Abstract
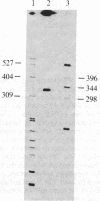
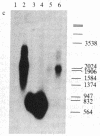
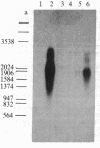
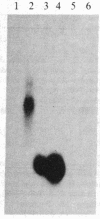
Vitamin D-dependent 28-kDa calcium-binding protein (CaBP28) cDNA clones were isolated from a chicken intestinal library. The nucleotide sequence analysis of the CaBP28 cDNA shows an open reading frame of 786 nucleotides, coding for a 262-amino acid 30.167-kDa protein. Interestingly, the protein contains six repeats of a domain with the feature of a calcium-binding site. In two of the six domains, oxygen-containing amino acids important for the positioning of calcium are absent, suggesting that these two sites have lost their calcium-binding capability and might have adopted a new function in evolution. In the chicken intestine, three different sized species of CaBP28 mRNA (2.0, 2.8, and 3.1 kilobases) are detected. Primer extension and S1 nuclease mapping show that the three CaBP28 mRNA species share a common 5' end but differ in the length of their 3' noncoding sequence. A similar triplet of CaBP28 mRNAs is identified in the rat kidney by the chicken probe, showing an interspecies conservation of the CaBP28. In the rat intestine, however, no CaBP28 mRNA could be detected. Instead, a vitamin D-dependent 9-kDa CaBP (CaBP9) is expressed, with an mRNA size of approximately equal to 0.7 kilobase that does not cross-hybridize with the CaBP28 probe. This indicates that the CaBP28 and CaBP9 are the product of two independent genes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bredderman P. J., Wasserman R. H. Chemical composition, affinity for calcium, and some related properties of the vitamin D dependent calcium-binding protein. Biochemistry. 1974 Apr 9;13(8):1687–1694. doi: 10.1021/bi00705a021. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Christakos S., Friedlander E. J., Frandsen B. R., Norman A. W. Studies on the mode of action of calciferol. XIII. Development of a radioimmunoassay for vitamin D-dependent chick intestinal calcium-binding protein and tissue distribution. Endocrinology. 1979 May;104(5):1495–1503. doi: 10.1210/endo-104-5-1495. [DOI] [PubMed] [Google Scholar]
- Christakos S., Norman A. W. Vitamin D-dependent calcium-binding protein synthesis by chick kidney and duodenal polysomes. Arch Biochem Biophys. 1980 Sep;203(2):809–815. doi: 10.1016/0003-9861(80)90242-8. [DOI] [PubMed] [Google Scholar]
- Chuvpilo S. A., Kravchenko V. V. A simple and rapid method for sequencing DNA. FEBS Lett. 1985 Jan 1;179(1):34–36. doi: 10.1016/0014-5793(85)80185-x. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Desplan C., Heidmann O., Lillie J. W., Auffray C., Thomasset M. Sequence of rat intestinal vitamin D-dependent calcium-binding protein derived from a cDNA clone. Evolutionary implications. J Biol Chem. 1983 Nov 25;258(22):13502–13505. [PubMed] [Google Scholar]
- Fullmer C. S., Wasserman R. H. The amino acid sequence of bovine intestinal calcium-binding protein. J Biol Chem. 1981 Jun 10;256(11):5669–5674. [PubMed] [Google Scholar]
- Goodman M., Pechère J. F., Haiech J., Demaille J. G. Evolutionary diversification of structure and function in the family of intracellular calcium-binding proteins. J Mol Evol. 1979 Nov;13(4):331–352. doi: 10.1007/BF01731373. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Guo L. H., Yang R. C., Wu R. An improved strategy for rapid direct sequencing of both strands of long DNA molecules cloned in a plasmid. Nucleic Acids Res. 1983 Aug 25;11(16):5521–5540. doi: 10.1093/nar/11.16.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann T., Kawakami M., Hitchman A. J., Harrison J. E., Dorrington K. J. The amino acid sequence of porcine intestinal calcium-binding protein. Can J Biochem. 1979 Jun;57(6):737–748. doi: 10.1139/o79-092. [DOI] [PubMed] [Google Scholar]
- Hunziker W., Siebert P. D., King M. W., Stucki P., Dugaiczyk A., Norman A. W. Molecular cloning of a vitamin D-dependent calcium-binding protein mRNA sequence from chick intestine. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4228–4232. doi: 10.1073/pnas.80.14.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilhoffer M. C., Haiech J., Demaille J. G. Ion binding to calmodulin. A comparison with other intracellular calcium-binding proteins. Mol Cell Biochem. 1983;51(1):33–54. doi: 10.1007/BF00215584. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H. Calcium-binding proteins. Annu Rev Biochem. 1976;45:239–266. doi: 10.1146/annurev.bi.45.070176.001323. [DOI] [PubMed] [Google Scholar]
- Laouari D., Pavlovitch H., Deceneux G., Balsan S. A vitamin D-dependent calcium-binding protein in rat skin. FEBS Lett. 1980 Mar 10;111(2):285–289. doi: 10.1016/0014-5793(80)80811-8. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman A. W., Roth J., Orci L. The vitamin D endocrine system: steroid metabolism, hormone receptors, and biological response (calcium binding proteins). Endocr Rev. 1982 Fall;3(4):331–366. doi: 10.1210/edrv-3-4-331. [DOI] [PubMed] [Google Scholar]
- Norman A. W., Wong R. G. Biological activity of the vitamin D metabolite 1,25-dihydroxycholecalciferol in chickens and rats. J Nutr. 1972 Dec;102(12):1709–1718. doi: 10.1093/jn/102.12.1709. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Poncz M., Solowiejczyk D., Ballantine M., Schwartz E., Surrey S. "Nonrandom" DNA sequence analysis in bacteriophage M13 by the dideoxy chain-termination method. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4298–4302. doi: 10.1073/pnas.79.14.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger P. R., Norman A. W., Heizmann C. W. Parvalbumin and vitamin D-dependent calcium-binding protein (Mr 28,000): comparison of their localization in the cerebellum of normal and rachitic rats. Neurosci Lett. 1985 Aug 16;59(1):97–103. doi: 10.1016/0304-3940(85)90221-6. [DOI] [PubMed] [Google Scholar]
- Siebert P., Hunziker W., Norman A. W. Cell-free translation analysis of the vitamin D-dependent calcium binding protein mRNA activity present in total RNA and polysomal extracts from chick intestine. Arch Biochem Biophys. 1982 Dec;219(2):286–296. doi: 10.1016/0003-9861(82)90159-x. [DOI] [PubMed] [Google Scholar]
- Spencer R., Charman M., Wilson P., Lawson E. Vitamin d-stimulated intestinal calcium absorption may not involve calcium-binding protein directly. Nature. 1976 Sep 9;263(5573):161–163. doi: 10.1038/263161a0. [DOI] [PubMed] [Google Scholar]
- Szebenyi D. M., Obendorf S. K., Moffat K. Structure of vitamin D-dependent calcium-binding protein from bovine intestine. Nature. 1981 Nov 26;294(5839):327–332. doi: 10.1038/294327a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman R. H., Taylor A. N. Vitamin d3-induced calcium-binding protein in chick intestinal mucosa. Science. 1966 May 6;152(3723):791–793. doi: 10.1126/science.152.3723.791. [DOI] [PubMed] [Google Scholar]
- Wilson P. W., Harding M., Lawson D. E. Putative amino acid sequence of chick calcium-binding protein deduced from a complementary DNA sequence. Nucleic Acids Res. 1985 Dec 20;13(24):8867–8881. doi: 10.1093/nar/13.24.8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eerd J. P., Takahshi K. Determination of the complete amino acid sequence of bovine cardiac troponin C. Biochemistry. 1976 Mar 9;15(5):1171–1180. doi: 10.1021/bi00650a033. [DOI] [PubMed] [Google Scholar]