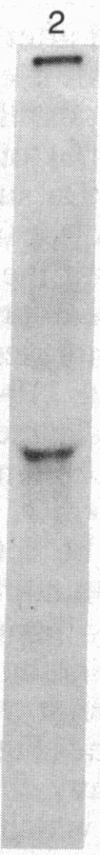
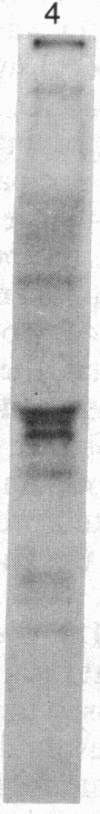
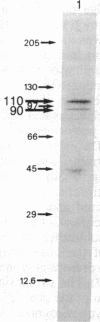
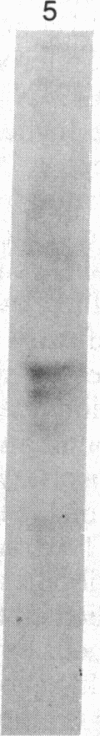
Abstract
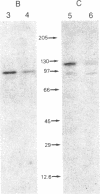
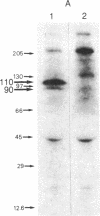
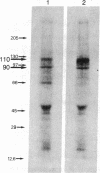
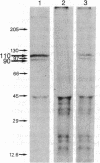
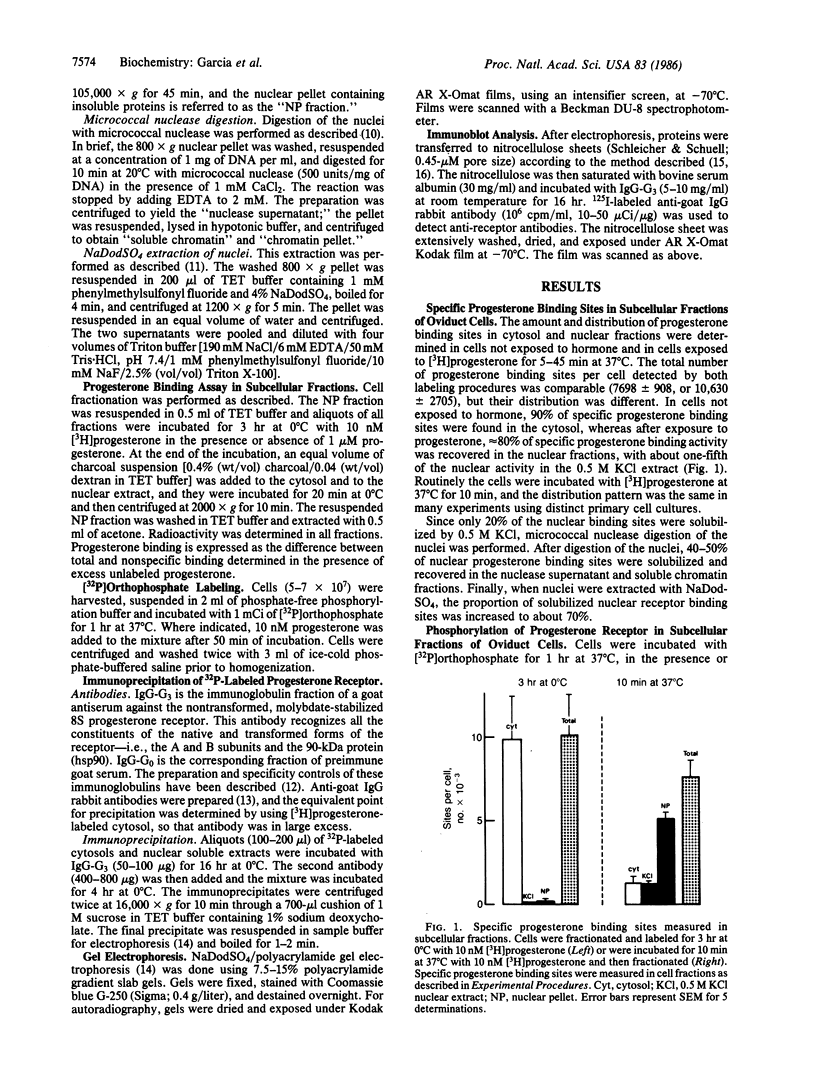
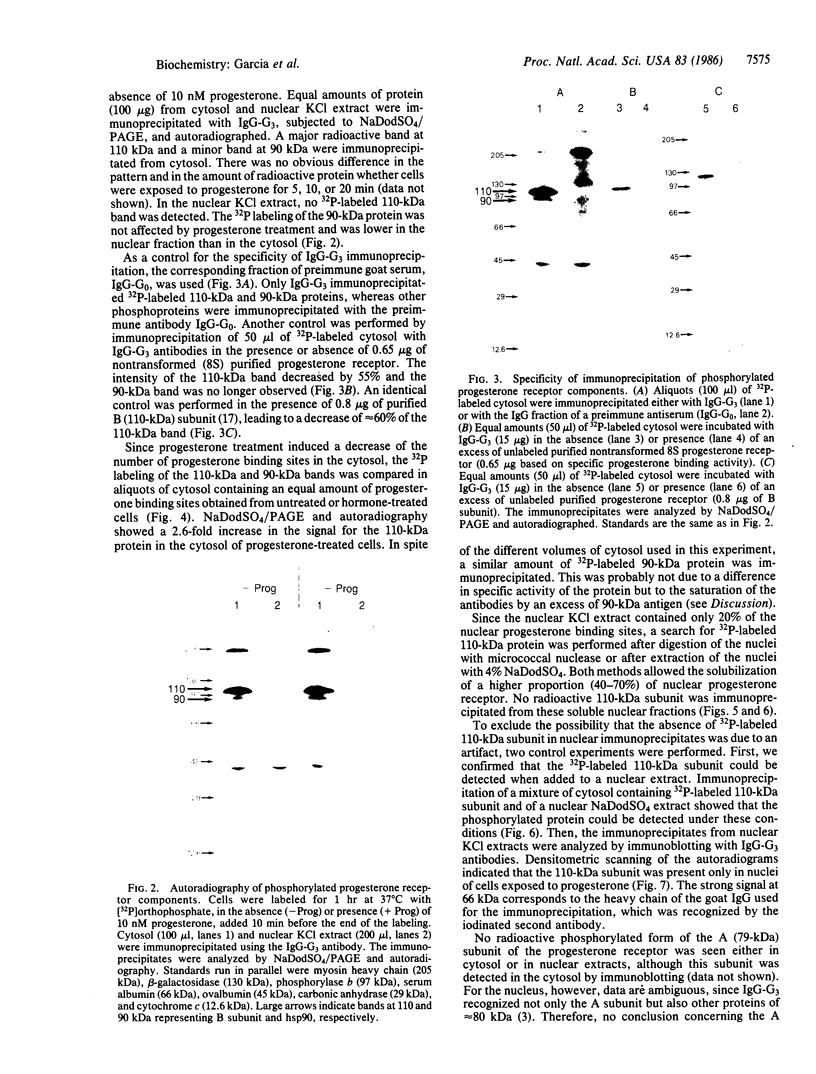
Oviduct cells from estradiol-treated chicks were grown in primary culture. After 3-5 days of culture in medium containing estradiol, 90% of the cellular progesterone binding sites were detected in the cytosol. After exposure to [3H]progesterone at 37 degrees C, 80% of the progesterone binding sites were found in nuclear fractions. Progesterone receptor phosphorylation was assessed after incubating the cells with [32P]orthophosphate. Receptor components were immunoprecipitated with a specific polyclonal antibody (IgG-G3) and analyzed by NaDodSO4/PAGE and autoradiography. In the cytosol, constant amounts of 32P-labeled 110-kDa subunit (the B subunit, one of the progesterone-binding components of the receptor) and of the non-steroid-binding heat shock protein hsp90 were found, whether cells had been exposed to progesterone or not. No 32P-labeled 79-kDa subunit (the A subunit, another progesterone-binding subunit) was detected. Various procedures were used to solubilize nuclear progesterone receptor (0.5 M KCl, micrococcal nuclease, NaDodSO4), and in no case was 32P-labeled B subunit detected in the extracts. However, nonradioactive B subunit was detected by immunoblot in a nuclear KCl extract of progesterone-treated cells. These results suggest that the fraction of the B subunit that becomes strongly attached to nuclear structures is not phosphorylated upon exposure of cells to progesterone.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Blobel G. Immunoprecipitation of proteins from cell-free translations. Methods Enzymol. 1983;96:111–120. doi: 10.1016/s0076-6879(83)96012-3. [DOI] [PubMed] [Google Scholar]
- Barnett C. A., Schmidt T. J., Litwack G. Effects of calf intestinal alkaline phosphatase, phosphatase inhibitors, and phosphorylated compounds on the rate of activation of glucocorticoid-receptor complexes. Biochemistry. 1980 Nov 11;19(23):5446–5455. doi: 10.1021/bi00564a046. [DOI] [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd-Leinen P., Gosse B., Rasmussen K., Martin-Dani G., Spelsberg T. C. Regulation of nuclear binding of the avian oviduct progesterone receptor. Changes during estrogen-induced oviduct development, withdrawal, and secondary stimulation. J Biol Chem. 1984 Feb 25;259(4):2411–2421. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Catelli M. G., Binart N., Jung-Testas I., Renoir J. M., Baulieu E. E., Feramisco J. R., Welch W. J. The common 90-kd protein component of non-transformed '8S' steroid receptors is a heat-shock protein. EMBO J. 1985 Dec 1;4(12):3131–3135. doi: 10.1002/j.1460-2075.1985.tb04055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty J. J., Puri R. K., Toft D. O. Phosphorylation in vivo of chicken oviduct progesterone receptor. J Biol Chem. 1982 Dec 10;257(23):14226–14230. [PubMed] [Google Scholar]
- Dougherty J. J., Puri R. K., Toft D. O. Polypeptide components of two 8 S forms of chicken oviduct progesterone receptor. J Biol Chem. 1984 Jun 25;259(12):8004–8009. [PubMed] [Google Scholar]
- Garcia T., Tuohimaa P., Mester J., Buchou T., Renoir J. M., Baulieu E. E. Protein kinase activity of purified components of the chicken oviduct progesterone receptor. Biochem Biophys Res Commun. 1983 Jun 29;113(3):960–966. doi: 10.1016/0006-291x(83)91092-6. [DOI] [PubMed] [Google Scholar]
- Gasc J. M., Ennis B. W., Baulieu E. E., Stumpf W. E. Récepteur de la progestérone dans l'oviducte de Poulet: double révélation par immunohistochimie avec des anticorps antirécepteur et par autoradiographie à l'aide d'un progestagène tritié. C R Seances Acad Sci III. 1983;297(9):477–482. [PubMed] [Google Scholar]
- Gasc J. M., Renoir J. M., Radanyi C., Joab I., Tuohimaa P., Baulieu E. E. Progesterone receptor in the chick oviduct: an immunohistochemical study with antibodies to distinct receptor components. J Cell Biol. 1984 Oct;99(4 Pt 1):1193–1201. doi: 10.1083/jcb.99.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh-Dastidar P., Coty W. A., Griest R. E., Woo D. D., Fox C. F. Progesterone receptor subunits are high-affinity substrates for phosphorylation by epidermal growth factor receptor. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1654–1658. doi: 10.1073/pnas.81.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley P. R., Dahmer M. K., Pratt W. B. Inactivation of glucocorticoid-binding capacity by protein phosphatases in the presence of molybdate and complete reactivation of dithiothreitol. J Biol Chem. 1982 Aug 10;257(15):8615–8618. [PubMed] [Google Scholar]
- Jung-Testas I., Garcia T., Baulieu E. E. Steroid hormones induce cell proliferation and specific protein synthesis in primary chick oviduct cultures. J Steroid Biochem. 1986 Jan;24(1):273–279. doi: 10.1016/0022-4731(86)90064-6. [DOI] [PubMed] [Google Scholar]
- Klein H., Maltzman W., Levine A. J. Structure-function relationships of the adenovirus DNA-binding protein. J Biol Chem. 1979 Nov 10;254(21):11051–11060. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lebeau M. C., Massol N., Baulieu E. E. Oestrogen and progesterone receptors in chick oviduct chromatin after administration of oestradiol, progesterone or anti-oestrogen. Biochem J. 1982 Jun 15;204(3):653–662. doi: 10.1042/bj2040653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linné T., Philipson L. Further characterization of the phosphate moiety of the adenovirus type 2 DNA-binding protein. Eur J Biochem. 1980 Jan;103(2):259–270. doi: 10.1111/j.1432-1033.1980.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Logeat F., Le Cunff M., Pamphile R., Milgrom E. The nuclear-bound form of the progesterone receptor is generated through a hormone-dependent phosphorylation. Biochem Biophys Res Commun. 1985 Aug 30;131(1):421–427. doi: 10.1016/0006-291x(85)91819-4. [DOI] [PubMed] [Google Scholar]
- Maggi A., Schrader W. T., O'Malley B. W. Progesterone-binding sites of the chick oviduct receptor. Presence of a weaker ligand site which is destroyed by phosphatase treatment. J Biol Chem. 1984 Sep 10;259(17):10956–10966. [PubMed] [Google Scholar]
- Mester J., Baulieu E. E. Progesterone receptors in the chick oviduct. Determination of the total concentration of binding sites in the cytosol and nuclear fraction and effect of progesterone on their distribution. Eur J Biochem. 1977 Jan;72(2):405–414. doi: 10.1111/j.1432-1033.1977.tb11265.x. [DOI] [PubMed] [Google Scholar]
- Migliaccio A., Lastoria S., Moncharmont B., Rotondi A., Auricchio F. Phosphorylation of calf uterus 17 beta-estradiol receptor by endogenous Ca2+-stimulated kinase activating the hormone binding of the receptor. Biochem Biophys Res Commun. 1982 Dec 15;109(3):1002–1010. doi: 10.1016/0006-291x(82)92039-3. [DOI] [PubMed] [Google Scholar]
- Migliaccio A., Rotondi A., Auricchio F. Calmodulin-stimulated phosphorylation of 17 beta-estradiol receptor on tyrosine. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5921–5925. doi: 10.1073/pnas.81.19.5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen C. J., Sando J. J., Pratt W. B. Evidence that dephosphorylation inactivates glucocorticoid receptors. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1398–1402. doi: 10.1073/pnas.74.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D. Regulation of protein synthesis in chick oviduct. I. Independent regulation of ovalbumin, conalbumin, ovomucoid, and lysozyme induction. J Biol Chem. 1972 Oct 25;247(20):6450–6461. [PubMed] [Google Scholar]
- Renoir J. M., Mester J., Buchou T., Catelli M. G., Tuohimaa P., Binart N., Joab I., Radanyi C., Baulieu E. E. Purification by affinity chromatography and immunological characterization of a 110kDa component of the chick oviduct progesterone receptor. Biochem J. 1984 Feb 1;217(3):685–692. doi: 10.1042/bj2170685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renoir J. M., Radanyi C., Yang C. R., Baulieu E. E. Antibodies against progesterone receptor from chick oviduct. Cross-reactivity with mammalian progesterone receptors. Eur J Biochem. 1982 Sep;127(1):81–86. doi: 10.1111/j.1432-1033.1982.tb06840.x. [DOI] [PubMed] [Google Scholar]
- Schrader W. T., Birnbaumer M. E., Hughes M. R., Weigel N. L., Grody W. W., O'Malley B. W. Studies on the structure and function of the chicken progesterone receptor. Recent Prog Horm Res. 1981;37:583–633. doi: 10.1016/b978-0-12-571137-1.50017-7. [DOI] [PubMed] [Google Scholar]
- Weigel N. L., Tash J. S., Means A. R., Schrader W. T., O'Malley B. W. Phosphorylation of hen progesterone receptor by cAMP dependent protein kinase. Biochem Biophys Res Commun. 1981 Sep 16;102(1):513–519. doi: 10.1016/0006-291x(81)91549-7. [DOI] [PubMed] [Google Scholar]
- Wilcox K. W., Kohn A., Sklyanskaya E., Roizman B. Herpes simplex virus phosphoproteins. I. Phosphate cycles on and off some viral polypeptides and can alter their affinity for DNA. J Virol. 1980 Jan;33(1):167–182. doi: 10.1128/jvi.33.1.167-182.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Ahe D., Renoir J. M., Buchou T., Baulieu E. E., Beato M. Receptors for glucocorticosteroid and progesterone recognize distinct features of a DNA regulatory element. Proc Natl Acad Sci U S A. 1986 May;83(9):2817–2821. doi: 10.1073/pnas.83.9.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]