SUMMARY
The nuclear lamina is a protein meshwork that lies under the inner nuclear membrane of metazoan cells. One function of the nuclear lamina is to organize heterochromatin at the inner nuclear periphery. However, very little is known about how heterochromatin attaches to the nuclear lamina and how such attachments are restored at mitotic exit. Here we show that a previously unstudied human protein, PRR14, functions to tether heterochromatin to the nuclear periphery during interphase, through associations with heterochromatin protein 1 (HP1) and the nuclear lamina. During early mitosis, PRR14 is released from the nuclear lamina and chromatin, and remains soluble. Strikingly, at the onset of anaphase, PRR14 is incorporated rapidly into chromatin through HP1 binding. Finally, in telophase, PRR14 relocalizes to the reforming nuclear lamina. This stepwise reassembly of PRR14 suggests a novel function in the selection of HP1–bound heterochromatin for reattachment to the nuclear lamina as cells exit mitosis.
INTRODUCTION
The nuclear envelope of metazoan cells is composed of an outer nuclear membrane (ONM), an inner nuclear membrane (INM), and the nuclear lamina. The nuclear lamina is a protein meshwork that attaches to the INM, and maintains nuclear shape by acting as a framework at the inner nuclear periphery (Dittmer and Misteli, 2011; Gerace and Huber, 2012; Simon and Wilson, 2011). The lamina is composed of the intermediate filament proteins Lamin A/C, Lamin B1, and Lamin B2, which form separate, but interconnected networks. In addition to its structural role in nuclear shape, the nuclear lamina functions as a scaffold for a variety cellular processes. Recent studies have shown that in interphase cells, the positioning of genes at the nuclear lamina promotes epigenetic gene silencing (Guelen et al., 2008; Peric-Hupkes et al., 2010; Peric-Hupkes and van Steensel, 2010; Reddy et al., 2008; Shevelyov and Nurminsky, 2012; Towbin et al., 2012). Furthermore, a repressive chromatin compartment at the inner nuclear periphery can be visualized as a layer of perinuclear heterochromatin. This layer is enriched for the defining features of repressed heterochromatin, the histone 3 lysine 9 di- and tri-methylation modifications (H3K9me2/3) (Towbin et al., 2012; Zhou et al., 2011). These H3K9me2/3 modifications are bound by members of the heterochromatin protein 1 (HP1) family (α,β,γ), whose functions are to promote heterochromatin formation, and also act as platform to recruit a variety of binding partners (Grewal and Jia, 2007; Zeng et al., 2010).
The mechanisms by which perinuclear heterochromatin attaches to the nuclear lamina are not well understood, and even less is known about how such attachments are disassembled and reassembled during cell division. An integral INM protein, the Lamin B receptor (LBR), serves to attach the nuclear lamina to the INM, and also binds to HP1-associated heterochromatin thereby connecting three concentric layers: the INM, the nuclear lamina, and heterochromatin (Makatsori et al., 2004; Olins et al., 2010; Solovei et al., 2013; Ye et al., 1997). LBR remains membrane-associated throughout mitosis, and a major function of this protein is to deliver nuclear membranes to chromatin during mitotic nuclear envelope reassembly.
Using a screen designed to detect epigenetic silencing factors (Poleshko et al., 2010), we have identified a previously unstudied human protein, Proline Rich Protein 14 (PRR14) (manuscript in preparation). Here we show that PRR14 localizes to the nuclear lamina in interphase, and functions to tether peripheral HP1-H3K9me3-marked heterochromatin to the nuclear lamina. Furthermore, PRR14 remains soluble during mitosis, and rapidly rebinds to chromatin in an HP1–dependent manner at the onset of anaphase. We suggest that an additional function of PRR14 may be to specify heterochromatin for reattachment to the nuclear lamina at mitotic exit.
RESULTS
PRR14 Localizes to the Nuclear Lamina and Knockdown Causes Defects in Nuclear Structure
The PRR14 gene is conserved in mammals and is widely expressed. The PRR14 full-length open reading frame encodes a predicted protein of 585 amino acids with high proline content. Bioinformatic approaches failed to detect any known functional domains, although a region near the C-terminus defines a paralogous relationship with another human gene, PRR14L.
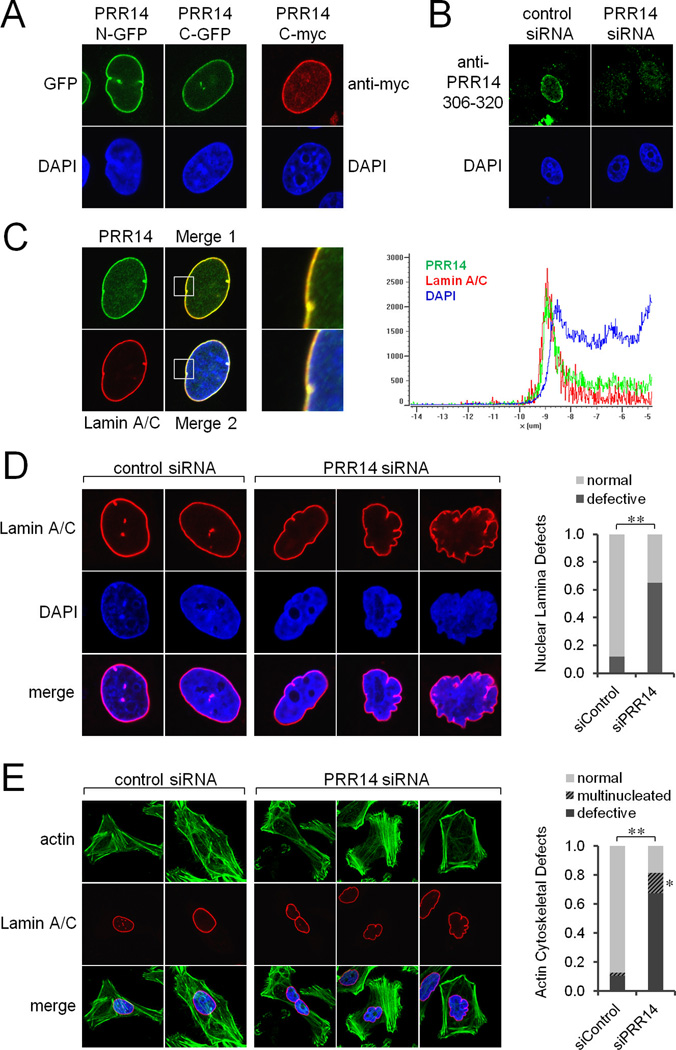
PRR14 N- and C-terminal GFP fusion proteins, a C-terminal myc-tagged version, and native PRR14 showed a nuclear rimming pattern in interphase HeLa cells consistent with concentration at the nuclear lamina (Figure 1A–C and Figure S1A–C). A similar pattern was seen in euploid human retinal pigment epithelial 1 (RPE1) cells (see Figure S3C). We determined that tagged PRR14 localizes to the inner nuclear periphery as opposed to the cytoplasmic face of the nuclear envelope, using a standard antibody accessibility approach (Figure S1A). High resolution imaging demonstrated a strict signal overlap between PRR14 and the nuclear lamina component Lamin A/C (Figure 1C). Notably, PRR14 has not been detected previously as a nuclear laminaassociated protein (Kubben et al., 2010; Roux et al., 2012).
Figure 1. Confocal Imaging Showing Localization of PRR14 at the Nuclear Lamina and Defects Produced by PRR14 Knockdown.
(A) N-terminal (mGFP) and C-terminal (turboGFP) -tagged PRR14 were expressed in HeLa cells, and detected by confocal microscopy. A C-terminal myc-tagged version of PRR14 was detected using an anti-myc antibody. DNA was stained with DAPI.
(B) Native PRR14 was detected in HeLa cells using a peptide antibody directed at amino acids 306–320. SiRNA knockdown of PRR14 was used to demonstrate specificity.
(C) HeLa cells stably expressing full length N-terminal GFP-tagged PRR14 were isolated. Cells were fixed and stained with anti-Lamin A/C (red). DNA was detected with DAPI (blue). Merge 1 combines the GFP and Lamin A/C signals, and Merge 2 also includes DAPI. Boxes in merged images indicate regions magnified at the right. A line profile of signal intensities is shown on the right, indicating colocalization of PRR14 and Lamin A/C.
(D) Nuclear defects produced after PRR14 siRNA transfection were monitored by confocal imaging after 72 hrs. Cells were fixed, and anti-Lamin A/C antibodies were used to detect the nuclear lamina structure. Fraction of cells displaying the indicated nuclear shape defects is shown (right panel), with n = 50 and n =100 for the control and PRR14 siRNA treated cells, respectively. DNA was stained with DAPI. Statistical analyses using Fisher’s Exact Test demonstrated that the knockdown phenotypes were statistically significant, with **p < 0.00001.
(E) Cells were treated with PRR14 siRNA and processed as in Panel C. F-actin was detected using Phalloidin conjugated to Alexa Fluor 488. Defects in the actin cytoskeleton and multinucleation phenotypes are shown. Fraction of cells displaying the indicated phenotypes is shown (right panel), with n = 47 and n = 80 for the control and PRR14 siRNA treated cells, respectively. The fraction of defective cells showing multinucleation defects is represented by the hatched area. Statistical analyses using Fisher’s Exact Test demonstrated that the knockdown phenotypes were statistically significant, with p-values of *p < 0.001 and **p < 0.00001. See Figure S1.
We next asked whether PRR14 contributes to nuclear lamina structure. SiRNA knockdown of PRR14 in HeLa cells resulted in nuclear distortions, as seen in diseases of the nuclear lamina (Figure 1D) (Butin-Israeli et al., 2012; Dittmer and Misteli, 2011; Gordon et al., 2012; Worman et al., 2010). Similar knockdown effects were observed in RPE1 cells (data not shown). Detachment of the nucleus from the actin cytoskeleton and high frequency multinucleation were also observed (Figures 1E and S1D–F). Again, these defects are similar to those seen in diseased or nuclear lamina-deficient cells (Gordon et al., 2012; Lee et al., 2007; Mejat and Misteli, 2010).
PRR14 Encodes Separable Nuclear Lamina and Heterochromatin Binding Domains
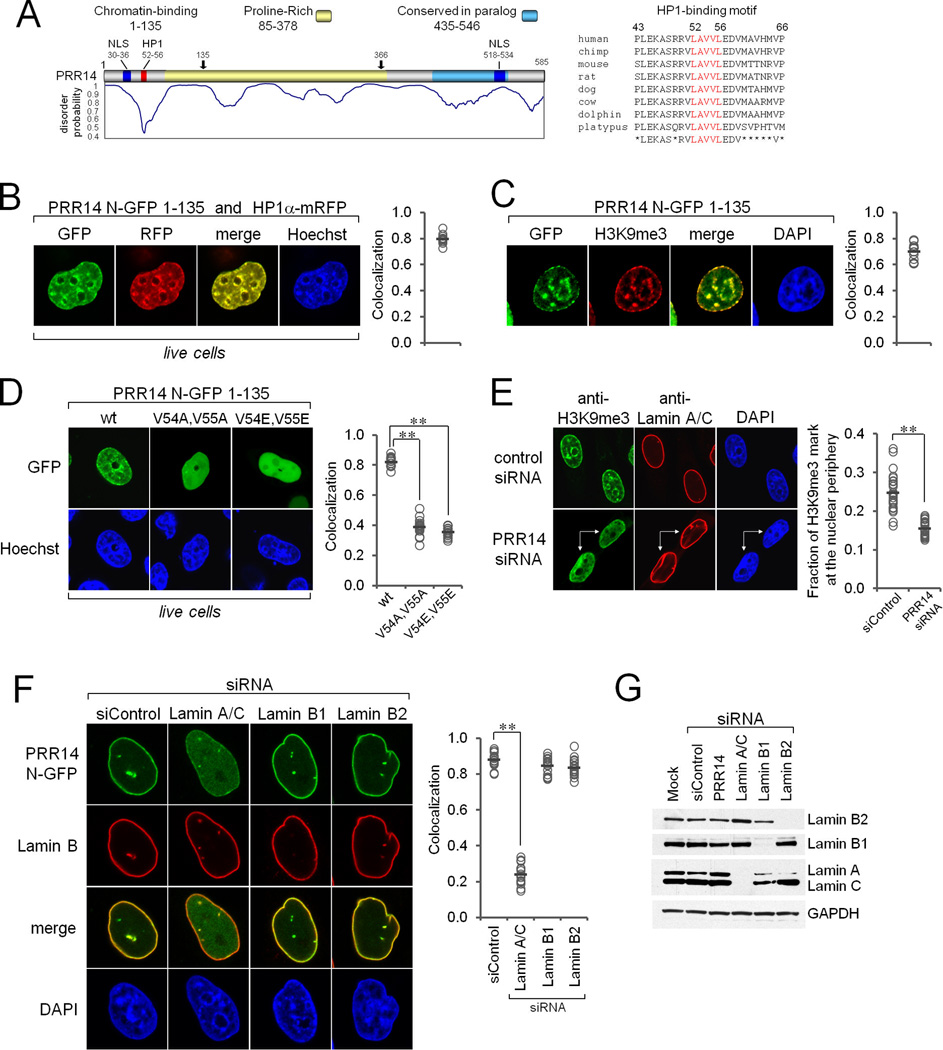
Bioinformatic analyses of the predicted PRR14 protein revealed areas of disorder based on PONDR-FIT (Xue et al., 2010), a proline-rich region defined by PROSITE (Sigrist et al., 2013), and an ordered, conserved C-terminal region (Figure 2A). An unbiased yeast-two hybrid matrix analysis, and a proteomics study had both detected PRR14 as a binding partner of human heterochromatin protein 1 alpha (HP1α) (Nozawa et al., 2010; Rual et al., 2005). Inspection of the PRR14 protein sequence revealed a conserved, variant HP1-binding motif within the ordered N-terminal region at positions 52–56 (LAVVL) (Figure 2A) (Lechner et al., 2005; Nozawa et al., 2010; Thiru et al., 2004). Deletion mapping showed that the N-terminal fragment (amino acids 1–135) accumulated in the nucleus (Figures 2B and S2A,B), while the central region was required for nuclear lamina localization (Figure S2A,B). Within the nucleus, the PRR14 N-terminal fragment bound to heterochromatin, based on colocalization with HP1α (Figure 2B). The N-terminal fragment also colocalized with the HP1 ligand, the H3K9me3 histone modification, in both perinuclear and intranuclear regions (Figures 2C). Substitutions of the candidate LAVVL HP1-binding motif, or siRNA knockdown of HP1, both resulted in loss of heterochromatin binding by the N-terminal fragment, and instead a diffuse nuclear-wide pattern was observed (Figures 2D, S2D). Thus, the PRR14 N-terminus, when expressed independently, was revealed to localize with HP1-H3K9me3-marked heterochromatin. We conclude that full length PRR14 is capable of binding to heterochromatin through the N-terminal LAVVL motif, while the central region is required for nuclear lamina localization.
Figure 2. PRR14 Functional Mapping and Evidence for a Heterochromatin-Nuclear Lamina Tethering Function.
(A) Map of the PRR14 protein. Shown are disorder probability, predicted NLS sequences (blue), the candidate HP1-binding motif (red) and the proline-rich region (yellow). Amino acid alignments showing conservation of the candidate HP1-binding motif is shown at the right. Arrows indicate demarcation of N-terminal, central, and C-terminal fragments (see Figure S2A,B for functional mapping).
(B) The GFP-tagged N-terminal PRR14 fragment (amino acids 1–135) was stably introduced into HeLa cells, and mRFP-HP1α was introduced by transient transfection. DNA was stained with Hoechst. MetaXpress software was used to quantitate red and green signal colocalization. Graph indicates measurements of individual cells (circles, n =15) and the bar indicates average value. See Supplemental Methods.
(C) The GFP-tagged N-terminal PRR14 fragment was introduced into HeLa cells by transient transfection. Cells were fixed and stained with anti-H3K9me3 antibodies (red) followed by confocal imaging. Red and green signal colocalization was analyzed as in panel B (n = 15).
(D) The GFP-tagged N-terminal fragment containing substitutions in the candidate HP1- binding motif were introduced into HeLa cells by transient transfection. Live cell confocal imaging was carried out. Dense chromatin regions were detected with Hoechst (blue). The results are expressed as the fraction of colocalizing green and blue signals in individual cells (n = 15), as in panel B. **p < 0.00001 for differences between wt and each substituted protein.
(E) Cells were transfected with PRR14 siRNA and control siRNA for 72 hrs, followed by costaining with anti-H3K9me3 and Lamin A/C antibodies and confocal imaging. A binucleated cell (arrows) is indicated, corresponding to the PRR14 knockdown phenotype shown in Figure 1E. Detachment of H3K9me3 chromatin was quantitated as described in Supplemental Methods. Images of multiple cells were analyzed for each treatment (n = 25), and differences in H3K9me3-marked chromatin localization at the nuclear periphery between control siRNA and PRR14 siRNA-treated cells were highly significant **p < 0.00001. (See Figure S4 for additional representative images and image analysis).
(F) HeLa cells stably expressing full length N-terminal GFP-tagged PRR14 were treated with the indicated siRNAs targeting the nuclear lamins. After 72 hrs, cells were fixed, the nuclear lamina was stained using an antibody that detects both Lamins B1 and B2 (red), and confocal imaging was carried out. Release of PRR14 from the nuclear periphery after Lamin A/C knockdown was quantitated as described in panel B (n = 16 for each sample). Highly significant differences **p < 0.00001 between the control and Lamin A/C siRNA knockdown cells were observed, as presented graphically.
(G) Western blot analysis of siRNA knockdown efficiencies, and specificities, is shown. See Figure S2.
PRR14 Functions in Nuclear Lamina-Heterochromatin Tethering
Substitutions in the LAVVL HP1-binding motif had no effect on nuclear lamina localization of full length PRR14 in interphase cells (Figure S2B). These findings suggested that, in interphase, PRR14 is tightly bound to the nuclear lamina through the central domain, with the N-terminus providing an attachment site to tether heterochromatin. Consistent with this proposal, siRNA knockdown of PRR14 resulted in a partial loss of perinuclear H3K9me3 heterochromatin staining, and a concomitant shift towards a more diffuse nuclear-wide pattern (Figure 2E). Additional representative images, and image analysis, are provided in Figure S4.
Next, siRNA knockdown was used to identify the nuclear lamina components required for positioning and tethering function of PRR14 at the nuclear periphery. Knockdown of Lamin A/C, but not Lamins B1 and B2, resulted in redistribution of GFP-tagged PRR14 from the nuclear lamina region to the nucleoplasm without a significant change in overall GFP intensity (Figure 2F–G, Figure S2C). These findings suggested that PRR14 functions to tether HP1-marked heterochromatin directly, or indirectly, to Lamin A/C. As the known mechanisms of tethering heterochromatin to the nuclear periphery are largely redundant (see Discussion), we carried out single and pairwise siRNA-knockdown of the relevant tethering components Lamin A/C and LBR (Figure S2E). Knockdown of the LBR tethering protein had no significant effect on peripheral heterochromatin, while double knockdown of LBR and Lamin A/C resulted in significant disassociation of perinuclear H3K9me3 heterochromatin (Figure S2E). These results identify a Lamin A/C-dependent tethering function in HeLa cells. Furthermore, unlike knockdown of LBR, knockdown of Lamin A/C alone produced a mild, but statistically significant dissociation of peripheral H3K9me3 chromatin. Notably, PRR14 knockdown alone produced as dramatic effect as the LBR-Lamin A/C double knokdown (Figure S2E) (see Discussion).
Evidence that PRR14 Plays a Role in Heterochromatin Organization at Mitotic Exit
The proposed tethering function predicted that PRR14 would be highly regulated during mitosis. Live cell time-lapse, and fixed cell confocal imaging of HeLa cells demonstrated that PRR14 is released from the lamina in prophase, and remains highly dispersed in metaphase (Figure 3, Figure S3). Within minutes of the onset of anaphase, a dramatic association of PRR14 with chromatin was observed (Figure S3A, Supplemental Movie S1). This behavior was distinct from other nuclear lamina-associated factors that instead surround chromatin during late anaphase and telophase (Haraguchi et al., 2008; Olins et al., 2010; Tseng and Chen, 2011; Wandke and Kutay, 2013).
Figure 3. PRR14 Colocalizes with HP1 on Anaphase Chromatin.
(A) HeLa cells stably expressing full length N-terminal GFP-tagged PRR14 were isolated. Cells were fixed, stained with anti-GFP and anti-Lamin A/C antibodies, and imaged using confocal microscopy. Merge 1 excludes the DAPI signal. Images are representative of the highly uniform behavior of the GFP-tagged PRR14 protein.
(B) Cells were prepared similarly to Panel A, except that the anti-LBR staining was compared to GFP-tagged PRR14.
(C) (Left) HeLa cells stably expressing both full length N-terminal GFP-tagged PRR14 and mRFP-tagged HP1α were isolated. Live cell confocal imaging is shown. (Right) HeLa cells stably expressing PRR14 N-GFP 1–135 were transiently transfected with an expression plasmid encoding mRFP-HP1α, and live cell confocal imaging is shown. Arrows indicate lack of PRR14 1–135 and HP1α colocalization only at the spindle midzone and midbody. See Figure S3.
Fixed cells were used to compare directly the mitotic behavior of PRR14 with other nuclear lamina components. As shown in Figure 3A, PRR14 dissociated from the intact nuclear lamina in prophase and dispersed throughout the nucleus. After nuclear lamina disassembly in prometaphase, both PRR14 and Lamin A/C became largely diffuse and remained so in metaphase. Similar to what was seen in live cells (Figure S3A), PRR14 was observed to bind chromatin in anaphase (Figure 3A). Clearly, the PRR14 protein was present throughout the anaphase chromosome structures, as opposed to surrounding the chromatin. Lamina A/C remained distributed during anaphase, as expected. In early telophase, Lamin A/C reassembled around chromatin, while strikingly, PRR14 remained associated with chromatin. Finally, in late telophase, PRR14 and Lamin A/C colocalized entirely at the inner nuclear periphery, as in interphase cells. Thus, at the beginning of mitosis, PRR14 disassembles from the intact nuclear lamina, and after anaphase onset, it reassembles in two steps, first binding to chromatin, and then relocalizing to the nuclear lamina. This behavior was confirmed using an alternative tag, as well as a euploid human cell line, RPE1 (Figure S3B,C).
As introduced above, LBR is a mediator of heterochromatin-nuclear envelope tethering. We therefore compared the behaviors of LBR and PRR14 during mitosis (Figure 3B). PRR14 was detected as a component of anaphase and early telophase chromatin as described above. In contrast, LBR was concentrated with peripheral membranes in early anaphase, and assembled at the chromatin edges in telophase, as expected. Only in late telophase did LBR and PRR14 colocalize, at the nuclear lamina (Figure 3B). Bioinformatic analysis of PRR14 failed to detect membrane interaction domains, and in contrast to LBR, the behavior of PRR14 during mitosis indicates that it is largely soluble.
PRR14 Colocalizes with HP1 on Anaphase Chromatin
The PRR14 binding partner HP1 is known to be displaced from chromosome arms at the onset of mitosis (Dormann et al., 2006; Fischle et al., 2005; Hirota et al., 2005; Nozawa et al., 2010). The HP1 ligands, the H3K9me2/3 modifications, are stably retained through mitosis, providing a mechanism for subsequent rebinding of HP1 to chromosomes (Wang and Higgins, 2012). We therefore asked whether PRR14 might reassemble with HP1 on anaphase chromatin. The results were striking, as metaphase chromosome arms were largely devoid of both PRR14 and HP1α, while strict colocalization was seen on anaphase chromatin (Figure 3C). Furthermore, the PRR14 N-terminal domain, containing the LAVVL HP1-binding motif, also colocalized with HP1α on anaphase chromatin (Figure 3C). Some HP1α localized to the spindle midzone and midbody, as previously described (Kang et al., 2011; Schmiedeberg et al., 2004) (Figure 3C). PRR14 did not bind to this extrachromosomal form of HP1α, consistent with the fact that the predicted PRR14 binding surface on HP1, the chromoshadow domain (CSD) interface, is occupied in this HP1 binding mode (Kang et al., 2011).
The collective findings above indicated that PRR14 can associate with HP1 on both interphase heterochromatin (Figure 2B–D) and anaphase chromatin (Figure 3C). To confirm the latter finding, we examined the effects of the HP1-binding motif substitutions (V54E, V55E) on full length PRR14 during mitosis. These PRR14 substitutions caused a dramatic defect in PRR14 anaphase chromatin association, which was observed in essentially all mitotic cells within the culture (Figure 4). We conclude that PRR14 associates with anaphase chromatin through HP1. Interestingly, the substituted PRR14 protein remained excluded from chromatin during the early stages of telophase, which resulted in the partitioning of the protein outside of the newly formed nucleus (Figure 4A,B). This nuclear exclusion caused a delay in, but did not block, the relocalization of the substituted PRR14 to the nuclear lamina (Figure 4A,B). These results suggest that this chromatin-binding defective PRR14 protein enters the nucleus using an alternative NLS-based nuclear entry mechanism (Figure S2A,B).
Figure 4. The Association of PRR14 with Anaphase Chromatin Requires the PRR14 HP1- binding Motif.
(A) HeLa cells stably expressing the full length N-terminal GFP-tagged PRR14 containing V54E,V55E substitutions were isolated. Cells were fixed and stained with anti-Lamin A/C and anti-GFP, and confocal imaging of mitotic cells was carried out. Images are representative of the highly uniform behavior of the substituted GFP-tagged PRR14 protein.
(B) The relevant images from Panel A are enlarged and compared to wild type N-GFP PRR14.
(C) Live cell confocal image shows that PRR14 N-GFP V54E,V55E fails to associate with mRFP-HP1α in anaphase, as compared to the wild type N-GFP PRR14 shown in Figure 3C.
DISCUSSION
The role of PRR14 as a bivalent tether for attachment of HP1-H3K9me2/3-marked heterochromatin to the nuclear lamina was uncovered as follows. We initially found that full length PRR14 localized to the nuclear lamina in interphase cells (Figure 1A–C). However, independent expression of the PRR14 N-terminal fragment revealed heterochromatin binding activity, namely colocalization with HP1α and its ligand, H3K9me3 (Figure 2A–C). Substitutions in the candidate PRR14 HP1-binding motif (LAVVL), as well as knockdown of HP1, resulted in loss of heterochromatin localization of this fragment (Figures 2D, S2D). Furthermore, a recent independent study had identified PRR14 as one of many high affinity HP1α binding partners that interact through the CSD interface (Nozawa et al., 2010). Indeed, our molecular modeling has confirmed that the HP1α CSD interface can accommodate the PRR14 LAVVL motif (data not shown). Taken together, our findings indicate that the predicted ordered PRR14 N-terminus interacts directly with HP1. Based on bioinformatic analyses (Figure 2A), we speculate that PRR14 acts as a partially unstructured scaffold for other proteins at the inner nuclear periphery, and we note that human phosphatase PP2A has also been detected as a binding partner (Herzog et al., 2012).
The chromatin binding capability of full length PRR14 was revealed in live mitotic cells. After disassembly from the nuclear lamina and chromatin early in mitosis (Figure 3), PRR14 was found to rebind to chromatin within minutes after the onset of anaphase (Figure S3A, Supplemental Movie S1). This association of PRR14 with anaphase chromatin is through direct binding to HP1, based on a requirement for the PRR14 LAVVL HP1-binding motif (Figure 4) and colocalization with HP1α (Figure 3C, Figure 4C). Furthermore, the N-terminal fragment alone containing the LAVVL motif was found to be sufficient for anaphase chromatin binding (Figure 3C, right). We conclude that PRR14 interacts with chromatin through HP1, in both interphase and anaphase. Furthermore, the behavior of PRR14 during mitosis provides overall support for a bivalent tethering function, as PRR14 first binds to anaphase chromatin in an HP1- dependent manner, prior to its attachment to the nuclear lamina at mitotic exit.
The dynamics of PRR14 during mitosis revealed a two-step reassembly process: anaphase chromatin binding, followed by lamina association in telophase. The body of evidence above indicates that PRR14 is exclusively associated with HP1-marked chromatin in anaphase (Figures 3 and 4). PRR14 remains chromatin-associated at the time that the nuclear lamina begins to reform and only in mid- and late- telophase does PRR14 concentrate at the nuclear lamina (Figures 3A). One interpretation of these findings is that PRR14 binds HP1-marked chromatin as part of a nuclear entry mechanism, and is then released to allow it to traffic to, and reassemble, at the nuclear lamina. We can largely discount this possibility, as anaphase chromatin binding is not essential for PRR14 reassembly at the nuclear lamina (Figures 4). We propose a more interesting possibility, that PRR14 remains attached to HP1-H3K9me2/3-marked chromatin and then guides reassembly of this heterochromatin at the reforming nuclear lamina (Figure S5).
The dynamics of PRR14 during mitosis generally parallels the known behaviors of HP1, and thereby further supports a role for HP1 in the mitotic disassembly and reassembly of PRR14. We found that PRR14 is released from the nuclear lamina and chromatin during prophase (Figure 3A, Figure S3B,C). At this stage, HP1 is known to be ejected from H3K9me2/3 chromatin through phosphorylation of histone H3 serine 10 (H3S10P) by Aurora B kinase (Dormann et al., 2006; Fischle et al., 2005; Hirota et al., 2005). Consistent with HP1-dependent chromatin localization of PRR14, we have found that treatment with an Aurora B kinase inhibitor prevents release of both HP1α and PRR14 from chromatin (data not shown). In metaphase, HP1 is known to remain largely absent from chromatin, and become dispersed throughout the cell, and PRR14 was found to behave similarly (Figure 3C). In anaphase, both HP1α (Sugimoto et al., 2001) and PRR14 were observed to rebind to chromatin (Figure 3). HP1 rebinding is mediated by H3K9me2/3, which is retained through mitosis (Wang and Higgins, 2012). Thus, reassembly of PRR14 on anaphase chromatin is likely guided by H3K9me2/3 marks, through HP1.
Three types of adapter complexes have been described that mediate chromatin attachment at the nuclear periphery. All three complexes include an integral membrane protein. BAF is a wellstudied chromatin/DNA binding protein that associates largely with membranes through INM proteins (Brachner and Foisner, 2011; Wagner and Krohne, 2007). More recent studies have identified a mechanism though which the transcription factor cKrox/ThPOK participates in tethering of DNA sequences to membranes through another INM protein, Lap2β (Zullo et al., 2012). Lastly, the INM protein LBR attaches to LaminB1/2 and encodes several chromatin binding sequences, including an HP1-binding motif (Olins et al., 2010). As confirmed here (Figure 3B), LBR is largely associated with membranes until telophase, where it begins to surround chromatin as a driver of nuclear envelope reassembly (Olins et al., 2010). As a membrane-bound factor, one role of LBR is to recruit membranes to chromatin during nuclear envelope reassembly in telophase. In apparent contrast to LBR, cKrox/ThPOK, and BAF, PRR14 assembles into chromatin at the onset of anaphase, with no evidence of direct or indirect membrane association. The soluble nature of PRR14, and its partnering with HP1 are likely critical for its role in accessing chromatin in anaphase (Figure 3). Therefore, we propose that PRR14 provides unique functions in anaphase and telophase, as compared to the other tethering complexes described above.
The mechanisms by which heterochromatin is tethered to the nuclear periphery during interphase are largely redundant (Olins et al., 2010; Solovei et al., 2013), as confirmed here (Figure S2E). For example, although LBR is described as a Lamin B-dependent tethering protein, its depletion is not necessarily sufficient for heterochromatin release from the periphery. A recent study proposed a Lamin A-dependent tether that was revealed in the absence of LBR (Solovei et al., 2013). As PRR14 requires Lamin A/C for localization to the inner nuclear periphery (Figure 2F), it is a candidate Lamin A/C-dependent heterochromatin tethering protein. In a simple PRR14-Lamin A/C tethering model, it would be expected that Lamin A/C knockdown would phenocopy PRR14 knockdown, and that detection of tethering roles for either component would require co-depletion of LBR. This is not the case, as Lamin A/C knockdown has only a mild (but measurable) effect on perinuclear heterochromatin, while PRR14 knockdown has a much stronger effect, mimicking the double knockdown of Lamin A/C and LBR (Figure S2E). PRR14 thus remains a strong candidate for serving as a Lamin A/C heterochromatin tether, but clearly has additional nonredundant functions with respect to perinuclear heterochromatin organization. Our results implicate at least two such nonredundant roles for PRR14: first, in the specification of heterochromatin for lamina attachment at mitotic exit as described above (Figures 3,4) and second, a direct or indirect role in overall nuclear lamina structure (Figure 1D,E). With respect to the second point, PRR14 depletion could lead to widespread defects in heterochromatin attachment. Taken together our results indicate a fundamental role for PRR14 in lamina structure and heterochromatin attachment. Consistent with such a role, the PRR14 gene is conserved in mammals, including the early mammal, the duckbilled platypus. Furthermore, PRR14 mRNA is widely expressed in mammalian tissues, as well as in early mouse development (Guo et al., 2010; Tang et al., 2010).
In summary, we provide evidence that human PRR14 acts to specify HP1-marked heterochromatin for tethering to the nuclear lamina at mitotic exit, as well serving among the redundant tethers during interphase. We speculate that PRR14 thus has a unique and critical mitotic role in specifying heterochromatin destined for localization at the nuclear lamina. In this sense, PRR14 may act as part of the epigenetic machinery that ensures proper inheritance of heterochromatin positioning at the nuclear lamina as cells exit mitosis.
METHODS
Expression Vectors and Mutagenesis
Two PRR14 expression vectors were obtained from OriGene Technologies, Inc., encoding C-terminal fusions with TurboGFP (RG208696) or a Myc-DDK-tag (RC208696). The PRR14 orf was also transferred to an N-terminal mGFP vector from OriGene (PS100048). Deletion mutagenesis was carried out using restriction enzyme sites within the PRR14 orf. For all other constructs, site-specific mutagenesis was carried out using the Agilent QuikChange II XL Site-Directed Mutagenesis Kit (200521). The mRFP-HP1α expression plasmid was obtained from Martijn Luijsterburg (Luijsterburg et al., 2009).
Confocal Microscopy and Immunofluorescence
Cells were fixed using a 4% paraformaldehyde for 15 min at room temperature. Cells were then permeabilized with 0.25% Triton X-100 for 10 min (or with Digitonin f.c. 25 µg/ml, Figure S1A). A blocking step was performed with 1% BSA in PBS-Tween 20. Fixed cells were incubated with antibodies for 1 hr at room temperature. DNA was visualized with DAPI staining (D1306, Life Technologies 953577) in fixed cells, or Hoechst in live cells. Alexa Fluor 488 phalloidin (Life Technologies, 953561) was used for staining actin filaments. See Supplemental Methods for details of image acquisition and analysis.
Transfection
Lipofectamine 2000 (Invitrogen, 11668500) was used for transient and stable transfection of PRR14 and HP1α plasmids. For siRNA transfection, the DharmaFECT (Dharmacon) transfection reagents were used according to the manufacturer's protocol. Pools of 4 siRNAs or single siRNAs were used. The AllStar siControl non-targeting siRNA (Qiagen) was used as a negative control. Final concentration of siRNA in transfection mixes was 50nM. Cells were cultured with transfection mix for 48 hrs, and after additional 24 hrs, cells were processed for further analysis.
Supplementary Material
Highlights.
Human PRR14 was found to tether HP1-marked heterochromatin to the nuclear lamina.
PRR14 is required for maintenance of nuclear lamina structure.
At the onset of anaphase, PRR14 binds rapidly to HP1-marked chromatin.
PRR14 may specify HP1-marked chromatin-to-nuclear lamina reattachment in telophase.
ACKNOWLEDGEMENTS
This work was supported, in whole or in part, by National Institutes of Health Grants DK082498 (R.A.K.), CA71515, and CA06927. This project is also funded, in part, under a grant with the Pennsylvania Department of Health. The Department specifically disclaims responsibility for any analyses, interpretations or conclusions. This work was also supported by the Fox Chase Cancer Center Keystone Program in Epigenetics and Progenitor Cells. A.P. is a recipient of an AACR Centennial Predoctoral Fellowship in Cancer Research. We thank Tim Yen, Anna Marie Skalka, and Fabrice Roegiers for critical comments, and Marie Estes and Karen Trush for assistance in preparing this manuscript. We are particularly grateful to Anna Marie Skalka for providing continued support and encouragement. We also thank Kathy Wilson for helpful suggestions during the early stages of this work and Martijn Luijsterburg for providing the mRFP-HP1α expression plasmid. The following Fox Chase Cancer Center Facilities supported this work: Molecular Modeling, DNA Sequencing, Bioimaging, Translational, and Cell Sorting. Anna Pecherskaya provided critical assistance in image analysis. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute, or any other sponsoring organization.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Brachner A, Foisner R. Evolvement of LEM proteins as chromatin tethers at the nuclear periphery. Biochem. Soc. Trans. 2011;39:1735–1741. doi: 10.1042/BST20110724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butin-Israeli V, Adam SA, Goldman AE, Goldman RD. Nuclear lamin functions and disease. Trends Genet. 2012;28:464–471. doi: 10.1016/j.tig.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer TA, Misteli T. The lamin protein family. Genome Biol. 2011;12:222. doi: 10.1186/gb-2011-12-5-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormann HL, Tseng BS, Allis CD, Funabiki H, Fischle W. Dynamic regulation of effector protein binding to histone modifications: the biology of HP1 switching. Cell Cycle. 2006;5:2842–2851. doi: 10.4161/cc.5.24.3540. [DOI] [PubMed] [Google Scholar]
- Fischle W, Tseng BS, Dormann HL, Ueberheide BM, Garcia BA, Shabanowitz J, Hunt DF, Funabiki H, Allis CD. Regulation of HP1-chromatin binding by histone H3 methylation and phosphorylation. Nature. 2005;438:1116–1122. doi: 10.1038/nature04219. [DOI] [PubMed] [Google Scholar]
- Gerace L, Huber MD. Nuclear lamina at the crossroads of the cytoplasm and nucleus. J. Struct. Biol. 2012;177:24–31. doi: 10.1016/j.jsb.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon LB, Cao K, Collins FS. Progeria: translational insights from cell biology. J Cell Biol. 2012;199:9–13. doi: 10.1083/jcb.201207072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal SI, Jia S. Heterochromatin revisited. Nat. Rev. Genet. 2007;8:35–46. doi: 10.1038/nrg2008. [DOI] [PubMed] [Google Scholar]
- Guelen L, Pagie L, Brasset E, Meuleman W, Faza MB, Talhout W, Eussen BH, de Klein A, Wessels L, de Laat W, et al. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature. 2008;453:948–951. doi: 10.1038/nature06947. [DOI] [PubMed] [Google Scholar]
- Guo G, Huss M, Tong GQ, Wang C, Li Sun L, Clarke ND, Robson P. Resolution of cell fate decisions revealed by single-cell gene expression analysis from zygote to blastocyst. Dev. Cell. 2010;18:675–685. doi: 10.1016/j.devcel.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Haraguchi T, Kojidani T, Koujin T, Shimi T, Osakada H, Mori C, Yamamoto A, Hiraoka Y. Live cell imaging and electron microscopy reveal dynamic processes of BAF-directed nuclear envelope assembly. J. Cell Sci. 2008;121:2540–2554. doi: 10.1242/jcs.033597. [DOI] [PubMed] [Google Scholar]
- Herzog F, Kahraman A, Boehringer D, Mak R, Bracher A, Walzthoeni T, Leitner A, Beck M, Hartl FU, Ban N, et al. Structural probing of a protein phosphatase 2A network by chemical cross-linking and mass spectrometry. Science. 2012;337:1348–1352. doi: 10.1126/science.1221483. [DOI] [PubMed] [Google Scholar]
- Hirota T, Lipp JJ, Toh BH, Peters JM. Histone H3 serine 10 phosphorylation by Aurora B causes HP1 dissociation from heterochromatin. Nature. 2005;438:1176–1180. doi: 10.1038/nature04254. [DOI] [PubMed] [Google Scholar]
- Kang J, Chaudhary J, Dong H, Kim S, Brautigam CA, Yu H. Mitotic centromeric targeting of HP1 and its binding to Sgo1 are dispensable for sister-chromatid cohesion in human cells. Mol. Biol. Cell. 2011;22:1181–1190. doi: 10.1091/mbc.E11-01-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubben N, Voncken JW, Demmers J, Calis C, van Almen G, Pinto Y, Misteli T. Identification of differential protein interactors of lamin A and progerin. Nucleus. 2010;1:513–525. doi: 10.4161/nucl.1.6.13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner MS, Schultz DC, Negorev D, Maul GG, Rauscher FJ., 3rd The mammalian heterochromatin protein 1 binds diverse nuclear proteins through a common motif that targets the chromoshadow domain. Biochem. Biophys. Res. Commun. 2005;331:929–937. doi: 10.1016/j.bbrc.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Lee JS, Hale CM, Panorchan P, Khatau SB, George JP, Tseng Y, Stewart CL, Hodzic D, Wirtz D. Nuclear lamin A/C deficiency induces defects in cell mechanics, polarization, and migration. Biophys. J. 2007;93:2542–2552. doi: 10.1529/biophysj.106.102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijsterburg MS, Dinant C, Lans H, Stap J, Wiernasz E, Lagerwerf S, Warmerdam DO, Lindh M, Brink MC, Dobrucki JW, et al. Heterochromatin protein 1 is recruited to various types of DNA damage. J. Cell Biol. 2009;185:577–586. doi: 10.1083/jcb.200810035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makatsori D, Kourmouli N, Polioudaki H, Shultz LD, McLean K, Theodoropoulos PA, Singh PB, Georgatos SD. The inner nuclear membrane protein lamin B receptor forms distinct microdomains and links epigenetically marked chromatin to the nuclear envelope. J. Biol. Chem. 2004;279:25567–25573. doi: 10.1074/jbc.M313606200. [DOI] [PubMed] [Google Scholar]
- Mejat A, Misteli T. LINC complexes in health and disease. Nucleus. 2010;1:40–52. doi: 10.4161/nucl.1.1.10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa RS, Nagao K, Masuda HT, Iwasaki O, Hirota T, Nozaki N, Kimura H, Obuse C. Human POGZ modulates dissociation of HP1alpha from mitotic chromosome arms through Aurora B activation. Nat. Cell Biol. 2010;12:719–727. doi: 10.1038/ncb2075. [DOI] [PubMed] [Google Scholar]
- Olins AL, Rhodes G, Welch DB, Zwerger M, Olins DE. Lamin B receptor: multi-tasking at the nuclear envelope. Nucleus. 2010;1:53–70. doi: 10.4161/nucl.1.1.10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peric-Hupkes D, Meuleman W, Pagie L, Bruggeman SW, Solovei I, Brugman W, Graf S, Flicek P, Kerkhoven RM, van Lohuizen M, et al. Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol. Cell. 2010;38:603–613. doi: 10.1016/j.molcel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peric-Hupkes D, van Steensel B. Role of the nuclear lamina in genome organization and gene expression. Cold Spring Harb. Symp. Quant. Biol. 2010;75:517–524. doi: 10.1101/sqb.2010.75.014. [DOI] [PubMed] [Google Scholar]
- Poleshko A, Einarson MB, Shalginskikh N, Zhang R, Adams PD, Skalka AM, Katz RA. Identification of a functional network of human epigenetic silencing factors. J. Biol. Chem. 2010;285:422–433. doi: 10.1074/jbc.M109.064667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy KL, Zullo JM, Bertolino E, Singh H. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature. 2008;452:243–247. doi: 10.1038/nature06727. [DOI] [PubMed] [Google Scholar]
- Roux KJ, Kim DI, Raida M, Burke B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell Biol. 2012;196:801–810. doi: 10.1083/jcb.201112098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze M, Ayivi-Guedehoussou N, et al. Towards a proteome-scale map of the human protein-protein interaction network. Nature. 2005;437:1173–1178. doi: 10.1038/nature04209. [DOI] [PubMed] [Google Scholar]
- Schmiedeberg L, Weisshart K, Diekmann S, Meyer Zu Hoerste G, Hemmerich P. High- and low-mobility populations of HP1 in heterochromatin of mammalian cells. Mol. Biol. Cell. 2004;15:2819–2833. doi: 10.1091/mbc.E03-11-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevelyov YY, Nurminsky DI. The nuclear lamina as a gene-silencing hub. Curr. Issues Mol. Biol. 2012;14:27–38. [PubMed] [Google Scholar]
- Sigrist CJ, de Castro E, Cerutti L, Cuche BA, Hulo N, Bridge A, Bougueleret L, Xenarios I. New and continuing developments at PROSITE. Nucleic Acids Res. 2013;41:D344–D347. doi: 10.1093/nar/gks1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DN, Wilson KL. The nucleoskeleton as a genome-associated dynamic 'network of networks'. Nat. Rev. Mol. Cell Biol. 2011;12:695–708. doi: 10.1038/nrm3207. [DOI] [PubMed] [Google Scholar]
- Solovei I, Wang AS, Thanisch K, Schmidt CS, Krebs S, Zwerger M, Cohen TV, Devys D, Foisner R, Peichl L, et al. LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell. 2013;152:584–598. doi: 10.1016/j.cell.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Tasaka H, Dotsu M. Molecular behavior in living mitotic cells of human centromere heterochromatin protein HPLalpha ectopically expressed as a fusion to red fluorescent protein. Cell Struct. Funct. 2001;26:705–718. doi: 10.1247/csf.26.705. [DOI] [PubMed] [Google Scholar]
- Tang F, Barbacioru C, Bao S, Lee C, Nordman E, Wang X, Lao K, Surani MA. Tracing the derivation of embryonic stem cells from the inner cell mass by single-cell RNA-Seq analysis. Cell Stem Cell. 2010;6:468–478. doi: 10.1016/j.stem.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiru A, Nietlispach D, Mott HR, Okuwaki M, Lyon D, Nielsen PR, Hirshberg M, Verreault A, Murzina NV, Laue ED. Structural basis of HP1/PXVXL motif peptide interactions and HP1 localisation to heterochromatin. EMBO J. 2004;23:489–499. doi: 10.1038/sj.emboj.7600088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin BD, Gonzalez-Aguilera C, Sack R, Gaidatzis D, Kalck V, Meister P, Askjaer P, Gasser SM. Step-wise methylation of histone H3K9 positions heterochromatin at the nuclear periphery. Cell. 2012;150:934–947. doi: 10.1016/j.cell.2012.06.051. [DOI] [PubMed] [Google Scholar]
- Tseng LC, Chen RH. Temporal control of nuclear envelope assembly by phosphorylation of lamin B receptor. Mol. Biol. Cell. 2011;22:3306–3317. doi: 10.1091/mbc.E11-03-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner N, Krohne G. LEM-Domain proteins: new insights into lamin-interacting proteins. Int. Rev. Cytol. 2007;261:1–46. doi: 10.1016/S0074-7696(07)61001-8. [DOI] [PubMed] [Google Scholar]
- Wandke C, Kutay U. Enclosing chromatin: reassembly of the nucleus after open mitosis. Cell. 2013;152:1222–1225. doi: 10.1016/j.cell.2013.02.046. [DOI] [PubMed] [Google Scholar]
- Wang F, Higgins JM. Histone modifications and mitosis: countermarks, landmarks, and bookmarks. Trends Cell Biol. 2012 doi: 10.1016/j.tcb.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Worman HJ, Ostlund C, Wang Y. Diseases of the nuclear envelope. Cold Spring Harb. Perspect. Biol. . 2010;2:a000760. doi: 10.1101/cshperspect.a000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B, Dunbrack RL, Williams RW, Dunker AK, Uversky VN. PONDR-FIT: a meta-predictor of intrinsically disordered amino acids. Biochim. Biophys. Acta. 2010;1804:996–1010. doi: 10.1016/j.bbapap.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Callebaut I, Pezhman A, Courvalin JC, Worman HJ. Domain-specific interactions of human HP1-type chromodomain proteins and inner nuclear membrane protein LBRJ. Biol. Chem. 1997;272:14983–14989. doi: 10.1074/jbc.272.23.14983. [DOI] [PubMed] [Google Scholar]
- Zeng W, Ball AR, Jr, Yokomori K. HP1: heterochromatin binding proteins working the genome. Epigenetics. 2010;5:287–292. doi: 10.4161/epi.5.4.11683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou VW, Goren A, Bernstein BE. Charting histone modifications and the functional organization of mammalian genomes. Nat. Rev. Genet. 2011;12:7–18. doi: 10.1038/nrg2905. [DOI] [PubMed] [Google Scholar]
- Zullo JM, Demarco IA, Pique-Regi R, Gaffney DJ, Epstein CB, Spooner CJ, Luperchio TR, Bernstein BE, Pritchard JK, Reddy KL, et al. DNA sequence-dependent compartmentalization and silencing of chromatin at the nuclear lamina. Cell. 2012;149:1474–1487. doi: 10.1016/j.cell.2012.04.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.