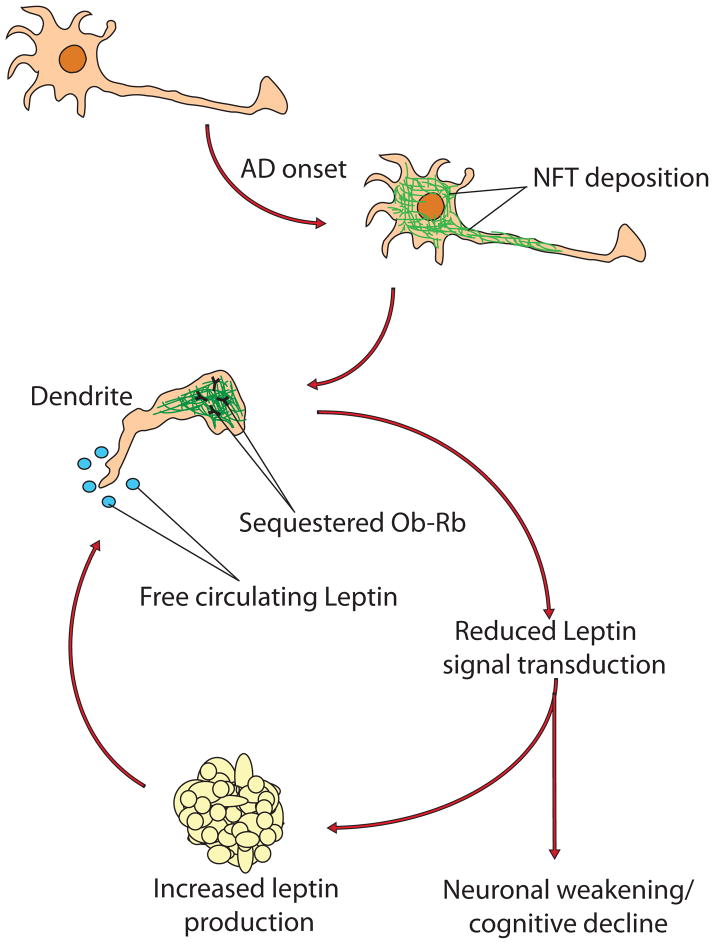
Figure 6.
Schematic depicting the proposed mechanism underlying leptin resistance in AD. Following onset of the disease, hyperphosphorylation of tau leads to the formation of NFT and corresponding impairment of intracellular trafficking networks. The leptin receptor (Ob-Rb) becomes unable to reach the cell membrane and thus becomes unable to gain access to circulating free leptin disrupting leptin signaling, and the cognitive/neuronal benefits associated with its activity do not manifest. Concurrently, the lack of leptin signal transduction within affected neurons creates the illusion of inadequate circulating leptin levels, ultimately communicating a physiological need for more leptin secretion. Ultimately, this cascade may cause a gradual weakening of affected neurons and cognitive decline even with increased level of leptin.
These data raise important questions as to the nature of the impact that these interactions have on the neurophysiology/cognitive phenotype of the affected patients. The disruption in leptin signaling in AD is clear; whether or not this is a manifestation of leptin resistance due to NFT development, or the result of some other neurological defects, however, is not clear. In relation to the reported benefits of leptin signaling in the hippocampus (Garza et al. 2008, Harvey 2003), the ultimate effect may be partly responsible for the dwindling cognitive capacity that characterizes AD. That is, perhaps the disjointed intracellular processing of Ob-Rb impairs leptin signaling in the affected regions (i.e., the hippocampal formation of the mediotemporal lobe), creating a leptin resistance that drastically reduces the benefits of leptin signaling (Perez-Gonzalez et al. 2011, Garza et al. 2008) in an already decaying brain state. The data listed here, in conjunction with the findings of Chiba et al. (Chiba et al. 2009), in which the JAK2/STAT3 signaling molecules of the leptin cascade were found to be altered in AD, support this postulation.
The goal of AD research is the generation of translational therapeutics that can prevent and reverse disease symptoms. Leptin has been previously demonstrated to exert neuroprotective effects in the hippocampus (Perez-Gonzalez et al. 2011, Garza et al. 2008) as well as an amelioration of the hyperphosphorylation of tau in these regions (Greco et al. 2009a, Greco et al. 2009b, Greco et al. 2008). Our findings seem to similarly point to the leptin pathway as a target for disease control. It may be possible to simultaneously reduce tau neuropathology and increase cognitive restoration through an increase in leptin signaling and appropriate leptin receptor trafficking.
Supplementary Material
Western blot analysis of purified leptin incubated in human CSF collected from 2 individuals, case A and case B, shows no degradation of either the monomer or dimer band even after 4 hours incubation at 37°C, mimicking the longest post-mortem interval of the subjects used for the leptin RIA analysis (A). The leptin receptor antibody, which detects NFT in AD brain by immunocytochemistry, detects the full length Ob-Rb at approximately 125kDa.
Acknowledgments
This study was supported by the National Institutes of Health (AG028679 to HGL and AG031852 to XWZ). UK-ADC database P30 AG028383. The funding source had no involvement in data collection, analysis or interpretation of the study.
Abbreviations
- AD
Alzheimer disease
- Aβ
amyloid-β
- BBB
blood-brain barrier
- BMI
body mass index
- CA
cornus ammonus
- CSF
cerebrospinal fluid
- MCI
mild cognitive impairment
- NFT
neurofibrillary tangle
- NGS
normal goat serum
- Ob-Rb
long form of the leptin receptor
Footnotes
Conflict of Interest
Dr. Xiongwei Zhu was a consultant for and received grant support from Medivation. Dr. Mark A. Smith was a consultant for Anavex Life Sciences Corporation, Eisai, Medivation, Neurotez, and Takeda Pharmaceuticals; owned stock options in Aria Neurosciences, Neurotez, Panacea and Voyager, and received lecture fees from GSK, Medivation and Pfizer.
References
- Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett FI, McFarlane-Anderson N, Wilks R, Luke A, Cooper RS, Forrester TE. Leptin concentration in women is influenced by regional distribution of adipose tissue. Am J Clin Nutr. 1997;66:1340–1344. doi: 10.1093/ajcn/66.6.1340. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Morphological criteria for the recognition of Alzheimer’s disease and the distribution pattern of cortical changes related to this disorder. Neurobiol Aging. 1994;15:355–356. doi: 10.1016/0197-4580(94)90032-9. discussion 379–380. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. discussion 278–284. [DOI] [PubMed] [Google Scholar]
- Castellani RJ, Nunomura A, Lee HG, Perry G, Smith MA. Phosphorylated tau: toxic, protective, or none of the above. J Alzheimers Dis. 2008;14:377–383. doi: 10.3233/jad-2008-14404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani RJ, Rolston RK, Smith MA. Alzheimer disease. Dis Mon. 2010;56:484–546. doi: 10.1016/j.disamonth.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T, Yamada M, Sasabe J, Terashita K, Shimoda M, Matsuoka M, Aiso S. Amyloid-beta causes memory impairment by disturbing the JAK2/STAT3 axis in hippocampal neurons. Mol Psychiatry. 2009;14:206–222. doi: 10.1038/mp.2008.105. [DOI] [PubMed] [Google Scholar]
- Costa A, Bini P, Hamze-Sinno M, Moglia A, Franciotta D, Sinforiani E, Ravaglia S, Bole-Feysot C, Hokfelt T, Dechelotte P, Fetissov SO. Galanin and alpha-MSH autoantibodies in cerebrospinal fluid of patients with Alzheimer’s disease. J Neuroimmunol. 2011;240–241:114–120. doi: 10.1016/j.jneuroim.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Deane R, Zlokovic BV. Role of the blood-brain barrier in the pathogenesis of Alzheimer’s disease. Curr Alzheimer Res. 2007;4:191–197. doi: 10.2174/156720507780362245. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- Erol A. An integrated and unifying hypothesis for the metabolic basis of sporadic Alzheimer’s disease. J Alzheimers Dis. 2008;13:241–253. doi: 10.3233/jad-2008-13302. [DOI] [PubMed] [Google Scholar]
- Fewlass DC, Noboa K, Pi-Sunyer FX, Johnston JM, Yan SD, Tezapsidis N. Obesity-related leptin regulates Alzheimer’s Abeta. FASEB J. 2004;18:1870–1878. doi: 10.1096/fj.04-2572com. [DOI] [PubMed] [Google Scholar]
- Flower L, Ahuja RH, Humphries SE, Mohamed-Ali V. Effects of sample handling on the stability of interleukin 6, tumour necrosis factor-alpha and leptin. Cytokine. 2000;12:1712–1716. doi: 10.1006/cyto.2000.0764. [DOI] [PubMed] [Google Scholar]
- Fulda S, Linseisen J, Wolfram G, Himmerich S, Gedrich K, Pollmacher T, Himmerich H. Leptin plasma levels in the general population: influence of age, gender, body weight and medical history. Protein Pept Lett. 2010;17:1436–1440. doi: 10.2174/0929866511009011436. [DOI] [PubMed] [Google Scholar]
- Garza JC, Guo M, Zhang W, Lu XY. Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J Biol Chem. 2008;283:18238–18247. doi: 10.1074/jbc.M800053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco SJ, Sarkar S, Casadesus G, Zhu X, Smith MA, Ashford JW, Johnston JM, Tezapsidis N. Leptin inhibits glycogen synthase kinase-3beta to prevent tau phosphorylation in neuronal cells. Neurosci Lett. 2009a;455:191–194. doi: 10.1016/j.neulet.2009.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco SJ, Sarkar S, Johnston JM, Tezapsidis N. Leptin regulates tau phosphorylation and amyloid through AMPK in neuronal cells. Biochem Biophys Res Commun. 2009b;380:98–104. doi: 10.1016/j.bbrc.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco SJ, Sarkar S, Johnston JM, Zhu X, Su B, Casadesus G, Ashford JW, Smith MA, Tezapsidis N. Leptin reduces Alzheimer’s disease-related tau phosphorylation in neuronal cells. Biochem Biophys Res Commun. 2008;376:536–541. doi: 10.1016/j.bbrc.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J. Leptin: a multifaceted hormone in the central nervous system. Mol Neurobiol. 2003;28:245–258. doi: 10.1385/MN:28:3:245. [DOI] [PubMed] [Google Scholar]
- Harvey J. Leptin: a diverse regulator of neuronal function. J Neurochem. 2007;100:307–313. doi: 10.1111/j.1471-4159.2006.04205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikita M, Bujo H, Hirayama S, Takahashi K, Morisaki N, Saito Y. Differential regulation of leptin receptor expression by insulin and leptin in neuroblastoma cells. Biochem Biophys Res Commun. 2000;271:703–709. doi: 10.1006/bbrc.2000.2692. [DOI] [PubMed] [Google Scholar]
- Holden KF, Lindquist K, Tylavsky FA, Rosano C, Harris TB, Yaffe K. Serum leptin level and cognition in the elderly: Findings from the Health ABC Study. Neurobiol Aging. 2009;30:1483–1489. doi: 10.1016/j.neurobiolaging.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurrimbux D, Gaffen Z, Farrell CL, Martin D, Thomas SA. The involvement of the blood-brain and the blood-cerebrospinal fluid barriers in the distribution of leptin into and out of the rat brain. Neuroscience. 2004;123:527–536. doi: 10.1016/j.neuroscience.2003.08.061. [DOI] [PubMed] [Google Scholar]
- Lee EB. Obesity, leptin, and Alzheimer’s disease. Ann N Y Acad Sci. 2011;1243:15–29. doi: 10.1111/j.1749-6632.2011.06274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HG, Ueda M, Miyamoto Y, Yoneda Y, Perry G, Smith MA, Zhu X. Aberrant localization of importin alpha1 in hippocampal neurons in Alzheimer disease. Brain Res. 2006a;1124:1–4. doi: 10.1016/j.brainres.2006.09.084. [DOI] [PubMed] [Google Scholar]
- Lee HG, Ueda M, Zhu X, Perry G, Smith MA. Ectopic expression of phospho-Smad2 in Alzheimer’s disease: uncoupling of the transforming growth factor-beta pathway? J Neurosci Res. 2006b;84:1856–1861. doi: 10.1002/jnr.21072. [DOI] [PubMed] [Google Scholar]
- Lieb W, Beiser AS, Vasan RS, Tan ZS, Au R, Harris TB, Roubenoff R, Auerbach S, DeCarli C, Wolf PA, Seshadri S. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA. 2009;302:2565–2572. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Gingerich RL, Santiago JV, Klein S, Smith CH, Landt M. Radioimmunoassay of leptin in human plasma. Clin Chem. 1996;42:942–946. [PubMed] [Google Scholar]
- Miller AA, Sharrock KC, McDade TW. Measurement of leptin in dried blood spot samples. Am J Hum Biol. 2006;18:857–860. doi: 10.1002/ajhb.20566. [DOI] [PubMed] [Google Scholar]
- Mitchell SE, Nogueiras R, Morris A, Tovar S, Grant C, Cruickshank M, Rayner DV, Dieguez C, Williams LM. Leptin receptor gene expression and number in the brain are regulated by leptin level and nutritional status. J Physiol. 2009;587:3573–3585. doi: 10.1113/jphysiol.2009.173328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondragon-Rodriguez S, Basurto-Islas G, Santa-Maria I, Mena R, Binder LI, Avila J, Smith MA, Perry G, Garcia-Sierra F. Cleavage and conformational changes of tau protein follow phosphorylation during Alzheimer’s disease. Int J Exp Pathol. 2008;89:81–90. doi: 10.1111/j.1365-2613.2007.00568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morash B, Li A, Murphy PR, Wilkinson M, Ur E. Leptin gene expression in the brain and pituitary gland. Endocrinology. 1999;140:5995–5998. doi: 10.1210/endo.140.12.7288. [DOI] [PubMed] [Google Scholar]
- Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. 2009;297:E1247–1259. doi: 10.1152/ajpendo.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson T, Nasman B, Rasmuson S, Ahren B. Dual relation between leptin and cortisol in humans is disturbed in Alzheimer’s disease. Biol Psychiatry. 1998;44:374–376. [PubMed] [Google Scholar]
- Perez-Gonzalez R, Antequera D, Vargas T, Spuch C, Bolos M, Carro E. Leptin induces proliferation of neuronal progenitors and neuroprotection in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2011;24:17–25. doi: 10.3233/JAD-2011-102070. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Power DA, Noel J, Collins R, O’Neill D. Circulating leptin levels and weight loss in Alzheimer’s disease patients. Dement Geriatr Cogn Disord. 2001;12:167–170. doi: 10.1159/000051252. [DOI] [PubMed] [Google Scholar]
- Raber J, Akana SF, Bhatnagar S, Dallman MF, Wong D, Mucke L. Hypothalamic-pituitary-adrenal dysfunction in Apoe(−/−) mice: possible role in behavioral and metabolic alterations. J Neurosci. 2000;20:2064–2071. doi: 10.1523/JNEUROSCI.20-05-02064.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley LJ, O’Malley D, Irving AJ, Ashford ML, Harvey J. Leptin inhibits epileptiform-like activity in rat hippocampal neurones via PI 3-kinase-driven activation of BK channels. J Physiol. 2002;545:933–944. doi: 10.1113/jphysiol.2002.029488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezapsidis N, Johnston JM, Smith MA, Ashford JW, Casadesus G, Robakis NK, Wolozin B, Perry G, Zhu X, Greco SJ, Sarkar S. Leptin: a novel therapeutic strategy for Alzheimer’s disease. J Alzheimers Dis. 2009;16:731–740. doi: 10.3233/JAD-2009-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoropoulou A, Metallinos IC, Psyrogiannis A, Vagenakis GA, Kyriazopoulou V. Ghrelin and leptin secretion in patients with moderate Alzheimer’s disease. J Nutr Health Aging. 2012;16:472–477. doi: 10.1007/s12603-012-0058-4. [DOI] [PubMed] [Google Scholar]
- Uotani S, Bjorbaek C, Tornoe J, Flier JS. Functional properties of leptin receptor isoforms: internalization and degradation of leptin and ligand-induced receptor downregulation. Diabetes. 1999;48:279–286. doi: 10.2337/diabetes.48.2.279. [DOI] [PubMed] [Google Scholar]
- Vidoni ED, Townley RA, Honea RA, Burns JM. Alzheimer disease biomarkers are associated with body mass index. Neurology. 2011;77:1913–1920. doi: 10.1212/WNL.0b013e318238eec1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcke M, Walum E. Characterization of leptin intracellular trafficking. Eur J Histochem. 2000;44:325–334. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Western blot analysis of purified leptin incubated in human CSF collected from 2 individuals, case A and case B, shows no degradation of either the monomer or dimer band even after 4 hours incubation at 37°C, mimicking the longest post-mortem interval of the subjects used for the leptin RIA analysis (A). The leptin receptor antibody, which detects NFT in AD brain by immunocytochemistry, detects the full length Ob-Rb at approximately 125kDa.