Abstract
Purpose
To determine whether a nurse navigator intervention improves quality of life and patient experience with care for people recently given a diagnosis of breast, colorectal, or lung cancer.
Patients and Methods
Adults with recently diagnosed primary breast, colorectal, or lung cancer (n = 251) received either enhanced usual care (n = 118) or nurse navigator support for 4 months (n = 133) in a two-group cluster randomized, controlled trial with primary care physicians as the units of randomization. Patient-reported measures included the Functional Assessment of Cancer Therapy–General (FACT-G) Quality of Life scale, three subscales of the Patient Assessment of Chronic Illness Care (PACIC), and selected subscales from a cancer adaptation of the Picker Institute's patient experience survey. Self-report measures were collected at baseline, 4 months, and 12 months. Automated administrative data were used to assess time to treatment and total health care costs.
Results
There were no significant differences between groups in FACT-G scores. Nurse navigator patients reported significantly higher scores on the PACIC and reported significantly fewer problems with care, especially psychosocial care, care coordination, and information, as measured by the Picker instrument. Cumulative costs after diagnosis did not differ significantly between groups, but lung cancer costs were $6,852 less among nurse navigator patients.
Conclusion
Compared with enhanced usual care, nurse navigator support for patients with cancer early in their course improves patient experience and reduces problems in care, but did not differentially affect quality of life.
INTRODUCTION
Patients recently given a diagnosis of cancer must make life-altering decisions while negotiating an often fragmented care system. This adds to the distress of coping with a life-threatening diagnosis. In an earlier study, we collected data on the barriers and facilitators of high quality cancer care from experts,1 community cancer care providers, and patients and their families through interviews, site visits, and focus groups.2 All respondent groups consistently described three major challenges faced by patients and caregivers: delays in and lack of coordination of care; a lack of relevant information; and inadequate attention to their emotional and social problems. These challenges were particularly acute in the period immediately after a cancer diagnosis. When asked what might help patients address these challenges, respondents often mentioned the provision of a navigator or advocate to support patients during this difficult period.
In response, many cancer care organizations have developed patient support programs involving volunteers or staff, often nurses, to help patients navigate the system. Despite the proliferation of oncology navigation programs, and the formation of a national society, there have been few rigorous evaluations of their effectiveness and only two published randomized trials that we could identify.3,4 One used lay persons and the other used nurses as navigators. Neither intervention proved to be effective. Published descriptions of oncology nurse navigator (NN) programs suggest considerable variability in approach among them.5 In this article, we describe the design and results of a cluster randomized, controlled trial evaluating the effects of NN support on the quality of life, care experience, problems and delays in care, and health care costs of patients recently given a diagnosis of breast, colorectal, and lung cancer.
PATIENTS AND METHODS
Setting
The trial was conducted in the Seattle and Bellevue service areas of Group Health (GH), an integrated, nonprofit delivery system serving 640,000 enrollees in Washington and Northern Idaho. Most GH enrollees are paneled to a primary care physician (PCP) who coordinates their care. GH provides surgical, medical, and radiation oncology services. The trial is part of the National Cancer Institute-funded Cancer Communication Research Center affiliated with the Cancer Research Network.6
PCPs and Randomization
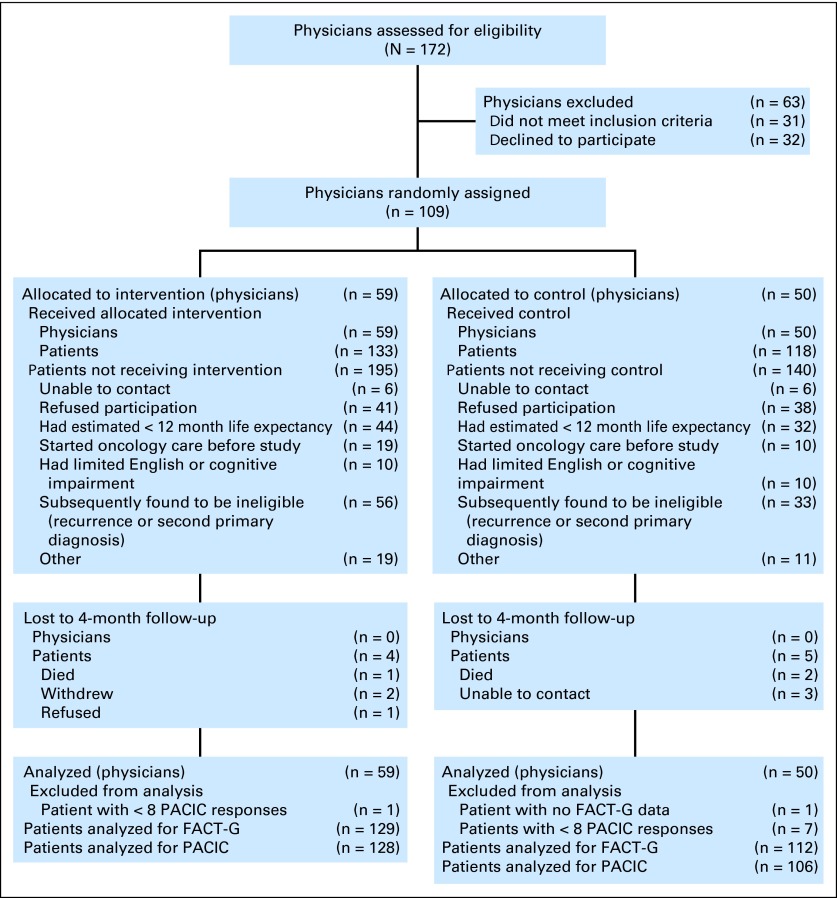
We chose cluster randomization to avoid burdening patients with newly diagnosed cancer with uncertainty about group assignment. Because essentially every GH patient with cancer has an assigned PCP, we randomly assigned PCPs. We identified 172 PCPs in the 11 Seattle and Bellevue area GH clinics, 141 of whom cared for 300 people 50 years or older, our pre-established minimum for randomization (Fig 1). Through meetings with PCPs at each clinic, we received signed consents from 109 PCPs (77%). We randomly assigned consenting PCPs within each clinic by using a computerized adaptive randomization program that sought to balance the number of patients older than age 50 between groups. Physicians were aware of their group assignment to facilitate communication with the NN.
Fig 1.
CONSORT diagram. FACT-G, Functional Assessment of Cancer Therapy–General; PACIC, Patient Assessment of Chronic Illness Care.
Patient Population
We identified potentially eligible (> 18 years) patients from computerized pathology reports by using an algorithm to identify reports suggesting malignant lesions in the breast, colon, rectum, or lung. A research assistant reviewed each suspicious report to confirm a primary cancer diagnosis. Research staff then contacted the PCP to see if the patient met eligibility criteria: was aware of the diagnosis, likely to survive at least 12 months, had not had an initial oncology visit, was not cognitively impaired, and could speak English (Fig 1). We contacted eligible patients by letter followed by a telephone call to obtain consent and collect baseline information. The consent form informed each patient to which group they were assigned. Pathology review was continuous, and patients received their initial contact usually within 1 week of diagnosis. Patients were recruited from July 2009 to September 2011.
We identified 586 patients with breast, colorectal, or lung cancers occurring among the practice panels of randomly assigned physicians. Figure 1 shows that 43% were enrolled, 13% refused to participate, and the remainder was ineligible or unable to be contacted. The major reasons for ineligibility were index cancer was a recurrence or second primary (35%), and life expectancy estimate was < 12 months (39%). Ninety (83%) randomly assigned physicians had at least one patient enrolled in the trial. The median number of patients per physician was two, and 14 (13%) had five or more patients enrolled.
Intervention
A description of the intervention has been reported previously.7 Briefly, the 4-month long intervention aimed to create a trusting relationship between the patient and the NN who served as each patient's advocate and source of support. The Institute of Medicine's model for the delivery of psychosocial health services8 and nurse interventions for patients with cancer and depression9–12 guided intervention development. Three registered nurses working in the GH delivery system each devoted 20% of their time to the intervention and actively managed an average of eight patients at any one time. The nurses received approximately 25 hours of training in empathic listening skills and behavioral strategies for assessing and addressing patients' distress, for example, through increasing empowerment, participation in rewarding activities, and actively engaging in problem-solving. Nurse training also included best practice pathways for the three cancers.
Nurses initially contacted patients within 24 hours of their enrollment onto the study, usually within 2 weeks of the date of their cancer diagnoses. In weekly nurse-initiated telephone calls, the nurses used the Distress13,14 and Fatigue Thermometers15 to identify problems and monitor progress. The nurses sought opportunities to meet in-person at least once with each patient—for example, accompanying them to a physician appointment. For patients reporting distress, the nurses assessed the sources of distress and collaborated with patients to develop goals and action plans. Action plans generally included increasing or sustaining physical or social activity, coordinating appointments and cancer care, and referrals to GH or community resources such as peer support. NN patients interacted with their nurse an average of 18 times. Nurse interactions, treatment plans, and a structured patient summary were all entered into GH's electronic medical record. The NNs generally discharged patients at the end of 16 weeks of intervention when most patients were involved in active cancer treatment.
Nurse Supervision
The nurses received weekly supervision via conference call with a clinical psychologist, oncology nurse specialist, and medical oncologist. During these calls, the nurses discussed new patients, hard to reach patients, and patients with complex psychological, clinical, or social problems.
Control Intervention
A Patient Advisory Committee provided input into study design. They recommended that patients in both intervention groups receive more tailored patient education or enhanced usual care. The committee helped develop a two-phase approach by providing patients with a brief quick start guide as well as a detailed manual.
Outcome Measures
Trained interviewers administered patient consent and collected all patient-reported data by telephone. Because the consent process identified the subject's group allocation, the interviewers were not blinded. The primary outcome measures were patient reported: the Functional Assessment of Cancer Therapy–General (FACT-G) Quality of Life measure,16 selected subscales of the Patient Assessment of Chronic Illness Care (PACIC),17 and problems in care from selected subscales of a patient experience measure designed by the Picker Institute and adapted for patients with cancer.18,19 The PACIC evaluates the extent to which care involves and activates patients, is well organized, and consistent with the patient's situation and values. Representative items are shown in the Appendix (online only). For this study, we replaced each mention of chronic illness in questions with cancer. Picker Institute items were scored as a problem in care if patients gave less than optimal responses (see Appendix). We also assessed depressive symptoms by using the Patient Health Questionnaire-920 and pain.21 Most patient-reported measures were assessed at baseline, 4 months, and 12 months. However, we asked the PACIC and Picker items at 4 and 12 months only because the questions asked directly about their cancer care.
GH automated administrative data provided measures of comorbidity, cancer stage, and the length of the interval between the date of diagnosis and the dates of key events in treatment. We also estimated the overall costs of care, employing a micro-costing approach that identified services from Current Procedural Terminology codes in GH claims data and estimated their direct costs by using the Medicare fee-for-service fee schedule (on the basis of the Resource Based Relative Value Scale). Costs included all billed services provided by GH and external providers. We examined total costs in the 3 months before through to 12 months after study enrollment. Labor costs of the intervention were derived from time logs kept by the nurses and the registered nurse wage scale at GH plus fringe benefits. We did not include training costs.
Statistical Analysis
Justification for our proposed sample size of 250 is provided in the Appendix. Our primary analysis assessed the differences in overall FACT-G, PACIC subscale and summary scores, and Picker subscales between intervention and control groups at 4 months. We created a PACIC summary score by computing the average score (from one to five) of the 10 items in the three subscales. Secondary analyses evaluated the difference in scores between groups at 12 months. We estimated the effect of randomization group on the mean overall FACT-G and PACIC scores by using linear regression. To account for nested clustering of patients within primary care providers, and longitudinal measurements within patients, we used generalized estimating equations, assuming an independence working correlation structure with a robust covariance adjustment, on the basis of residuals. Because we had relatively few patients per provider, we adjusted estimates for small cluster sizes. All analyses were intent-to-treat.
Models were adjusted for baseline characteristics of age and education (which differed between randomization groups), and baseline patient-reported outcome scores. All statistical tests were two-sided and P < .05 were considered statistically significant.
We used generalized estimating equations (with log link) to calculate adjusted total costs per patient in each study month (−3 months to +12 months of study enrollment). Separate models were estimated for each month and controlled for the same baseline covariates as in the main outcomes models. Using adjusted total costs for each patient, we then calculated the median total cost for each month in the NN and control arms, stratified by cancer type.
Study Oversight
A data safety monitoring board reviewed methods initially and outcomes every 6 months thereafter. The trial was approved by the GH Institutional Review Board.
RESULTS
Table 1 shows the characteristics of the enrolled patient population. Three quarters of participating patients had breast cancer, which accounts for the preponderance of women. The remaining patients were roughly split between colorectal and lung cancers. The mean age of patients was 62 (range, 36 to 92). The study population was 82% white and 53% were college graduates, reflective of local demographics and insurance characteristics of the GH enrollees.
Table 1.
Final Characteristics of Enrolled Sample by Randomly Assigned Group
| Characteristic | Randomly Assigned Group |
P for Difference Between Groups | |||
|---|---|---|---|---|---|
| Control |
Intervention |
||||
| No. | % | No. | % | ||
| Total No. enrolled | 118 | 133 | |||
| Age, years | |||||
| Mean | 64.4 | 60.4 | |||
| SD | 11.3 | 12.2 | |||
| Sex | .26 | ||||
| Male | 16 | 13.6 | 12 | 9.0 | |
| Female | 102 | 86.4 | 121 | 91.0 | |
| Race* | .59 | ||||
| White | 100 | 84.8 | 106 | 79.7 | |
| African American | 3 | 2.5 | 7 | 5.3 | |
| Asian | 5 | 4.2 | 10 | 7.5 | |
| American Indian/Alaska Native | 5 | 4.2 | 7 | 5.3 | |
| Native Hawaiian/Other Pacific Islander | 1 | 0.9 | 0 | 0.0 | |
| Other | 3 | 2.5 | 3 | 2.3 | |
| Hispanic† | 5 | 4.2 | 4 | 3.0 | .11 |
| Cancer site | .64 | ||||
| Breast | 87 | 73.7 | 103 | 77.4 | |
| Colorectal | 17 | 14.4 | 14 | 10.5 | |
| Lung | 14 | 11.9 | 16 | 12.0 | |
| Cancer stage (AJCC) | .54 | ||||
| 0 | 17 | 14.5 | 14 | 10.5 | |
| I | 48 | 41.0 | 50 | 37.6 | |
| II | 35 | 29.9 | 39 | 29.3 | |
| III | 13 | 11.1 | 22 | 16.5 | |
| IV | 4 | 3.4 | 8 | 6.0 | |
| Marital status | .59 | ||||
| Married | 73 | 61.9 | 78 | 58.7 | |
| Living with partner | 9 | 7.6 | 14 | 10.5 | |
| Widowed | 11 | 9.3 | 11 | 8.3 | |
| Divorced/separated | 18 | 15.3 | 16 | 12.0 | |
| Never married/single | 7 | 5.9 | 14 | 10.5 | |
| Education† | .003 | ||||
| < High school | 3 | 2.5 | 2 | 1.5 | |
| High school graduate or GED | 23 | 19.5 | 13 | 9.8 | |
| Some college | 40 | 33.9 | 34 | 25.8 | |
| College graduate | 24 | 20.3 | 56 | 42.4 | |
| Postgraduate degree | 28 | 23.7 | 27 | 20.5 | |
| No. of people living with patient | .95 | ||||
| 0 | 22 | 18.6 | 29 | 21.8 | |
| 1 | 61 | 51.7 | 65 | 48.9 | |
| 2 | 19 | 16.1 | 18 | 13.5 | |
| 3 | 10 | 8.5 | 11 | 8.3 | |
| ≥ 4 | 6 | 5.1 | 8 | 6.8 | |
| Varies | 0 | 0.0 | 1 | 0.8 | |
| Charlson comorbidity score | .06 | ||||
| 0 | 76 | 69.7 | 88 | 74.0 | |
| 1 | 10 | 9.2 | 19 | 16.0 | |
| 2 | 14 | 12.8 | 9 | 7.6 | |
| 3+ | 9 | 8.3 | 3 | 2.5 | |
| Unknown | 9 | 14 | |||
Abbreviations: AJCC, American Joint Committee on Cancer TNM staging system; GED, General Educational Development; SD, standard deviation.
Primary race reported.
One person responded “don't know.”
Baseline mean FACT-G scores for the entire cohort were somewhat higher (83 v 80) (Table 2) than normative data from samples of patients with mixed cancers as well as a random sample of US adults.16 Similarly, depressive symptoms (Patient Health Questionnaire-9 scores) were reported less commonly than in other studies of patients with cancer.22 The relatively high quality of life and low prevalence of depressive symptoms of our study population likely reflects the exclusion of individuals not expected to survive for 12 months, refusals by ill patients, and the relatively high socioeconomic status of GH enrollees.
Table 2.
Impact of Nurse Navigator Intervention on Quality of Life and Depressive Symptoms
| Mean Scores at 4 Months |
Difference in Scores Between Groups at 4 Months |
Mean Scores at 12 Months |
Difference in Scores Between Groups at 12 Months |
|||||
|---|---|---|---|---|---|---|---|---|
| Randomly Assigned Group |
Randomly Assigned Group |
|||||||
| Control | Intervention | Adjusted β | 95% CI | Control | Intervention | Adjusted β | 95% CI | |
| FACT-G surveys, No. | 112 | 129 | 107 | 122 | ||||
| Baseline scores | ||||||||
| Mean | 83.6 | 82.6 | 83.6 | 82.6 | ||||
| SD | 13.5 | 13.4 | 13.5 | 13.4 | ||||
| PHQ-9 surveys, No. | 113 | 129 | 107 | 123 | ||||
| Baseline scores | ||||||||
| Mean | 4.7 | 5.0 | 4.7 | 5.0 | ||||
| SD | 4.1 | 4.6 | 4.1 | 4.6 | ||||
| FACT-G overall* | 0.8 | −2.6 to 4.3 | 0.6 | −2.2 to 3.5 | ||||
| Mean | 87.0 | 86.7 | 90.4 | 90.8 | ||||
| SD | 15.5 | 14.1 | 11.5 | 13.8 | ||||
| Physical well-being | 0.5 | −0.9 to 1.8 | 0.3 | −0.6 to 1.2 | ||||
| Mean | 22.1 | 22.3 | 24.3 | 24.4 | ||||
| SD | 5.9 | 5.1 | 3.7 | 4.4 | ||||
| Social well-being | 2.3 | −0.8 to 1.3 | 0.1 | −1.0 to 1.2 | ||||
| Mean | 23.1 | 23.3 | 22.9 | 23.1 | ||||
| SD | 4.7 | 4.4 | 4.6 | 4.7 | ||||
| Emotional well-being | −0.2 | −1.1 to 0.6 | 0.5 | −0.1 to 1.2 | ||||
| Mean | 21.0 | 20.4 | 20.8 | 21.1 | ||||
| SD | 3.7 | 3.7 | 3.1 | 3.0 | ||||
| Functional well-being | 0.3 | −1.0 to 1.7 | −0.3 | −1.4 to 0.8 | ||||
| Mean | 20.7 | 20.7 | 22.4 | 22.3 | ||||
| SD | 5.8 | 5.2 | 4.4 | 5.2 | ||||
| PHQ-9 overall† | −0.4 | −1.4 to 0.6 | 0.1 | −0.9 to 1.1 | ||||
| Mean | 4.5 | 4.5 | 3.1 | 3.3 | ||||
| SD | 4.4 | 3.9 | 3.4 | 3.8 | ||||
Abbreviations: FACT-G, Functional Assessment of Cancer Therapy–General; PHQ-9, Patient Health Questionnaire-9, SD, standard deviation.
Adjusted for patient age, education, FACT-G baseline score, and small cluster sizes.
Adjusted for patient age, education, PHQ-9 baseline score, and small cluster sizes.
Table 2 shows that FACT-G total and subscale scores increased from baseline in both groups at 4 months and again at 12 months with no significant differences noted between NN and control groups. The summary PACIC score and all subscale scores were higher among NN patients at 4 months and 12 months (Table 3). The differences in the summary scores and problem-solving/contextual subscale were statistically significant.
Table 3.
Effect of Nurse Navigator Intervention on Quality of Care and Problems in Care
| Difference Between Groups at 4 Months* |
Difference Between Groups at 12 Months* |
|||||||
|---|---|---|---|---|---|---|---|---|
| Randomly Assigned Group |
Control |
Randomly Assigned Group |
Adjusted β | 95% CI | ||||
| Control | Intervention | Adjusted β | 95% CI | Control | Intervention | |||
| PACIC summary score* | 0.3 | 0.02 to 0.6 | 0.3 | 0.01 to 0.5 | ||||
| Mean | 3.7 | 3.9 | 3.6 | 4.0 | ||||
| SD | 1.0 | 0.8 | 1.0 | 0.8 | ||||
| Patient activation | 0.3 | −0.01 to 0.6 | 0.3 | −0.04 to 0.7 | ||||
| Mean | 3.7 | 4.0 | 3.7 | 4.0 | ||||
| SD | 1.2 | 1.0 | 1.2 | 1.0 | ||||
| Delivery system/practice design | 0.2 | −0.1 to 0.4 | 0.1 | −0.1 to 0.4 | ||||
| Mean | 3.8 | 3.9 | 3.8 | 4.0 | ||||
| SD | 1.1 | 0.8 | 1.0 | 0.9 | ||||
| Problem solving/contextual | 0.4 | 0.04 to 0.7 | 0.3 | 0.03 to 0.6 | ||||
| Mean | 3.5 | 3.9 | 3.4 | 3.9 | ||||
| SD | 1.1 | 1.0 | 1.1 | 1.0 | ||||
| Picker subscales | ||||||||
| % reporting problems at 4 months | ||||||||
| Coordination of care | −7.0 | −13.0 to −1.0 | −6.0 | −12.1 to 0.2 | ||||
| Mean | 19.7 | 14.7 | 18.5 | 12.9 | ||||
| SD | 25.8 | 20.6 | 26.6 | 18.7 | ||||
| Confidence in providers | −2.0 | −8.0 to 4.1 | −1.5 | −5.4 to 2.4 | ||||
| Mean | 7.1 | 6.2 | 4.9 | 4.1 | ||||
| SD | 22.0 | 19.8 | 14.9 | 16.4 | ||||
| Treatment information | −2.3 | −9.2 to 4.7 | NA | NA | ||||
| Mean | 18.5 | 18.5 | NA | NA | ||||
| SD | 25.9 | 25.0 | NA | NA | ||||
| Health information | −11.7 | −20.2 to −3.1 | NA | NA | ||||
| Mean | 50.7 | 40.8 | NA | NA | ||||
| SD | 34.2 | 32.8 | NA | NA | ||||
| Access to cancer care | −1.3 | −5.3 to 2.7 | NA | NA | ||||
| Mean | 6.9 | 6.1 | NA | NA | ||||
| SD | 16.5 | 17.2 | NA | NA | ||||
| Psychosocial care | −10.0 | −16.8 to −3.2 | −9.2 | −15.9 to −2.5 | ||||
| Mean | 26.6 | 17.8 | 23.6 | 14.9 | ||||
| SD | 26.0 | 21.8 | 27.5 | 24.1 | ||||
NOTE. Scores listed as “NA” could not be calculated because questions were not asked during 12-month interview.
Abbreviations: NA, not available; PACIC, Patient Assessment of Chronic Illness Care; SD, standard deviation.
Adjusted for patient age, education, and small cluster sizes.
Table 3 shows the prevalence of various problems in care reported by each intervention group. The most commonly occurring problems related to the receipt of health information, psychosocial care, and coordination of care. Compared with control patients, NN patients were significantly less likely to report problems in coordination of care and psychosocial care at both 4 and 12 months, and for health information at 4 months (not measured at 12 months).
We found no significant differences between groups in the number of days between diagnosis and first oncology visit, and onset of treatment (chemo- or radiotherapy) (data not shown). Control patients received their first surgery significantly earlier than NN patients (24 days v 30 days after diagnosis, respectively).
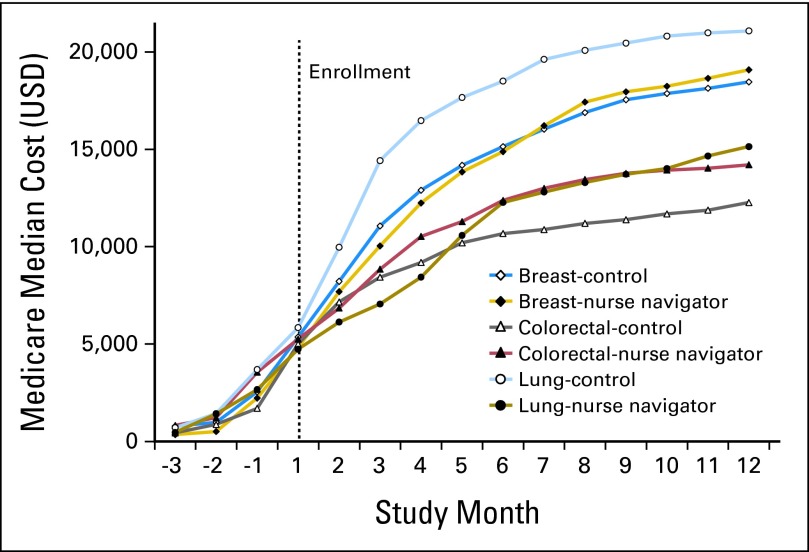
Figure 2 shows the median adjusted cumulative costs of cancer care from 3 months before the date of diagnosis through to the end of the first year after diagnosis by cancer type and intervention group. The costs shown include the costs of the intervention. Cumulative costs were nearly identical in the 3 months before study enrollment. At 12 months of follow-up, cumulative costs in the breast cancer and colorectal cancer NN groups tended to be slightly higher; however, cumulative costs in the NN arm of the lung cancer group were $6,852 lower than the control group. None of the differences in median cumulative costs between groups were statistically significant.
Fig 2.
Median cumulative costs by cancer type and study group. USD, US dollars.
DISCUSSION
Patient navigation programs were developed to address the barriers that low income individuals face gaining timely access to cancer screening and diagnostic services.23 Substantial evidence now confirms the utility of such programs.5 Professional navigators who provide support to patients with cancer after diagnosis are a more recent phenomenon.3,24 Two randomized trials have evaluated the impact of patient navigators on patients with recently diagnosed breast, lung, and colorectal cancer.3,4,25 Both studies used the FACT-G to assess quality of life. Neither study found significant differences between intervention and control patients in quality of life, and Fiscella et al4 and Hendren et al25 found no differences in satisfaction with care or time to treatment.
We too found that our NN intervention did not impact quality of life or delays in receiving care. But we did find that the intervention significantly improved patient experience with their cancer care and reduced the prevalence of problems in care. The PACIC results indicate that NN patients felt more involved in their care, more informed as to how cancer affects their life, and better prepared for the future. Significantly fewer NN patients reported problems in their care, especially in the areas of health information, care coordination, and psychosocial care. These three aspects of cancer care correspond closely to the major challenges identified in our earlier study.2 The largest differences between groups involved psychosocial care, which was the focus of our nurse training. When asked “did a doctor, nurse, or social worker go out of their way to make you feel better emotionally,” 89% of NN patients and 59% of control patients answered “definitely.”
Why didn't the improvements in care and patient experience contribute to improvements in quality of life? A recent review of the psychometric properties of the FACT-G indicates that the evidence of the responsiveness of the instrument is mixed.26 This raises the possibility that the FACT-G may not be sensitive enough to pick up the effects of psychosocial/informational interventions among relatively healthy patients with cancer.
We also assessed the total costs of cancer care for each subject from 3 months before diagnosis to 12 months after diagnosis. Although the lung cancer results are interesting, we cannot infer any difference in the cumulative total cost of care between groups because of the small sample sizes. It should be noted that the participants in the NN intervention reported significantly better experience and fewer problems with care without incurring higher costs.
What characteristics of our intervention may help explain why we saw positive effects? Our navigators were nurses who had experience with patients with cancer and were familiar with the care system in which they were working. They were trained to deal with psychosocial distress. The data point to their ability to help patients coordinate their care, provide emotional support, and answer clinical questions. This suggests the importance of a clinical background, psychosocial training, as well as local care system savvy.
Limitations of our study include the atypicality of the setting, the lack of baseline data for the PACIC and Picker questions, the random assignment of physicians rather than patients, and the limited sample size. The GH integrated system likely reduces the care coordination challenges faced by patients with cancer because providers share the same electronic medical record and often are located in the same building. However, those advantages may also have made it more difficult to find a difference between groups. The potential benefits of having an NN should increase in more fragmented settings.
Because we did not ask PACIC and Picker questions in the baseline survey, there is a possibility that NN patients were generally more satisfied with their health care. The baseline survey did include general questions about satisfaction with care that suggested no important baseline differences in patient experience; eg, “From the time it was suspected you had cancer, how satisfied are you with your health care?” Seventy-four percent of control patients and 70% of NN patients reported being very satisfied with their care (data not shown).
Cluster randomization contributed to some significant differences in the characteristics of the two groups. Hopefully, adjusting for covariates reduced any bias associated with the differences in age and education. Cluster randomization also precluded random assignment of patients within cancer type.
We found that NN support of patients with recently diagnosed breast, lung, or colorectal cancers improved patient experience and reduced problems related to psychosocial support, care coordination, and obtaining information. In comparison with control patients, NN patients reported feeling better supported emotionally, more involved in their care, better able to plan ahead, and better informed. These differences in patient experience were evident at 4 months, the end of the intervention period for NN patients, and again at 12 months. The persistence of the positive effects for 8 months after the last NN contact suggests that NN involvement did more than just buttress patients at a stressful time. It appeared to help patients develop the confidence and skills to more effectively manage their illness and its treatment. Further research will be needed to clarify how well nurse navigation works in more typical, fragmented care systems, and whether it can reduce the costs of cancer care.
Supplementary Material
Acknowledgment
We thank the superb nurse navigators: Ellen Canfield, Lynn Flaherty, and Jennifer Min; and Kathryn Horner and Eric Chen, MD, for their leadership in developing the project and supporting the intervention; and Beth Lapham, Leah Tuzzio, and Janice Miyoshi for their administrative and data collection support.
Appendix
Patient Assessment of Chronic Illness Care
Responses range from “none of the time” to “always”
“Over the past 4 months, when I received care for my cancer, I was:
- Patient activation subscale
- 1. Asked for my ideas when we made a treatment plan.
- 2. Given choice about treatment to think about.
- 3. Asked to talk about any problems with my medicines or their effects
- Delivery system/practice design subscale
- 4. Given a written list of things I should do to improve my health.
- 5. Satisfied that my care was well organized.
- 6. Shown how what I did to take care of myself influenced my condition.
- Problem solving/contextual subscale
- 12. Sure that my doctor or nurse thought about my values, beliefs, and traditions when they recommended treatment to me.
- 13. Helped to make a treatment plan that I could carry out in my daily life.
- 14. Helped to plan ahead so I could take care of my condition in hard times.
- 15. Asked how cancer affects my life.
Cancer Adaptation of the Picker Institute Survey (representative items by subscale)
- Coordination of care subscale
- How often were the doctors, nurses, and other providers who cared for you familiar with your most recent medical history?
- Confidence in providers subscale
- Do you think your doctors knew enough about cancer treatment?
- Treatment information subscale
- Were you given as much information as you wanted about the treatment options for treating your cancer?
- Health information subscale
- Did you get as much information as you wanted about your nutritional needs?
- Access to cancer care
- How often were you able to see the specialists you wanted to see?
- Psychosocial care
- Did a doctor, nurse, or social worker go out of their way to make you feel better emotionally?
Footnotes
See accompanying editorial on page 3
Supported by Grant No. P20CA137219 from the National Cancer Institute of the National Institutes of Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00921713.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Robert J. Reid, Group Health Physicians (C) Consultant or Advisory Role: None Stock Ownership: Robert J. Reid, Group Health Physicians Honoraria: None Research Funding: None Expert Testimony: None Patents: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Edward H. Wagner, Evette J. Ludman, Erin J. Aiello Bowles, Robert Penfold, Carolyn M. Rutter, Jessica Chubak, Ruth McCorkle
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Aiello Bowles EJ, Tuzzio L, Wiese CJ, et al. Understanding high-quality cancer care: A summary of expert perspectives. Cancer. 2008;112:934–942. doi: 10.1002/cncr.23250. [DOI] [PubMed] [Google Scholar]
- 2.Wagner EH, Aiello Bowles EJ, Greene SM, et al. The quality of cancer patient experience: Perspectives of patients, family members, providers and experts. Qual Saf Health Care. 2010;19:484–489. doi: 10.1136/qshc.2010.042374. [DOI] [PubMed] [Google Scholar]
- 3.Skrutkowski MSA, Eades M, Swidzinski M, et al. Impact of a pivot nurse in oncology on patients with lung or breast cancer: Symptom distress, fatigue, quality of life, and use of healthcare resources. Oncol Nurs Forum. 2008;35:948–954. doi: 10.1188/08.ONF.948-954. [DOI] [PubMed] [Google Scholar]
- 4.Fiscella K, Whitley E, Hendren S, et al. Patient navigation for breast and colorectal cancer treatment: A randomized trial. Cancer Epidemiol Biomarkers Prev. 2012;21:1673–1681. doi: 10.1158/1055-9965.EPI-12-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paskett ED, Harrop JP, Wells KJ. Patient navigation: An update on the state of the science. CA Cancer J Clin. 2011;61:237–249. doi: 10.3322/caac.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner EH, Greene SM, Hart G, et al. Building a research consortium of large health systems: The Cancer Res Network. J Natl Cancer Inst Monogr. 2005:3–11. doi: 10.1093/jncimonographs/lgi032. [DOI] [PubMed] [Google Scholar]
- 7.Horner K, Ludman EJ, McCorkle R, et al. An oncology nurse navigator program designed to eliminate gaps in early cancer care. Clin J Oncol Nurs. 2013;17:43–48. doi: 10.1188/13.CJON.43-48. [DOI] [PubMed] [Google Scholar]
- 8.Institute of Medicine. Cancer Care for the Whole Patient. Meeting Psychosocial Health Needs; Washington, DC: The National Academies Press; 2007. [PubMed] [Google Scholar]
- 9.McCorkle R, Benoliel JQ, Donaldson G, et al. A randomized clinical trial of home nursing care for lung cancer patients. Cancer. 1989;64:1375–1382. doi: 10.1002/1097-0142(19890915)64:6<1375::aid-cncr2820640634>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Given C, Given B, Rahbar M, et al. Effect of a cognitive behavioral intervention on reducing symptom severity during chemotherapy. J Clin Oncol. 2004;22:507–516. doi: 10.1200/JCO.2004.01.241. [DOI] [PubMed] [Google Scholar]
- 11.Katon WJ, Von Korff M, Lin EH, et al. The Pathways Study: A randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry. 2004;61:1042–1049. doi: 10.1001/archpsyc.61.10.1042. [DOI] [PubMed] [Google Scholar]
- 12.Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363:2611–2620. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103:1494–1502. doi: 10.1002/cncr.20940. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell AJ. Short screening tools for cancer-related distress: A review and diagnostic validity meta-analysis. J Natl Compr Canc Netw. 2010;8:487–494. doi: 10.6004/jnccn.2010.0035. [DOI] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network CRF Panel. Fort Washington, PA: National Comprehensive Cancer Network; 2005. Practice Guidelines in Oncology, volume 2: Cancer-Related Fatigue. [DOI] [PubMed] [Google Scholar]
- 16.Brucker PS, Yost K, Cashy J, et al. General population and cancer patient norms for the Functional Assessment of Cancer Therapy-General (FACT-G) Eval Health Prof. 2005;28:192–211. doi: 10.1177/0163278705275341. [DOI] [PubMed] [Google Scholar]
- 17.Glasgow RE, Wagner EH, Schaefer J, et al. Development and validation of the Patient Assessment of Chronic Illness Care (PACIC) Med Care. 2005;43:436–444. doi: 10.1097/01.mlr.0000160375.47920.8c. [DOI] [PubMed] [Google Scholar]
- 18.Ayanian JZ, Zaslavsky AM, Guadagnoli, et al. Patients' perceptions of quality of care for colorectal cancer by race, ethnicity, and language. J Clin Oncol. 2005;23:6576–6586. doi: 10.1200/JCO.2005.06.102. [DOI] [PubMed] [Google Scholar]
- 19.Malin JL, Ko C, Ayanian JZ, et al. Understanding cancer patients' experience and outcomes: Development and pilot study of the Cancer Care Outcomes Research and Surveillance patient survey. Support Care Cancer. 2006;14:837–848. doi: 10.1007/s00520-005-0902-8. [DOI] [PubMed] [Google Scholar]
- 20.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Von Korff MOJ, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50:133–149. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell AJ, Chan M, Bhatti H, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: A meta-analysis of 94 interview-based studies. Lancet Oncol. 2011;12:160–174. doi: 10.1016/S1470-2045(11)70002-X. [DOI] [PubMed] [Google Scholar]
- 23.Freeman HP. The origin, evolution, and principles of patient navigation. Cancer Epidemiol Biomarkers Prev. 2012;21:1614–1617. doi: 10.1158/1055-9965.EPI-12-0982. [DOI] [PubMed] [Google Scholar]
- 24.Fillion L, Cook S, Veillette AM, et al. Professional navigation: A comparative study of two Canadian models. Can Oncol Nurs J. 2012;22:257–277. doi: 10.5737/1181912x224257266. [DOI] [PubMed] [Google Scholar]
- 25.Hendren S, Griggs JJ, Epstein R, et al. Randomized controlled trial of patient navigation for newly diagnosed cancer patients: Effects on quality of life. Cancer Epidemiol Biomarkers Prev. 2012;21:1682–1690. doi: 10.1158/1055-9965.EPI-12-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luckett TKM, Butow PN, Oguchi M, et al. Choosing between the EORTC QLQ-C30 and FACT-G for measuring health-related quality of life in cancer clinical research: Issues, evidence and recommendations. Ann Oncol. 2011;22:2179–2190. doi: 10.1093/annonc/mdq721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.