Abstract
Purpose
In contrast to the classic form, variant hairy cell leukemia (HCLv) responds poorly to single-agent purine analogs, expresses unmutated BRAF, has shorter overall survival, and lacks effective standard therapy. No treatment has achieved a high complete remission rate even in small series, and of 39 reported cases from 6 studies, overall response rate after cladribine was 44% with 8% complete remissions. Rituximab has been found to increase the sensitivity of malignant cells to cladribine, suggesting that combination with cladribine might improve response in HCLv. To test this hypothesis, HCLv patients were treated with simultaneous cladribine and rituximab.
Experimental design
HCLv patients with 0-1 prior courses of cladribine received cladribine 0.15 mg/Kg days 1–5, with 8 weekly doses of rituximab 375 mg/m2 beginning day 1. Restaging was performed, and minimal residual disease (MRD) in blood and marrow was quantified using PCR, immunohistochemistry, and flow cytometry.
Results
By 6 months, 9 (90%) of 10 patients achieved complete remission (CR), compared to 3 (8%) of 39 reported cases treated with cladribine alone (p<0.0001). Of the 9 CRs, 8 remain free of MRD at 12–48 (median 27) months of follow-up. No dose-limiting toxicities were observed when beginning cladribine and rituximab on the same day, although most patients required short-term steroids to prevent and treat rituximab infusion reactions. Cytopenias in CRs resolved in 7–211 (median 34) days without major infections.
Conclusion
Although cladribine alone lacks effectiveness for early or relapsed HCLv, cladribine with immediate rituximab achieves CRs without MRD and is feasible to administer.
Keywords: Hairy cell leukemia, monoclonal antibody, CD20, CD22, minimal residual disease, chemoimmunotherapy
INTRODUCTION
Hairy cell leukemia (HCL), a B-cell malignancy comprising about 2% of leukemias, was noted in 1980 to contain a variant form comprising 10–20% of patients, called HCLv (1). The World Health Organization (WHO) now recognizes HCLv as an entity distinct from HCL within the category ‘splenic lymphoma/leukemia, unclassifiable’, resembling HCL immunophenotypically except lacking CD25, tartrate-resistant acid phosphatase (TRAP), and annexin A1 (2). Unlike classic HCL, which achieves high complete remission (CR) and overall response rates (ORR) with single-agent purine analogs cladribine or pentostatin (3–5), HCLv is primarily resistant. Among 39 patients with HCLv reported from six retrospective studies of 3–15 patients each (6–11), the CR rate with cladribine was only 8%, and ORR 44%. Response was similarly poor with pentostatin (6). BRAF inhibitors such as Vemurafenib may be useful in classic HCL (12), but patients with HCLv have wild-type BRAF (13, 14) and hence would not be expected to benefit. Median overall survival from diagnosis is only about 9 years for HCLv compared to over 25 years for classic HCL (6, 11). Thus, alternative treatment approaches are urgently needed for this disease.
In classic HCL, Ravandi et al. recently reported a 100% CR rate in 31 purine analog-naïve patients treated with cladribine followed by 8 weekly doses of rituximab begun 1 month after cladribine (15, 16). In addition, 5 with HCLv were treated, 2 of whom died of secondary malignancies and 1 relapsed at 6 months before dying of disease, but 2 remained in CR for 12 and 35 months (16). The status of minimal residual disease (MRD) in these 2 or the other 3 HCLv patients was not reported. Rituximab is reported to sensitize malignant B-cells to cladribine (17). To exploit such synergy in patients with HCLv, we prospectively treated patients with rituximab begun on the same day as cladribine. We report here clinical results in 10 consecutive patients with HCLv treated prospectively with cladribine and immediate rituximab.
METHODS
Treatment protocol
Patients with HCLv were enrolled on a trial (NCT00923013) comparing immediate with delayed rituximab after cladribine for previously untreated or once-relapsed HCL. Patients with HCLv constituted a separate non-randomized stratification receiving cladribine and immediate rituximab, with additional rituximab at least 6 months later if MRD is detected. Patients required therapy based on cytopenias, lymphocytosis, or symptomatic splenomegaly, and provided written informed consent approved by the NCI Institutional Review Board (IRB).
Administration
Cladribine was administered in 5 daily doses at 0.15 mg/Kg/day by 2-hour intravenous infusion. On day 1, 8 weekly doses of rituximab were begun at 375 mg/m2/dose. To prevent or treat rituximab infusion reactions, patients usually received diphenhydramine, acetaminophen, meperidine, and methylprednisolone.
Response assessment
Disease was assessed by bone marrow studies before and 1 and 6 months after cladribine, yearly still 2.5 years, and then every 2 years. Blood studies were done more frequently, and flow cytometry and PCR for immunoglobulin rearrangements were performed on blood and bone marrow specimens as described (18). CR required absence of malignant cells in the blood or bone marrow by non-immunologic stains and resolution of cytopenias and splenomegaly, as described (19). Normal blood counts required for CR included neutrophil count of 1500/mm3, platelets 100,000/mm3, and hemoglobin 11 g/dL. CR duration was defined from the date where all criteria of CR had been fulfilled, until the last assessment (either blood count or restaging) consistent with CR or the first assessment not consistent with CR. MRD in CR was defined as the presence of suspicious cells in the bone marrow biopsy by immunohistochemistry (IHC) as previously reported (20), or the presence of leukemic cells in the marrow aspirate or blood by flow cytometry or PCR. Statistical comparisons were performed using the Fisher’s exact test.
Immunologic studies
IHC was performed on bone marrow biopsy sections using antibodies to CD20, CD79a, CD3, TRAP, mutated BRAF, and Annexin A1. For flow cytometry on bone marrow or peripheral blood, panels included antibodies to B-cell markers CD19, CD20, and CD22, HCL-associated antigens CD11c, and CD103, HCL-associated and HCLv-non-associated antigens CD123 and CD25, and non-associated antigens CD5, CD10, and CD23, as described (21).
Molecular studies
Immunoglobulin gene rearrangement studies targeting both the heavy chain and the kappa light chain gene were performed as follows. For the immunoglobulin heavy chain (IGH) locus, two separate PCR reactions were performed, using consensus primers to the joining region and either framework region II or III (FRII-IGHJ and FRIII-IGHJ PCR), as described (22). For the kappa light chain, two additional PCR reactions were performed using the Biomed II primer set described by van Dongen et al. (23). The PCR products were analyzed on an ABI 3130xl Genetic Analyzer. To characterize IGH variable gene (IGHV) sequences, total RNA was prepared from PAXgene tubes (PreAnalytiX) and the resulting first-strand cDNA subjected to PCR using 6 IGHV FRI primers combined with 1 IGHJ consensus primer, as described (24).
RESULTS
Patient demographics and prior therapy
At the time of treatment, patient ages ranged from 43 to 80 years, median 65 (Table 1), and the male to female ratio was 6 to 4. Patients were enrolled 3 to 148 (median 15) months after diagnosis, representing a wide variation in both progression of HCLv and the prior therapies used. Prior therapy included none in 2 cases, cladribine alone in 5 cases, rituximab alone in 1 case, and cladribine followed by rituximab and splenectomy in 2 cases. The 2 patients receiving cladribine and rituximab received the rituximab either 5 (RG08) or 2 (RG10) months after cladribine, with RG08 receiving only part of 2 doses of rituximab due to infusion reactions, and RG10 receiving 4 doses of rituximab. For those 8 patients with prior therapy, best response to prior therapy was judged based on standard HCL response criteria (25, 26) using available retrospective blood counts and spleen measurements. Five of the patients were considered primarily refractory to cladribine with either no response or response lasting less than 1 year, and one patient progressed on initial rituximab. One patient had CR after cladribine lasting 16 months followed by partial response (PR) lasting 7 months, and another patient had PR lasting 37 months (Table 1). Blood counts and tumor assessments were taken at intervals often exceeding a year, thus overestimating true response times to prior therapy in some patients. Thus, prior to enrollment, demographics and response to 1st line purine analog were as expected for HCLv, with lower male-female ratio, higher age at diagnosis, and much lower durable response rate to single agent purine analog therapy as compared with classic HCL (27).
Table 1.
Clinical Characteristics of patients with hairy cell leukemia variant
| Patient No. | Age (yr)/Sex | Months After Diagnosis | Prior treatment (response) | Months after last therapy | HCL Count (Cells/mm3) | Spleen size (max) (mm) |
|---|---|---|---|---|---|---|
| RG01 | 43/M | 67 | Cladribine (CR x16 mo) | 54 | 29700 | 185 |
| RG02 | 62/M | 148 | Cladribine (PR x37 mo) | 49 | 4 | 218 |
| RG03 | 51/F | 16 | Cladribine (PR x11 mo) | 15 | 440 | 236 |
| RG04 | 75/M | 9 | Cladribine (PD) | 6 | 370 | 280 |
| RG05 | 54/F | 14 | Cladribine (PR x9 mo) | 12 | 4300 | 213 |
| RG06 | 59/F | 36 | None | - | 15300 | 174 |
| RG07 | 80/M | 3 | None | - | 3130 | 220 |
| RG08 | 70/M | 11 | Cladribine (PR x5mo), Rituximab (PD), Splenectomy (PD) | 1.5 | 15000 | 0* |
| RG09 | 68/M | 5 | Rituximab (PD) | 2 | 28000 | 370 |
| RG10 | 75/F | 50 | Cladribine (PR x2 mo), rituximab (SD), Splenectomy (PD) | 15 | 241000 | 0* |
Maximum (max) spleen height and weight 271 mm, 6 Kg (RG08), and 210 mm, 3.2 Kg (RG10), prior to splenectomy
Patient characteristics at the time of enrollment
Consistent with the relatively high lymphocyte count observed in HCLv, the circulating hairy cell leukemic count was less than 350 cells/mm3 in just 1 case, ranging from 4 to 241,000 with a median of 9700 cells/mm3. Spleen size for the 8 without prior splenectomy, represented by maximum diameter, was 174–370 (median 215) mm. As shown in Table 2, all 10 patients met immunophenotypic criteria for HCLv based on the 2008 WHO definition (2), including negativity for CD25, TRAP or annexin A1, and strong positivity for B-cell antigens (particularly CD20), and positivity for CD103 and CD11c. CD123 was negative as expected (18, 28–30) in all but 1 case. The positivity for CD103 in each case helped establish the diagnosis of HCLv in contradistinction to splenic marginal zone lymphoma (18, 28–31). Malignant cells were abundant enough in 9 of 10 cases to perform molecular characterization. Three of these 9 patients expressed the IGHV4-34 immunoglobulin rearrangement, all unmutated between 99.58 and 100% homologous to germline. Three other patients expressed unmutated rearrangements, IGHV1-69, IGHV3-30, and VH4-4 with 99.17–100% homology to germline. The IGHV3-30 was an unproductive rearrangement containing a stop codon and out-of-frame junction in the third complementarity determining region. All 10 patients expressed wild-type BRAF, as expected for HCLv (13, 14, 32). As shown in Table I, patient RG02 was unusual in that enrollment was 148 months after diagnosis and 49 months after his first and only therapy. Nevertheless, his initial presentation was consistent clinically with that of HCLv with an absolute lymphocyte count of 22,900 cells/mm3. His white blood cell count remained in the 20–50,000 cell/mm3 range during the years that he postponed therapy, until he presented with massive splenomegaly and renal failure. He had a PR to single-agent cladribine but relapsed with progressive splenomegaly and transfusion dependence 37 months later (Table 1). Bone marrow samples were examined prior to initial cladribine, and the leukemic infiltrate was negative as expected for annexin A1 and mutated BRAF, confirming the original diagnosis as HCLv. Thus, while HCLv can be difficult to differentiate from HCL and other splenic B-cell malignancies, including splenic marginal zone lymphoma, the presence of B-cell markers, CD103, CD11c, and absence of CD25, TRAP, BRAF V600E and annexin A1 in all cases firmly established the diagnosis of HCLv in these patients (2, 29, 31).
Table 2.
Pretreatment Phenotypic and Molecular characteristics of patients
| Patient No. | CD25 | CD123 | CD103 | CD11c | CD20 sites/cell | IGHV | %homology | BRAF | Annexin A1 |
|---|---|---|---|---|---|---|---|---|---|
| RG01 | − | − | + | ++ | 256,000 | 6-1 | 87.65% | Wild type | − |
| RG02 | − | − | + | + | 98,000 | Not done | Not done | Wild type | − |
| RG03 | − | − | + | ++ | 195,000 | 3-9 | 94.45% | Wild type | − |
| RG04 | − | − | + | ++ | 124,000 | 4-34 | 99.58% | Wild type | − |
| RG05 | − | − | + | ++ | 127,000 | 4-34 | 100% | Wild type | − |
| RG06 | − | − | + | ++ | 133,000 | 4-34 | 100% | Wild type | − |
| RG07 | − | − | + | ++ | 185,000 | 3-30 | 100% | Wild type | − |
| RG08 | − | + | + | + | 192,000 | 1-69 | 99.17% | Wild type | − |
| RG09 | − | − | + | + | 189,000 | 4-4 | 99.17% | Wild type | − |
| RG10 | − | − | + | ++ | 227,000 | 1-46 | 97.13% | Wild type | − |
Response
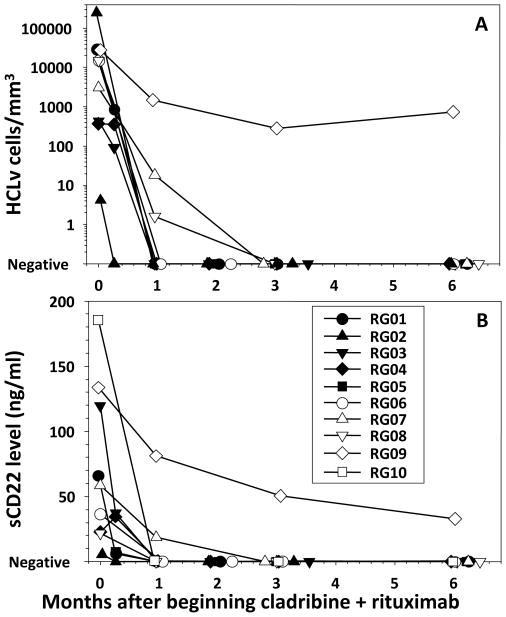
To determine response to cladribine with immediate rituximab, patients were evaluated before and at several time points after beginning 5 daily doses of cladribine and 8 weekly doses of rituximab, the rituximab starting the first day. Of the 10 patients, 9 (90%) achieved CR by 6 months after beginning cladribine, defined as absence of leukemic cells in the blood and bone marrow by non-immunologic stains, resolution of cytopenias, and resolution of splenomegaly. Bone marrow biopsies and aspirates were performed at 1 and 6 months after beginning cladribine, and were negative by morphology for HCL at the 1 month time point in all patients except RG07 and RG09, and RG07 was negative by 6 months. Besides RG08 and RG10, who had prior splenectomy, and RG09, who did not respond, splenomegaly decreased in the other 7 patients by 1 month and spleens were nonpalpable by 6 months. Of the 9 CRs, the median time to resolution of cytopenias and lymphocytosis to the level required for CR was 34 days, with RG07 requiring 211 days and the other 8 patients requiring 7–91 days. As shown in Figure 1A, circulating leukemia cells quantified by flow cytometry decreased rapidly, remaining detectable in only 3 of 10 patients by 1 month and in 1 patient by 3 months. Due to lack of CD25 expression in these HCLv patients, soluble CD25, as expected, was not helpful as a tumor marker (data not shown). Therefore, soluble CD22, previously reported to correlate with HCL tumor burden and response (33), was followed instead. As shown in Figure 1B, decreases in soluble CD22 paralleled those in circulating HCLv cells (Figure 1A), with only 2 patients remaining elevated at 1 month and 1 at 2 or 3 months. One of the patients (RG04) had a slight decrease in circulating hairy cells and a slight increase in soluble CD22 during the first week of treatment, but by 1 month both parameters were undetectable. Patient RG09 had a slight increase in circulating HCLv cells but continued decrease in sCD22, consistent with significant response in the spleen, the major source of HCLv generating sCD22, despite beginning to relapse in the peripheral blood. Thus 9 (90%) of 10 patients achieved CR with cladribine and immediate rituximab, compared to only 8% of 39 historical HCLv patients reported from 6 retrospective studies using cladribine alone (6–11) (p<0.0001). These retrospective reports of cladribine alone in HCLv included 4 PRs out of 8 (6), 2 PRs out of 6 (34), 1 CR and 2 PRs out of 4 (8), 1 PR out of 3 (9), 1 CR and 2 PRs out of 3 (10), and 1 CR and 3 PRs out of 15 (11) patients. In the present study with Cladribine combined with immediate rituximab, all 9 CRs maintained evidence of CR by restaging studies at 12–48 (median 30) months of follow-up.
Figure 1. Time course of response after cladribine plus rituximab.
For each of the indicated patients, tumor burden is quantified either by flow cytometry (A) or by soluble CD22 level (B).
Minimal residual disease (MRD)
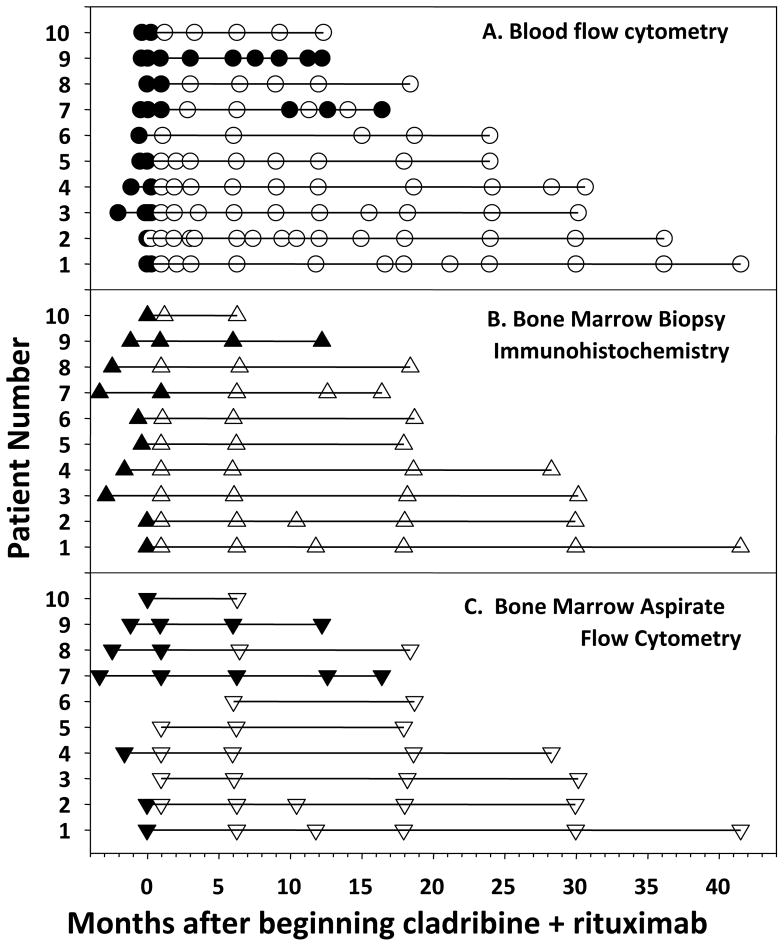
Since HCL patients achieving CR with cladribine commonly have leukemic cells detectable in the marrow presenting a risk factor for early relapse (20, 35–37), MRD after cladribine and rituximab was followed in the HCLv patients. Flow cytometry of the blood became negative in 9 (90%) of 10 patients by 3 months (Figures 1A, 2A). Bone marrow IHC became negative in 8 (80%) of 10 patients at 1 month, and in 9 (90%) of 10 patients by 6 months (Figure 2B). Of 8 patients with sufficient follow-up, all were still negative at 16–18 months and negativity at 28–30 months was documented in 4 patients. Finally, as shown in Figure 2C, bone marrow aspirate flow cytometry became negative in 4 (57%) of 7 evaluable (aspirable) patients by 1 month. By 6 months, all marrows were aspirable and 8 (80%) of 10 patients were negative by bone marrow aspirate flow cytometry. RG07 remained positive (0.1% of the marrow cells) at 6 months but was negative by flow cytometry of blood and IHC of the bone marrow biopsy. At 9 months, this patient had detectable leukemic cells by flow cytometry of blood, still met criteria for CR, and per protocol began a 2nd 8-week course of rituximab, resulting in continued CR with negative marrow IHC (Figure 2B). Thus 9 (90%) of the patients achieved a negative bone marrow biopsy by IHC. Although consensus PCR was previously reported to be less sensitive than flow cytometry (21), in the 10 HCLv patients, PCR and flow cytometry agreed (data not shown) when comparing tests on the same patient, time point, and tissue (bone marrow aspirate or blood). Thus cladribine with early rituximab achieved CRs in 9 (90%) of the 10 HCLv patients, and was associated in 8 (89%) of 9 CRs with absence of MRD. As shown in Figure 2, 8 remain MRD-free at 12–48 (median 27) months of follow-up.
Figure 2. Minimal residual disease before and after cladribine plus rituximab.
HCLv patients, identified as in Fig. 1, were tested for flow cytometry of blood (A), immunohistochemistry of bone marrow biopsy (B) and flow cytometry of bone marrow aspirate (C), with positive results indicated by solid and negative results by open markers.
Toxicity of rituximab immediately following cladribine
Cladribine followed immediately by rituximab was tolerated well, with the first 2 patients reporting minimal infusional side effects without steroid prophylaxis. Due to infusional side effects observed with the 3rd patient, prophylactic methylprednisolone was instituted for the last 5 patients to prevent infusion reactions. Maximum toxicity grades in each of the patients for each toxicity experienced are listed in Table 3. Based on NCI Common Toxicity Criteria Version 3.0, the adverse events occurring in over 30% of patients were lymphopenia (100%), leukopenia (70%), neutropenia, chills, hypoalbuminemia, and nausea (60% each), thrombocytopenia and aspartate aminotransferase (50% each), and arthralgia and cough (40% each). The grade 3–4 events seen in >1 patient included lymphopenia (70%), neutropenia (40%), thrombocytopenia (30%), and leukopenia (20%). Patient RG10 had the highest circulating count prior to enrollment (241,000/mm3) and had transient laboratory evidence of mild tumor lysis syndrome including potassium 5.3 mmol/L, uric acid 14.1 mg/dL, and phosphorus 7.2 mg/dL, but without associated clinical symptoms or evidence of renal insufficiency. Lymphopenia and leukopenia reflected decreases in both normal and malignant cells and in most cases did not represent true adverse events. Only 1 patient had febrile neutropenia, which was limited to a temperature of 38.3°C, neutrophil count of 0.4, and resolution on i.v. antibiotics without evidence of serious infection. Thus cladribine followed immediately by cladribine had an acceptable safety profile, and the lack of infections despite combination therapy may be related to the lower rate of cytopenias in HCLv as compared with classic HCL (6).
Table 3.
Maximum toxicity grade for each patient
| Toxicity | RG01 | RG02 | RG03 | RG04 | RG05 | RG06 | RG07 | RG08 | RG09 | RG10 | %Grd 1-4 | %Grd 3-4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lymphopenia | 1 | 4 | 3 | 3 | 4 | 3 | 3 | 3 | 1 | 2 | 100% | 70% |
| Leukopenia | 2 | 2 | 4 | 2 | 2 | 2 | 3 | 70% | 20% | |||
| Neutropenia | 3 | 4 | 3 | 2 | 2 | 4 | 60% | 40% | ||||
| Chills | 2 | 1 | 2 | 1 | 2 | 2 | 60% | 0% | ||||
| Hypoalbuminemia | 1 | 1 | 1 | 1 | 1 | 2 | 60% | 0% | ||||
| Nausea | 1 | 1 | 1 | 1 | 1 | 1 | 60% | 0% | ||||
| Platelets | 3 | 2 | 3 | 2 | 4 | 50% | 30% | |||||
| SGOT (AST) | 1 | 2 | 1 | 2 | 2 | 50% | 0% | |||||
| Arthralgia | 1 | 1 | 1 | 1 | 40% | 0% | ||||||
| Cough | 1 | 1 | 1 | 1 | 40% | 0% | ||||||
| Fatigue | 2 | 1 | 2 | 30% | 0% | |||||||
| Rash | 1 | 1 | 2 | 30% | 0% | |||||||
| GGT | 1 | 1 | 1 | 30% | 0% | |||||||
| Rhinitis | 1 | 1 | 1 | 30% | 0% | |||||||
| Vomiting | 1 | 1 | 1 | 30% | 0% | |||||||
| Hyperuricemia | 1 | 4 | 20% | 10% | ||||||||
| Myalgia | 2 | 2 | 20% | 0% | ||||||||
| Fever | 2 | 2 | 20% | 0% | ||||||||
| Bronchospasm | 2 | 2 | 20% | 0% | ||||||||
| Flushing | 2 | 1 | 20% | 0% | ||||||||
| Dyspnea | 1 | 2 | 20% | 0% | ||||||||
| SGPT (ALT) | 1 | 1 | 20% | 0% | ||||||||
| Dizziness | 1 | 1 | 20% | 0% | ||||||||
| Hematuria | 1 | 1 | 20% | 0% | ||||||||
| Hypotension | 1 | 1 | 20% | 0% | ||||||||
| Febrile neutropenia | 3 | 10% | 10% | |||||||||
| Haptoglobin | 3 | 10% | 10% | |||||||||
| Anemia | 3 | 10% | 10% | |||||||||
| Hypophosphatemia | 3 | 10% | 10% | |||||||||
| Tumor lysis | 3 | 10% | 10% | |||||||||
| Bilirubin | 2 | 10% | 0% | |||||||||
| Bone pain | 2 | 10% | 0% | |||||||||
| Flu syndrome | 2 | 10% | 0% | |||||||||
| Sweating | 2 | 10% | 0% | |||||||||
| Back pain | 2 | 10% | 0% | |||||||||
| Alk phos | 1 | 10% | 0% | |||||||||
| Headache | 1 | 10% | 0% | |||||||||
| Pruritus | 1 | 10% | 0% | |||||||||
| Throat Pain | 1 | 10% | 0% | |||||||||
| Diarrhea | 1 | 10% | 0% | |||||||||
| Oral mucositis | 1 | 10% | 0% | |||||||||
| Systolic hypertension | 1 | 10% | 0% |
Thrombocytopenia immediately following cladribine and rituximab
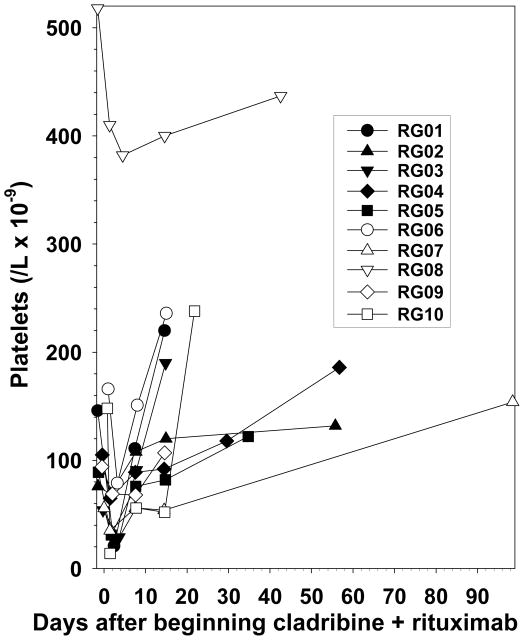
When beginning rituximab the same day as cladribine, a previously unreported transient decrease in platelets was observed, shown in Figure 3, which was noted immediately after beginning cladribine and rituximab. Platelets decreased by 7–136 (median 49) cells/L x10−9 to platelet nadirs of 14–382 (median 50) cells/L x10−9 with a percentage decrease of 9–91 (median 42%), and resolved more rapidly than expected of chemotherapy-induced marrow toxicity. In 50% of cases, the platelet count nadir was not worse than the baseline grade. Patient RG08 was one of 2 asplenic patients and as expected had a higher platelet count than other patients prior to treatment. Thus cladribine and immediate rituximab had an acceptable safety profile and the rapid thrombocytopenia observed was not significant enough to lead to bleeding or platelet transfusions.
Figure 3. Thrombocytopenia after cladribine plus rituximab.
Patients identified as in Fig 1. The beginning of treatment is considered day 1.
DISCUSSION
Our goal was to determine whether cladribine followed by rituximab would be effective for treating HCLv patients who were either newly diagnosed or had 1 prior course of cladribine. Our approach was to use the regimen of rituximab following cladribine, with which Ravandi et al., using rituximab delayed by 1 month, reported excellent results in early classic HCL (15, 16), but durable (greater than 1 year) CR in only 2 of 5 with HCLv (16). Due to the aggressiveness of HCLv, and preclinical data demonstrating the ability of rituximab to sensitize leukemia and lymphoma cells to cladribine (17), we began rituximab the same day as cladribine, rather than wait 1 month (16). A regimen almost identical to this, where the 1st dose of rituximab began the day before cladribine, was reported by Forconi et al. to achieve an MRD-free CR in a patient with multiply relapsed classic HCL (38). We found that of 10 HCLv patients enrolled and staged 6 months after treatment, 9 (90%) had CR, and 8 (80%) had CR without MRD. Tumor burden responded rapidly to combination treatment and no patient relapsed from CR at 12–48 (median 30) months of follow-up.
Up until now, a consistently effective treatment strategy for HCLv is unknown. Cladribine alone achieves CR in less than 10% and overall response in less than 50% (6–11), and CRs to rituximab in HCLv have been reported only anecdotally (39–41). The 90% CR rate in the present series of 10 HCLv patients is clearly superior to 3 out of 39 historical patients (6–11) completely responding to cladribine alone (p<0.0001). In fact, the 90% ORR is also significantly better than the 44% ORR in the historical group (p=0.012). However, it is not possible to determine if rituximab is significantly more effective if begun immediately with cladribine or 1 month later, since Ravandi et al. reported that 2 of the 5 HCLv patients treated died of secondary malignancies and only 1 died of rapidly recurrent HCLv (16). Theoretically, immediate rituximab might be advantageous for enhancing synergy between rituximab and cladribine, and 1-month delayed rituximab might be advantageous for avoiding infusion reactions in high-risk, medically fragile patients. Regardless, we believe HCLv should be treated with combined therapy with purine analog (cladribine or pentostatin) and monoclonal antibody therapy, such as rituximab.
In the classic or typical form of HCL, CR, overall response, and progression-free survival are excellent with purine analog alone, so that any incremental improvement achieved by addition of rituximab would require very long follow-up to assess its benefit. Nevertheless, recent data reporting median relapse-free survivals of 16, 11, and 6.5 years after 1st, 2nd and 3rd line purine analog, respectively (5), argue that even classic HCL patients will likely become treatment-refractory if diagnosed at young age and if treatment is restricted to purine analog as single-agent. To examine the benefit of rituximab in newly diagnosed or once-relapsed classic HCL, using MRD 6 months after cladribine as a surrogate endpoint, a randomized trial is underway in which classic HCL patients receive cladribine with rituximab begun either immediately or delayed at least 6 months. In patients with classic HCL, unlike those with the more rapidly-progressive HCLv, delayed rituximab may be as effective as immediate rituximab in eliminating MRD and preventing or at least delaying relapse.
It has been reported that HCLv is often difficult to differentiate from splenic marginal zone lymphoma (SMZL), each having similar B-cell antigens and HCL scores (42, 43), but this differentiation is important since SMZL is more indolent than HCLv and responds better to treatment (44). It is unlikely that our patients had SMZL rather than HCLv not only based on their morphology, but also their expression of CD103 in all 10 cases, an antigen expressed in <10% of cases of SMZL (28), including none of 43 patients from 3 studies (29, 45, 46). In our 2 patients who had splenectomy, splenic pathology was consistent with HCLv in both cases. Thus while the ability to differentiate SMZL from HCLv is not 100% reliable, it is unlikely that our efficacy data with cladribine and rituximab are significantly affected by the sensitivity of SMZL to this regimen.
While classic HCL is associated with the BRAF V600E mutation in a high percentage of cases (14, 32), HCLv, as well as IGHV4-34-expressing classic HCL cells, lack this mutation (13). IGHV4-34-expressing HCL patients have been shown to have a poor prognosis with single-agent cladribine as initial therapy, and poor overall survival, whether they are classified as classic HCL or HCLv immunophenotypically (11). These patients, like the others in this study, would not be appropriate for therapy with BRAF inhibition (12). It is notable that of the 3 patients in this study expressing IGHV4-34 (Table 2), all achieved CR with no MRD (Figure 2). Two of our patients (RG07 and RG08) were noted to have a 17p13.1 abnormality resulting in p53 deletion, which as previously noted is also a poor-prognostic factor (44, 47). Although RG07 was the one patient with MRD detectable in CR, RG08 achieved elimination of MRD by the cladribine-rituximab combination. Patient RG09, the only one without response, had normal p53 but had loss of 14q32, expressing IgH, by fluorescent in situ hybridization (FISH). It will be important to determine whether the addition of rituximab or possibly other antibody-based therapies to purine analog will improve the outcome of both poor-risk and good-risk HCL.
Statement of Translational Relevance.
While great progress had been achieved in treating classic hairy cell leukemia (HCL), with purine analogs, rituximab, immunotoxins, and BRAF inhibition, progress has been less for the more aggressive variant, HCLv, which lacks mutated BRAF, and no series has reported a high complete remission (CR) rate to any regimen. Based on the prior observation that rituximab increases malignant cell sensitivity to purine analog, we used cladribine with simultaneous rituximab in either newly diagnosed or relapsed HCLv. We observed a complete remission rate in 90% of 10 patients and in 8 of 9 CRs, minimal residual disease became undetectable, without relapse after a median follow-up of 27 months. Soluble CD22 levels also resolved, evidence against persistent splenic disease. These results suggest that patients with HCLv should receive monoclonal antibody therapy in addition to purine analog. Further follow-up will be needed to determine if this strategy can eradicate the malignant clone.
Acknowledgments
We thank our clinical staff Rita Mincemoyer, Elizabeth Maestri, Natasha Kormanik, Sonya Duke, and Barbara Debrah, and all other medical staff who helped care for the patients. We also thank Dr. Constance Yuan in the Laboratory of Pathology and Drs. Raul Braylan, Irina Maric and Roger Kurlander in the Department of Laboratory Medicine for sample analysis. This work was supported by the Intramural Research Program, NCI, NIH and the Hairy Cell Leukemia Foundation. The rituximab for the trial was sponsored by Genentech.
Conflicts of Interests. Genentech supplied the rituximab used in this study, and is currently arranging partial financial support of lab research. All of the authors were employed by the National Institutes of Health (NIH) at the time of the study.
References
- 1.Cawley JC, Burns GF, Hayhoe FG. A chronic lymphoproliferative disorder with distinctive features: a distinct variant of hairy-cell leukaemia. Leuk Res. 1980;4:547–59. doi: 10.1016/0145-2126(80)90066-1. [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4. Vol. 2. World Health Organization; 2008. [Google Scholar]
- 3.Grever M, Kopecky K, Foucar MK, Head D, Bennett JM, Hutchison RE, et al. Randomized comparison of pentostatin versus interferon alfa-2a in previously untreated patients with hairy cell leukemia: an intergroup study. J Clin Oncol. 1995;13:974–82. doi: 10.1200/JCO.1995.13.4.974. [DOI] [PubMed] [Google Scholar]
- 4.Saven A, Burian C, Koziol JA, Piro LD. Long-term follow-up of patients with hairy cell leukemia after cladribine treatment. Blood. 1998;92:1918–26. [PubMed] [Google Scholar]
- 5.Else M, Dearden CE, Matutes E, Garcia-Talavera J, Rohatiner AZ, Johnson SA, et al. Long-term follow-up of 233 patients with hairy cell leukaemia, treated initially with pentostatin or cladribine, at a median of 16 years from diagnosis. Br J Haematol. 2009;145:733–40. doi: 10.1111/j.1365-2141.2009.07668.x. [DOI] [PubMed] [Google Scholar]
- 6.Matutes E, Wotherspoon A, Brito-Babapulle V, Catovsky D. The natural history and clinico-pathological features of the variant form of hairy cell leukemia. Leukemia. 2001;15:184–6. doi: 10.1038/sj.leu.2401999. [DOI] [PubMed] [Google Scholar]
- 7.Robak T, Blasinska-Morawiec M, Blonski J, Hellmann A, Halaburda K, Konopka L, et al. 2-chlorodeoxyadenosine (cladribine) in the treatment of hairy cell leukemia and hairy cell leukemia variant: 7-year experience in Poland. Eur J Haematol. 1999;62:49–56. doi: 10.1111/j.1600-0609.1999.tb01114.x. [DOI] [PubMed] [Google Scholar]
- 8.Tetreault SA, Robbins BA, Saven A. Treatment of hairy cell leukemia-variant with cladribine. Leuk Lymphoma. 1999;35:347–54. doi: 10.3109/10428199909145739. [DOI] [PubMed] [Google Scholar]
- 9.Machii T, Chou T, Suzuki M, Ohe Y, Katagiri S, Kitano EK, et al. Phase II clinical study of cladribine in the treatment of hairy cell leukemia. Int J Hematol. 2005;82:230–5. doi: 10.1532/IJH97.04128. [DOI] [PubMed] [Google Scholar]
- 10.Palomera L, Domingo JM, Sola C, Azaceta G, Calvo MT, Gutierrez M. Cladribine (2-chlorodeoxyadenosine) therapy in hairy cell leukemia variant. A report of three cases. Haematologica. 2002;87:107–8. [PubMed] [Google Scholar]
- 11.Arons E, Suntum T, Stetler-Stevenson M, Kreitman RJ. VH4-34+ hairy cell leukemia, a new variant with poor prognosis despite standard therapy. Blood. 2009;114:4687–95. doi: 10.1182/blood-2009-01-201731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dietrich S, Glimm H, Andrulis M, von Kalle C, Ho AD, Zenz T. BRAF inhibition in refractory hairy-cell leukemia. N Engl J Med. 2012;366:2038–40. doi: 10.1056/NEJMc1202124. [DOI] [PubMed] [Google Scholar]
- 13.Xi L, Arons E, Navarro W, Calvo KR, Stetler-Stevenson M, Raffeld M, et al. Both variant and IGHV4-34-expressing hairy cell leukemia lack the BRAF V600E mutation. Blood. 2012;119:3330–2. doi: 10.1182/blood-2011-09-379339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiacci E, Schiavoni G, Forconi F, Santi A, Trentin L, Ambrosetti A, et al. Simple genetic diagnosis of hairy cell leukemia by sensitive detection of the BRAF-V600E mutation. Blood. 2012;119:192–5. doi: 10.1182/blood-2011-08-371179. [DOI] [PubMed] [Google Scholar]
- 15.Ravandi F, Jorgensen JL, O’Brien SM, Verstovsek S, Koller CA, Faderl S, et al. Eradication of minimal residual disease in hairy cell leukemia. Blood. 2006;107:4658–62. doi: 10.1182/blood-2005-11-4590. [DOI] [PubMed] [Google Scholar]
- 16.Ravandi F, O’Brien S, Jorgensen J, Pierce S, Faderl S, Ferrajoli A, et al. Phase 2 study of cladribine followed by rituximab in patients with hairy cell leukemia. Blood. 2011;118:3818–23. doi: 10.1182/blood-2011-04-351502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chow KU, Sommerlad WD, Boehrer S, Schneider B, Seipelt G, Rummel MJ, et al. Anti-CD20 antibody (IDEC-C2B8, rituximab) enhances efficacy of cytotoxic drugs on neoplastic lymphocytes in vitro: role of cytokines, complement, and caspases. Haematologica. 2002;87:33–43. [PubMed] [Google Scholar]
- 18.Venkataraman G, Aguhar C, Kreitman RJ, Yuan CM, Stetler-Stevenson M. Characteristic CD103 and CD123 Expression Pattern Defines Hairy Cell Leukemia: Usefulness of CD123 and CD103 in the Diagnosis of Mature B-Cell Lymphoproliferative Disorders. Am J Clin Pathol. 2011;136:625–30. doi: 10.1309/AJCPKUM9J4IXCWEU. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreitman RJ, Stetler-Stevenson M, Margulies I, Noel P, FitzGerald DJP, Wilson WH, et al. Phase II trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with hairy cell leukemia. J Clin Oncol. 2009;27:2983–90. doi: 10.1200/JCO.2008.20.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tallman MS, Hakimian D, Kopecky KJ, Wheaton S, Wollins E, Foucar K, et al. Minimal residual disease in patients with hairy cell leukemia in complete remission treated with 2-chlorodeoxyadenosine or 2- deoxycoformycin and prediction of early relapse. Clin Cancer Res. 1999;5:1665–70. [PubMed] [Google Scholar]
- 21.Sausville JE, Salloum R, Sorbara L, Kingma DW, Raffeld M, Kreitman RJ, et al. Minimal residual disease detection in hairy cell leukemia. Comparison of flow cytometric immunophenotyping with clonal analysis using consensus primer polymerase chain reaction for the heavy chain gene. Am J Clin Pathol. 2003;119:213–7. doi: 10.1309/G629-9513-NGLC-UB1K. [DOI] [PubMed] [Google Scholar]
- 22.Ramasamy I, Brisco M, Morley A. Improved PCR method for detecting monoclonal immunoglobulin heavy chain rearrangement in B cell neoplasms. J Clin Pathol. 1992;45:770–5. doi: 10.1136/jcp.45.9.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Dongen JJ, Langerak AW, Bruggemann M, Evans PA, Hummel M, Lavender FL, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17:2257–317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 24.Arons E, Roth L, Sapolsky J, Suntum T, Stetler-Stevenson M, Kreitman RJ. Evidence of Canonical Somatic Hypermutation in Hairy Cell Leukemia. Blood. 2011;117:4844–51. doi: 10.1182/blood-2010-11-316737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kreitman RJ, Tallman MS, Robak T, Coutre S, Wilson WH, Stetler-Stevenson M, et al. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol. 2012;30:1822–8. doi: 10.1200/JCO.2011.38.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nieva J, Bethel K, Saven A. Phase 2 study of rituximab in the treatment of cladribine-failed patients with hairy cell leukemia. Blood. 2003;102:810–3. doi: 10.1182/blood-2003-01-0014. [DOI] [PubMed] [Google Scholar]
- 27.Robak T. Hairy-cell leukemia variant: recent view on diagnosis, biology and treatment. Cancer Treat Rev. 2011;37:3–10. doi: 10.1016/j.ctrv.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Matutes E, Oscier D, Montalban C, Berger F, CalletBauchu E, Dogan A, et al. Splenic marginal zone lymphoma proposals for a revision of diagnostic, staging and therapeutic criteria. Leukemia. 2008;22:487–95. doi: 10.1038/sj.leu.2405068. [DOI] [PubMed] [Google Scholar]
- 29.Shao H, Calvo KR, Grönborg M, Tembhare PR, Kreitman RJ, Stetler-Stevenson M, et al. Distinguishing Hairy Cell Leukemia Variant from Hairy Cell Leukemia: Development and Validation of Diagnostic Criteria. Leuk Res. 2012;37:401–9. doi: 10.1016/j.leukres.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DelGiudice I, Matutes E, Morilla R, Morilla A, OwusuAnkomah K, Rafiq F, et al. The diagnostic value of CD123 in B-cell disorders with hairy or villous lymphocytes. Haematologica. 2004;89:303–8. [PubMed] [Google Scholar]
- 31.Iannitto E, Tripodo C. How I diagnose and treat splenic lymphomas. Blood. 2011;117:2585–95. doi: 10.1182/blood-2010-09-271437. [DOI] [PubMed] [Google Scholar]
- 32.Tiacci E, Trifonov V, Schiavoni G, Holmes A, Kern W, Martelli MP, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med. 2011;364:2305–15. doi: 10.1056/NEJMoa1014209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsushita K, Margulies I, Onda M, Nagata S, Stetler-Stevenson M, Kreitman RJ. Soluble CD22 as a Tumor Marker for Hairy Cell Leukemia. Blood. 2008;112:2272–7. doi: 10.1182/blood-2008-01-131987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robak T, Blasinska-Morawiec M, Krykowski E, Hansz J, Komarnicki M, Kazimierczak M, et al. 2-chlorodeoxyadenosine (2-CdA) in 2-hour versus 24-hour intravenous infusion in the treatment of patients with hairy cell leukemia. Leuk Lymphoma. 1996;22:107–11. doi: 10.3109/10428199609051736. [DOI] [PubMed] [Google Scholar]
- 35.Bastie JN, CazalsHatem D, Daniel MT, MFDA, Rabian CL, Glaisner S, et al. Five years follow-up after 2-chloro deoxyadenosine treatment in thirty patients with hairy cell leukemia: Evaluation of minimal residual disease and CD4+lymphocytopenia after treatment. Leuk Lymphoma. 1999;35:555–65. doi: 10.1080/10428199909169620. [DOI] [PubMed] [Google Scholar]
- 36.Wheaton S, Tallman MS, Hakimian D, Peterson L. Minimal residual disease may predict bone marrow relapse in patients with hairy cell leukemia treated with 2-chlorodeoxyadenosine. Blood. 1996;87:1556–60. [PubMed] [Google Scholar]
- 37.Mhawech-Fauceglia P, Oberholzer M, Aschenafi S, Baur A, Kurrer M, Von Rohr A, et al. Potential predictive patterns of minimal residual disease detected by immunohistochemistry on bone marrow biopsy specimens during a long-term follow-up in patients treated with cladribine for hairy cell leukemia. Arch Pathol Lab Med. 2006;130:374–7. doi: 10.5858/2006-130-374-PPPOMR. [DOI] [PubMed] [Google Scholar]
- 38.Forconi F, Toraldo F, Sozzi E, Amato T, Raspadori D, Lauria F. Complete molecular remission induced by concomitant cladribine--rituximab treatment in a case of multi-resistant hairy cell leukemia. Leuk Lymphoma. 2007;48:2441–3. doi: 10.1080/10428190701647903. [DOI] [PubMed] [Google Scholar]
- 39.Narat S, Gandla J, Dogan A, Mehta A. Successful treatment of hairy cell leukemia variant with rituximab. Leuk Lymphoma. 2005;46:1229–32. doi: 10.1080/10428190500083433. [DOI] [PubMed] [Google Scholar]
- 40.Imamura T, Ohtsuka E, Ogata M, Oka F, Kashima K, Kikuchi H, et al. Successful induction of long-term remission using rituximab in a patient with refractory hairy cell leukemia-Japanese variant. Int J Hematol. 2004;80:432–4. doi: 10.1532/ijh97.04078. [DOI] [PubMed] [Google Scholar]
- 41.Quach H, Januszewicz H, Westerman D. Complete remission of hairy cell leukemia variant (HCL-v) complicated by red cell aplasia post treatment with rituximab. Haematologica. 2005;90(Suppl):ECR26. [PubMed] [Google Scholar]
- 42.Sun T, Dittmar K, Koduru P, Susin M, Teichberg S, Brody J. Relationship between hairy cell leukemia variant and splenic lymphoma with villous lymphocytes: presentation of a new concept. Am J Hematol. 1996;51:282–8. doi: 10.1002/(SICI)1096-8652(199604)51:4<282::AID-AJH6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 43.Ya-In C, Brandwein J, Pantalony D, Chang H. Hairy cell leukemia variant with features of intrasinusoidal bone marrow involvement. Arch Pathol Lab Med. 2005;129:395–8. doi: 10.5858/2005-129-395-HCLVWF. [DOI] [PubMed] [Google Scholar]
- 44.Hockley SL, Else M, Morilla A, Wotherspoon A, Dearden C, Catovsky D, et al. The prognostic impact of clinical and molecular features in hairy cell leukaemia variant and splenic marginal zone lymphoma. Br J Haematol. 2012;158:347–54. doi: 10.1111/j.1365-2141.2012.09163.x. [DOI] [PubMed] [Google Scholar]
- 45.Traverse-Glehen A, Baseggio L, Bauchu EC, Morel D, Gazzo S, Ffrench M, et al. Splenic red pulp lymphoma with numerous basophilic villous lymphocytes: a distinct clinicopathologic and molecular entity? Blood. 2008;111:2253–60. doi: 10.1182/blood-2007-07-098848. [DOI] [PubMed] [Google Scholar]
- 46.Kost CB, Holden JT, Mann KP. Marginal zone B-cell lymphoma: A retrospective immunophenotypic analysis. Cytometry Part B Clin Cytom. 2008;74B:282–6. doi: 10.1002/cyto.b.20426. [DOI] [PubMed] [Google Scholar]
- 47.Forconi F, Sozzi E, Cencini E, Zaja F, Intermesoli T, Stelitano C, et al. Hairy cell leukemias with unmutated IGHV genes define the minor subset refractory to single-agent cladribine and with more aggressive behavior. Blood. 2009;114:4696–702. doi: 10.1182/blood-2009-03-212449. [DOI] [PubMed] [Google Scholar]