Abstract
A 17-year-old man was admitted with a complaint of knee pain. He was diagnosed with Wilson disease by ophthalmologic and laboratory studies during hospitalization. Initial plain radiography of both knees showed multiple osteochondritis dissecans (OCD) on the medial and lateral femoral condyles of both knees. Subsequent magnetic resonance imaging showed multiple OCDs, which were symmetric on both knees. Subchondral cysts on the medial condyle and trochlear dysplasia were additionally evident on both femurs. We report this case with a focus on the imaging findings.
Keywords: Osteochondritis dissecans, Magnetic resonance imaging, Hepatolenticular degeneration
Wilson disease is a rare autosomal recessive disorder in which an elevated level of copper accumulates in the liver and other organs, which can eventually result in serious harm to the nervous system, kidneys, and eyes1,2). Wilson disease is present at birth but symptoms generally take years to appear, usually between the ages of four and late teens. Symptoms include abdominal pain and jaundice. It is most common in people of Eastern European and Southern Italian descent. Since Warnock (1952) first reported osteoporosis and spontaneous fractures in a patient with Wilson disease, various osteoarticular lesions related to the condition have been described3). We report a 17-year-old man with Wilson disease, who had a complaint of knee pain. To our knowledge, this is a first report on the magnetic resonance imaging (MRI) of multiple and symmetric osteochondritis dissecans (OCDs) of both knees in a patient with Wilson disease.
Case Report
A 17-year-old man visited our orthopedic department with an acute attack of the left knee pain that had begun 2 days previously. The patient had experienced discomfort in both knees for the previous 7 months. A physical examination revealed diffuse swelling and tenderness of the left knee and motion limitation due to pain. Positive Wilson sign was evident and there seemed no other joint involvement but the both knees.
The patient had no record of previous medical history or family history of other diseases.
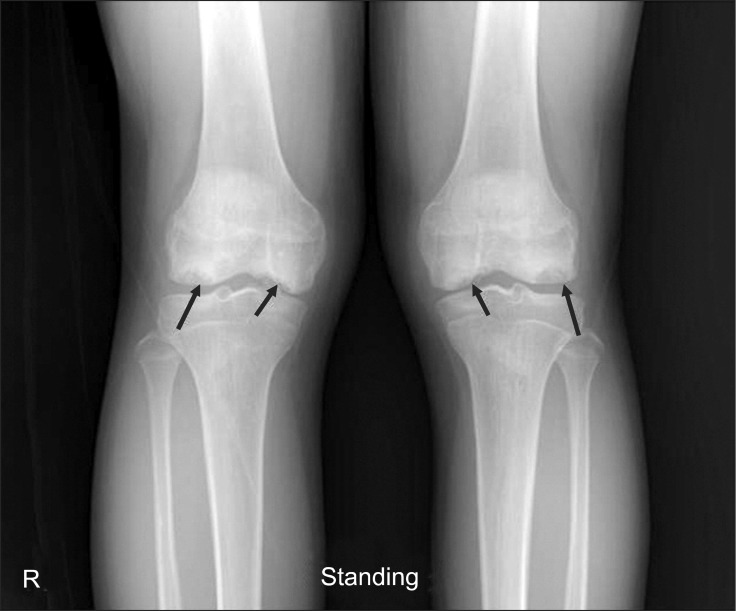
Plain radiography was performed, which revealed symmetrical multiple OCD on femoral condyles of both knees (Fig. 1). Further evaluation was warranted for the unusual presentation of the disease. The patient was admitted and had a routine laboratory test prior to admission and it was his first time to take laboratory tests. His laboratory tests showed thrombocytopenia platelet count of 83×103/µL, prolonged prothrombin (PT)/activated partial prothrombin time (aPTT) of 14.7/45.3 sec. Further lab tests were carried out, and revealed deficit of coagulation factors V, XI, and XII (all 44%; normal range, 60% to 140%). Abdominal ultrasound revealed cirrhotic morphologic changes of the liver and splenomegaly. A suspicion of Wilson disease led to more additional laboratory tests for confirmation, which showed low ceruloplasmin (<4 mg/dL; normal range, 16.2 to 35.6 mg/dL), low serum copper (43 µg/dL; normal range, 70 to 145 µg/dL), and high urine copper (383 µg/dL: normal range, 15 to 60 µg/dL). Ophthalmologic examination showed Keyser-Fleischer rings in both corneas.
Fig. 1.
A 17-year-old man with Wilson disease who had a complaint of abrupt onset of knee pain. Plain knee anteroposterior view shows multiple, symmetric osteochondritis dissecans on the medial and lateral femoral condyles of both knees.
He was diagnosed with Wilson disease. Examinations of other commonly involved joints affected by Wilson disease, such as spine, hip, and wrists, were done and showed normal findings, and bone mineral density study also revealed normal values.
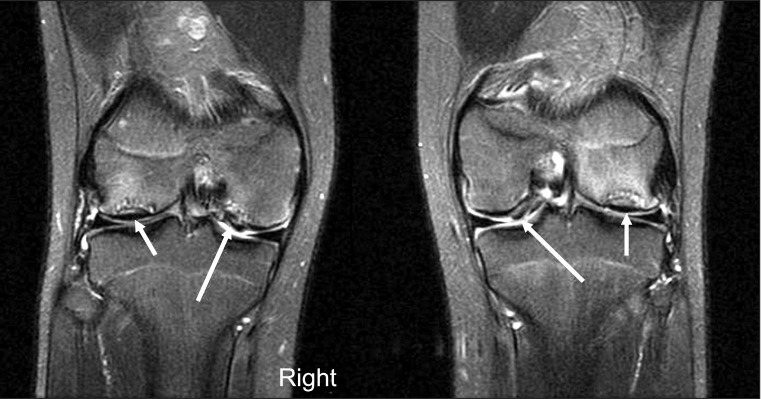
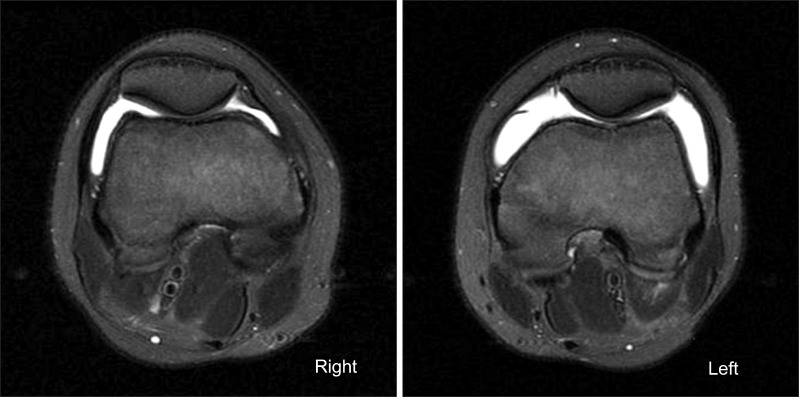
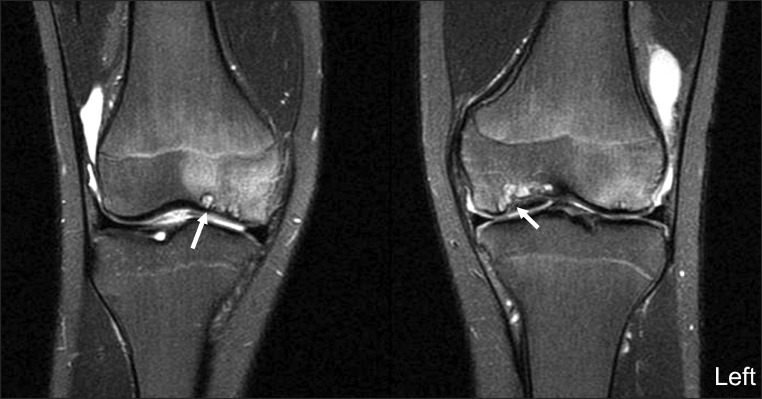
An MRI scan of the knee was performed to evaluate OCD lesions for its stage and treatment plans and to evaluate ligamentous or meniscal injury, if any. The MRI scan revealed characteristic bilateral symmetric OCD on both knees and symmetric detached OCD on the medial condyle of the bilateral femurs. Subchondral cysts were also seen on the medial femoral condyle of both knees and the trochlear dysplasia was observed in both femurs (Figs. 2-4).
Fig. 2.
T2-weighted image with fat saturation coronal images of both knees showed multiple, symmetric osteochondritis dissecans (OCD) (arrows) of both knees, detached OCD (long arrows) was found on the medial femoral condyle symmetrically on both knees.
Fig. 4.
Axial T2-weighted image with fat saturation images of the both femur showed shallow trochlea, suggestive of trochlea dysplasia.
Arthroscopic debridement was performed for treatment of OCD.
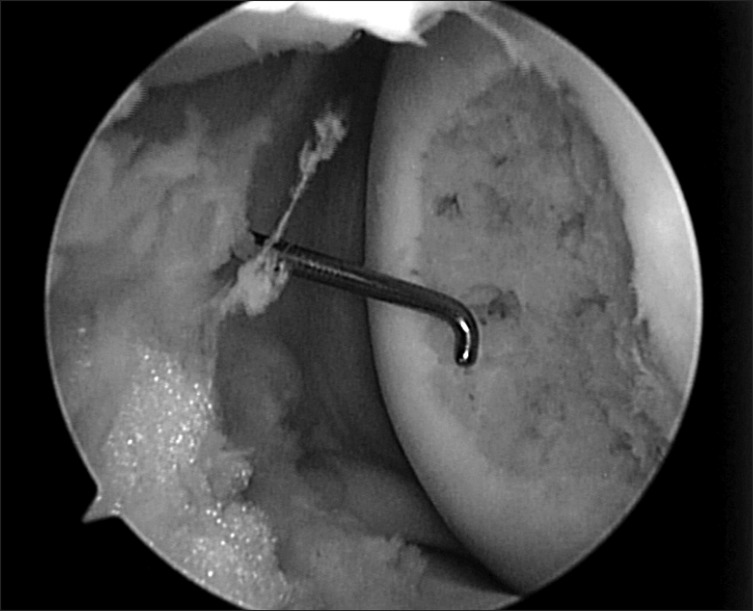
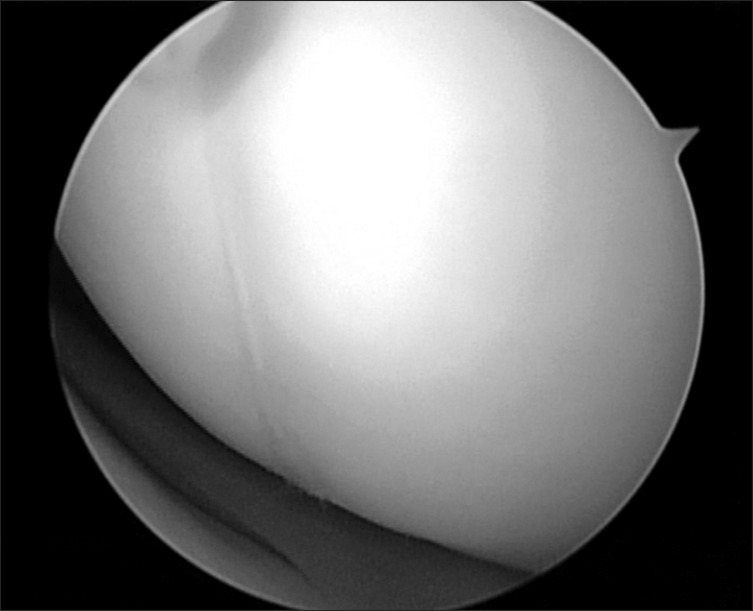
Arthroscopic findings revealed OCD lesions on both medial and lateral femoral condyles, which were not apparently unstable at first under direct arthroscopic visualization. Using an arthroscopic probe, the medial femoral condyle lesion was found at the inferocentral portion of the medial femoral condyle; the lesion was unstable and already in a detached state (Fig. 5). The detached fragment too fragile to fixate was excised, and curettage and multiple drilling were performed subsequently (Fig. 6). The lesion on the lateral femoral condyle, also located at the inferocentral aspect, was stable and undetached, and was left untouched with the expectation of spontaneous healing (Fig. 7).
Fig. 5.
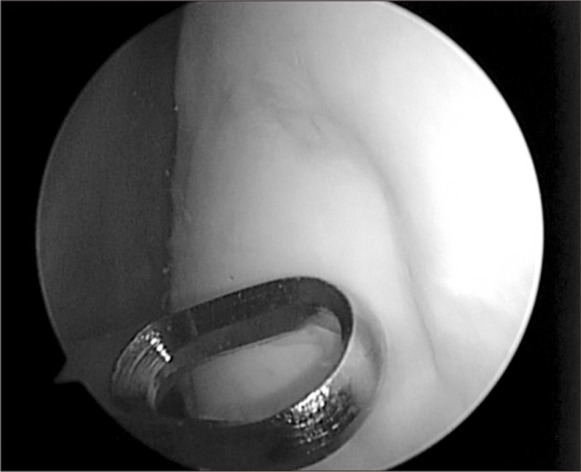
Arthroscopic finding of an osteochondritis dissecans (OCD) lesion on the inferocentral portion of the medial femoral condyle, notice the unstable state of OCD lesion.
Fig. 6.
Arthroscopic finding of the lesion on the medial femoral condyle; after excision of fragment and multiple drilling of the lesion.
Fig. 7.
Arthroscopic finding of a osteochondritis dissecans lesion on the inferocentral portion of the lateral femoral condyle which was stable and healed state.
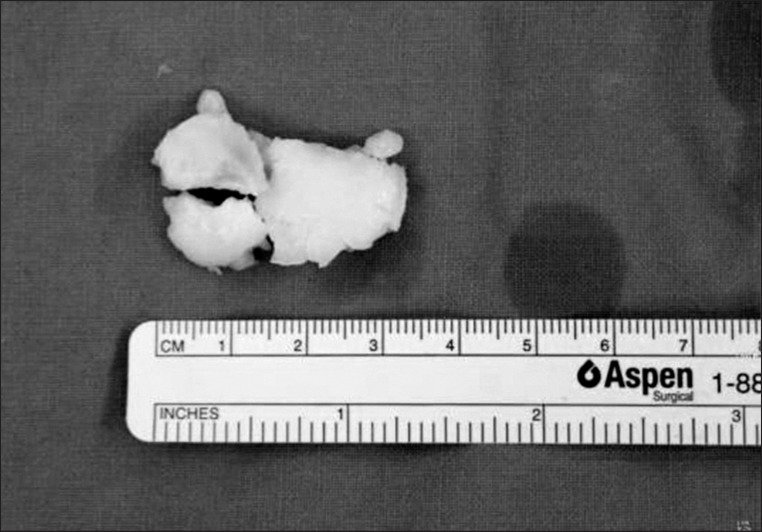
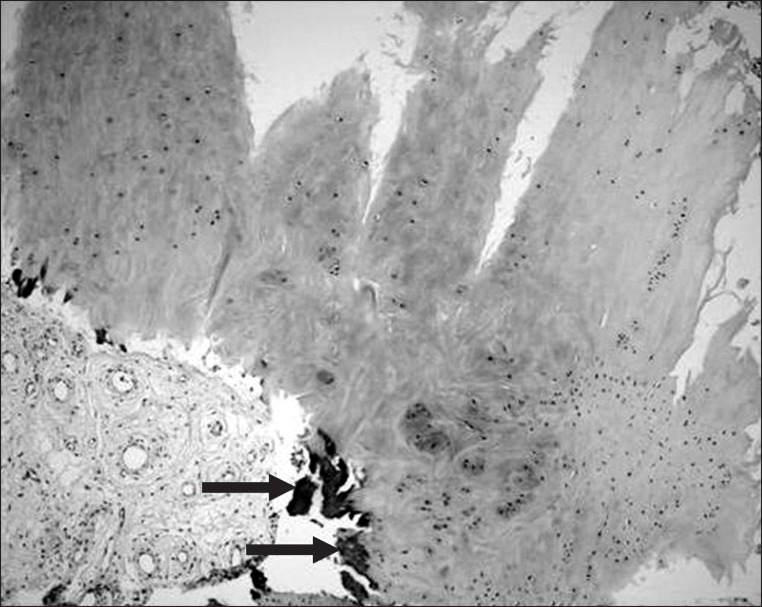
Microscopically, the section revealed articular cartilage with secondary calcification and synovial tissue (Figs. 8, 9).
Fig. 8.
Gross pathologic specimen showed yellowish debridement of osteochondritis dissecans.
Fig. 9.
Microscopically, the section revealed several fragments of articular cartilage with secondary calcification (arrows) and multiple fragments of synovial tissue (H&E, ×100).
After the arthroscopic treatment, the symptoms slowly diminished and at the 2-month postoperative follow-up, the patient was pain free with full range of motion of the knee. Ever since the diagnosis of the Wilson disease, the patient was on chelation therapy and had no recurrence of knee joint pain within the total 6 months of follow-up period.
Discussion
Hepatolenticular degeneration is a rare inborn disease of defective copper metabolism and is inherited as an autosomal recessive trait1).
The pathogenesis of abnormal copper retention is unclear. One possibility is increased intestinal absorption of copper in patients with Wilson disease. However, studies with 64Cu and 67Cu isotopes showed decreased copper excretion from the bile duct, supporting the view that abnormal copper metabolism is not due to increased absorption. The disease is commonly diagnosed in patients with a liver disease, aged 5 to 40 years, with a decreased serum ceruloplasmin (<20 mg/dL) and detectable Kayser-Fleischer rings, and Wilson disease.
Osteoarticular changes related to Wilson disease that have been reported previously comprise bone changes including osteoporosis and osteomalacia, and degenerative changes of the articular bone and cartilage that occur mainly in small joints. Radiographic changes include marginal bone fragments, osteoarthritis incompatible with age, calcification of the joint capsule or tendon insertion, articular sclerosis and subarticular cysts, and osteochondrosis4-7). Although all these findings may not be present in every patient, if these findings are observed in the small joints of the hands and wrists, clinicians should be alerted to the possibility of this rare disease.
The mechanism of these bone and joint changes is unknown. A clinical and laboratory study involving 40 Chinese patients8) suggested the involvement of two important factors. First, the high incidence of osteoporosis and occasional rickets is probably secondary to marked hyperphosphaturia and resultant hypophophatemia. The second factor is chronic trauma. Patients with Wilson disease are clumsy and more likely to sustain trauma, and underlying demineralization makes them more prone to develop deformities. However, this hypothesis has not been confirmed in other movement disorders9,10), and there is no correlation between severity of neurologic impairment and arthropathy2). An additional factor is that renal tubular acidosis may itself contribute to osteomalacia.
In one report, arthropathy became milder secondary to earlier diagnosis of the disease and more intensive chelation therapy2,9). The authors also demonstrated copper in the cartilage and synovium in patients with Wilson disease. So the arthropathy of Wilson disease may be caused by copper deposition in joints, with tissue damage mediated by oxygen-derived free radicals2,9).
Radiographic changes of knee joint include degenerative changes, OCD, erosion of lateral borders of patellae, and appearances resembling Sudeck's atrophy3). OCD of the knee has been previously reported in only one study3). In this prior study, three patients among 32 with Wilson disease demonstrated OCD of the knee. However, details of this condition were not provided, and it appeared as a solitary lesion in one figure. Our case featured multiple OCDs in both knees that could be analyzed using MRI.
MRI revealed that the OCDs were multiple and symmetric on both femoral condyles of both knees; one OCD was detached and displaced from the medial femoral condyle of both knees, which was symmetric and a very unique finding. Subchondral cysts were also evident in the medial femoral condyles of both knees. In general, OCD usually occurs in the lateral aspect of the medial femoral condyle in the general population. This case and the previously reviewed cases in the literatures demonstrate OCDs in the inferocentral portion of the condyles.
The limitation of this study is that the mechanism of association of OCDs with Wilson disease was not identified.
The cases involving Wilson disease show a connection between the disease and the osteoarticular manifestations. OCD is a disease whose pathogenesis has not been clearly revealed. These rare findings of multiple OCDs in the inferocentral portion of both femoral condyles in patient with Wilson disease may provide useful information for understanding the true nature of OCD lesions in the future.
Fig. 3.
T2-weighted image with fat saturation coronal images of both knees revealed subchondral cysts on the medial femoral condyle of both knees.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Xie YZ, Zhang XZ, Xu XH, Zhang ZX, Feng YK. Radiologic study of 42 cases of Wilson disease. Skeletal Radiol. 1985;13:114–119. doi: 10.1007/BF00352081. [DOI] [PubMed] [Google Scholar]
- 2.Menerey KA, Eider W, Brewer GJ, Braunstein EM, Schumacher HR, Fox IH. The arthropathy of Wilson's disease: clinical and pathologic features. J Rheumatol. 1988;15:331–337. [PubMed] [Google Scholar]
- 3.Golding DN, Walshe JM. Arthropathy of Wilson's disease. Study of clinical and radiological features in 32 patients. Ann Rheum Dis. 1977;36:99–111. doi: 10.1136/ard.36.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feller ER, Schumacher HR. Osteoarticular changes in Wilson's disease. Arthritis Rheum. 1972;15:259–266. doi: 10.1002/art.1780150307. [DOI] [PubMed] [Google Scholar]
- 5.Mindelzun R, Elkin M, Scheinberg IH, Sternlieb I. Skeletal changes in Wilson's disease: a radiological study. Radiology. 1970;94:127–132. doi: 10.1148/10.1148/94.1.127. [DOI] [PubMed] [Google Scholar]
- 6.Rosenoer VM, Michell RC. Skeletal changes in Wilson's disease (hepato-lenticular degeneration) Br J Radiol. 1959;32:805–809. doi: 10.1259/0007-1285-32-384-805. [DOI] [PubMed] [Google Scholar]
- 7.Xie YZ, Zhang XZ, Xu XH. Roentgenologic study of 41 cases of Wilson's disease. Chin Med J (Engl) 1982;95:674–678. [PubMed] [Google Scholar]
- 8.Strickland GT, Leu ML. Wilson's disease. Clinical and laboratory maniestations in 40 patients. Medicine (Baltimore) 1975;54:113–137. [PubMed] [Google Scholar]
- 9.Kaklamanis P, Spengos M. Osteoarticular changes and synovial biopsy findings in Wilson's disease. Ann Rheum Dis. 1973;32:422–427. doi: 10.1136/ard.32.5.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finby N, Bearn AG. Roentgenographic abnormalities of the skeletal system in Wilson's disease (hepatolenticular degeneration) Am J Roentgenol Radium Ther Nucl Med. 1958;79:603–611. [PubMed] [Google Scholar]