Abstract
Chimeric antigen receptors (CAR)-transduced T cells hold great promise in the treatment of malignant disease. Here, we demonstrate that intracerebral injection with a human, epidermal growth factor receptor variant III (EGFRvIII)-specific, third generation CAR successfully treats glioma in mice. Importantly, these results endorse clinical translation of this CAR in patients with EGFRvIII-expressing brain tumors.
Keywords: Central nervous system neoplasms, Epidermal growth factor receptor, Glioblastoma, Immunotherapy, T-lymphocytes
1. Introduction
Glioblastoma (GBM) is the most common and deadly primary malignant brain tumor, for which there are limited treatment options.1 Chimeric antigen receptors (CAR) represent a promising technology that redirects T cells to treat tumors via surface antibody-based domains translated in tandem with intracellular T cell signaling moieties. Costimulatory 4-1BB signalling has been shown to significantly improve the ability of CAR-tranduced T cells to persist and achieve antitumor T cell responses.2 However, such “third generation” CAR have not been tested for efficacy against intracerebral tumors. A mutation of the epidermal growth factor receptor, variant III (EGFRvIII), is frequently expressed on the surface of GBM but is completely absent from all normal tissues.3 Here, we demonstrate that an EGFRvIII-targeted, third generation CAR is specific and effective against human GBM cells in vitro and in vivo.
2. Materials and methods
2.1 Cell lines
Human glioma cell lines U87MG and U87MG.ΔEGFR are previously described.4
2.2 EGFRvIII CAR retroviral vector and analysis
Human peripheral blood lymphocytes were transduced with EGFRvIII CAR as described.5 Cytokine staining for interferon γ (IFNγ) was performed according to manufacturer’s instructions (Cytofix/Cytoperm; BD Bioscience, San Jose, CA, USA).
2.3 In vivo experiments
Efficacy was tested in non-obese diabetic scid gamma mice. Glioma cells (5 × 104) and T cells were implanted intracerebrally as described.6
2.4 Statistical methods
Frequencies of IFNγ+ cells with respect to groups defined by EGFRvIII specificity and peptide blockade were evaluated by two-way analysis of variance with interaction. The Kaplan – Meier estimator was used to generate survival curves, and groups were compared using the generalized Wilcoxon test.
3. Results
3.1 Third generation EGFRvIII CAR is specific for EGFRvIII+ glioma
One limitation of potent CAR-based therapies has been lethal toxicity arising from affinity for antigens co-expressed on healthy tissues.7,8 Targeting EGFRvIII, however, greatly reduces this risk of autoimmunity. Demonstrating its specificity, EGFRvIII CAR-transduced T cells did not exhibit detectable immunity to EGFRvIII-negative cells above untransduced levels. However, on incubation with U87MG.ΔEGFR, EGFRvIII CAR-transduced T cells yielded a significantly elevated frequency of IFNγ-expressing cells, which was subsequently inhibited in a dose-response fashion by peptide blockade with PEPvIII (Fig. 1).
Fig. 1.
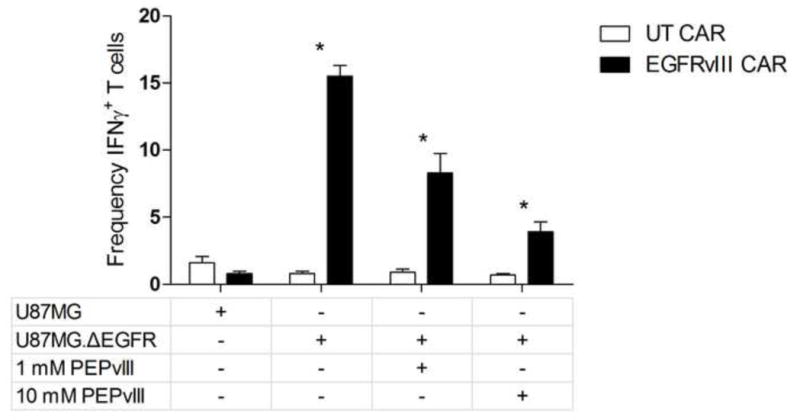
Graph showing epidermal growth factor receptor variant III (EGFRvIII) chimeric antigen receptors (CAR) T cell function and antitumor efficacy is specific for EGFRvIII+ tumors and is inhibited by soluble PEPvIII blockade. CAR-transduced or untransduced T cells with glioblastoma targets and soluble PEPvIII peptide was performed. Frequency of interferon γ+ cells was significantly greater in the presence of the EGFRvIII CAR, as well as reduced in a dose-dependent manner in the presence of increasing concentrations of PEPvIII.
*p < 0.001.
CAR = chimeric antigen receptors, EGFRvIII = epidermal growth factor receptor variant III, IFN = interferon, UT = untrandsuced.
3.2 Third generation EGFRvIII CAR treat intracerebral tumors
To investigate the ability of EGFRvIII CAR-transduced T cells to treat glioma in the entral nervous system, mice were implanted intracerebrally either with U87MG.ΔEGFR alone, U87MG.ΔEGFR with untransduced (UT) T cells, or U87MG.ΔEGFR with EGFRvIII CAR-transduced T cells at various doses. Mice receiving tumor with UT T cells did not exhibit a significant change in survival compared to mice implanted with tumor alone. However, mice treated with CAR-transduced T-cell doses of 5 × 104 or greater showed a dose-dependent increase in survival (EGFRvIII CAR versus UT, p < 0.001, log-rank test; Fig. 2), without toxicity to adjacent normal brain upon histological analysis.
Fig. 2.
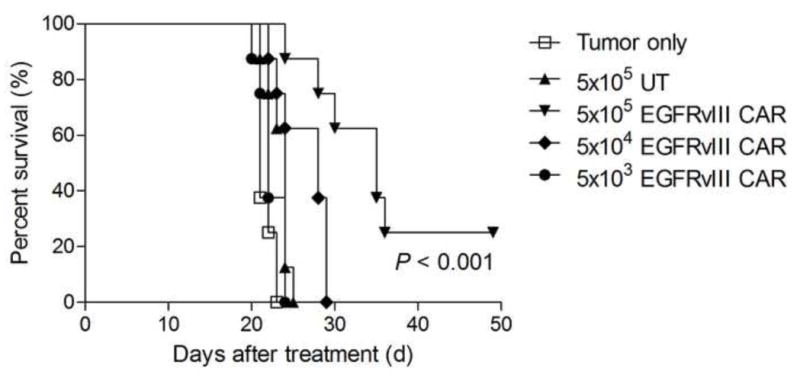
Graph showing epidermal growth factor receptor variant III (EGFRvIII) chimeric antigen receptors (CAR)-transduced T cell therapy treats intracerebral glioma in a dose dependent manner (p < 0.001; generalized Wilcoxon test). EGFRvIII CAR-transduced T cells were implanted intracerebrally. The log-rank test was used to determine statistical significance.
CAR = chimeric antigen receptors, EGFRvIII = epidermal growth factor receptor variant III, UT = untrandsuced.
4. Discussion
Here, we have demonstrated that intracerebral injection of T cells expressing third generation EGFRvIII CAR can mediate safe, therapeutic responses against EGFRvIII-expressing tumors in the brain. Additionally, soluble peptide blockade was shown to specifically inhibit the functional activity of CAR, and may serve as a potential antidote for CAR targeting less tumor-specific targets.
Our third generation EGFRvIII CAR promises to foster potent T cell function beyond what would otherwise be expected with earlier generation CAR constructs availing fewer costimulatory signaling domains.6,9 Together these data provide further rationale for the expedient translation of T cell based therapies for malignant glioma.
Acknowledgments
This work was supported by grants from the National Institutes of Health: SRC on Primary Tumors of the Central Nervous System P50 NS020023-28 and 3R01-CA-135272-02S1 supplement for Brain Tumor Stem Cell R01. Additional support was provided by 5P50-NS020023-29, 5R01-CA135272-02S1, 5R01-CA134844-03, 5UL1-RR024128-05, 5R21-NS067980-02, and 5R21-NS068057-02. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of Interest/Disclosures
The authors declare that they have no financial or other conflicts of interest in relation to this research and its publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smoll NR, Schaller K, Gautschi OP. Long-term survival of patients with glioblastoma multiforme (GBM) J Clin Neurosci. 2013 doi: 10.1016/j.jocn.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 2.Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17(8):1453–64. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi BD, Archer GE, Mitchell DA, et al. EGFRvIII-targeted vaccination therapy of malignant glioma. Brain Pathol. 2009;19(4):713–23. doi: 10.1111/j.1750-3639.2009.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishikawa R, Ji XD, Harmon RC, et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci U S A. 1994;91(16):7727–31. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan RA, Johnson LA, Davis JL, et al. Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Hum Gene Ther. 2012;23(10):1043–53. doi: 10.1089/hum.2012.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohno M, Natsume A, Ichiro Iwami K, et al. Retrovirally engineered T-cell-based immunotherapy targeting type III variant epidermal growth factor receptor, a glioma-associated antigen. Cancer Sci. 2010;101(12):2518–24. doi: 10.1111/j.1349-7006.2010.01734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843–51. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brentjens R, Yeh R, Bernal Y, et al. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 2010;18(4):666–8. doi: 10.1038/mt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bullain SS, Sahin A, Szentirmai O, et al. Genetically engineered T cells to target EGFRvIII expressing glioblastoma. J Neurooncol. 2009;94(3):373–82. doi: 10.1007/s11060-009-9889-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


