Abstract
Purpose
Aurora A kinase (AAK) is overexpressed in aggressive lymphomas and can correlate with more histologically aggressive forms of disease. We therefore designed a phase II study of alisertib, a selective AAK inhibitor, in patients with relapsed and refractory aggressive non-Hodgkin lymphomas.
Patients and Methods
Patients age ≥ 18 years were eligible if they had relapsed or refractory diffuse large B-cell lymphoma (DLBCL), mantle-cell lymphoma (MCL), transformed follicular lymphoma, Burkitt's lymphoma, or noncutaneous T-cell lymphoma. Alisertib was administered orally at 50 mg twice daily for 7 days in 21-day cycles.
Results
We enrolled 48 patients. Histologies included DLBCL (n = 21), MCL (n = 13), peripheral T-cell lymphoma (n = 8), transformed follicular lymphoma (n = 5), and Burkitt's (n = 1). Most common grade 3 to 4 adverse events were neutropenia (63%), leukopenia (54%), anemia (35%), thrombocytopenia (33%), stomatitis (15%), febrile neutropenia (13%), and fatigue (6%). Four deaths during the study were attributed to progressive non-Hodgkin lymphoma (n = 2), treatment-related sepsis (n = 1), and unknown cause (n = 1). The overall response rate was 27%, including responses in three of 21 patients with DLBCL, three of 13 with MCL, one of one with Burkitt's lymphoma, two of five with transformed follicular lymphoma, and four of eight with noncutaneous T-cell lymphoma. The alisertib steady-state trough concentration (n = 25) revealed the expected pharmacokinetic variability, with a trend for higher incidence of adverse event–related dose reductions at higher trough concentrations. Analysis for AAK gene amplification and total AAK protein revealed no differences between histologies or correlation with clinical response.
Conclusion
The novel AAK inhibitor alisertib seems clinically active in both B- and T-cell aggressive lymphomas. On the basis of these results, confirmatory single-agent and combination studies have been initiated.
INTRODUCTION
Despite significant improvements in outcome for patients with diffuse large B-cell lymphoma (DLBCL) with the incorporation of rituximab into induction therapy,1 most patients with relapsed or refractory DLBCL still succumb to their disease. Indeed, the ability to salvage patients with DLBCL refractory to rituximab is quite limited.2 In addition, most patients with mantle-cell lymphoma (MCL) and T-cell lymphoma remain incurable, and therefore, novel therapies are urgently needed for these groups of patients.
Aurora A kinase (AAK) is a member of the aurora kinase family and is expressed in all actively dividing cells. Several groups have reported increased AAK expression in aggressive lymphomas, as compared with normal B cells.3–6 Analysis of the Lymphoma/Leukemia Molecular Profiling Project database indicates degree of aurora kinase overexpression correlates with inferior prognosis in MCL.7 Forced overexpression of AAK in experimental model systems can result in transformation of normal cells, suggesting an important oncogenic role of this protein.
Alisertib (MLN8237; Millennium Pharmaceuticals, Cambridge, MA) is a selective, competitive, and reversible small-molecule inhibitor of AAK. Treatment of malignant cells with this inhibitor results in abnormal mitotic spindle formation, accumulation of mitotic cells, and decrease of tumor-cell proliferation, as would be expected with AAK inhibition.8 In a murine model of aggressive DLBCL, alisertib resulted in significant tumor growth inhibition.9 Alisertib has led directly to antiproliferation, polyploidy by endoreduplication, and direct apoptosis in cell-line studies of aggressive lymphomas.7 Two recently published phase I trials have tested this agent in advanced solid tumors, recommending a phase II dose of 50 mg twice daily for 7 days in 21-day cycles.10,11 Both of these studies demonstrated bioavailability of oral formulations, with modest antitumor activity.
We therefore designed a phase II study of single-agent alisertib in patients with relapsed or refractory aggressive non-Hodgkin lymphoma (NHL). The results detailed here indicate the tolerability and clinical activity of this regimen in a variety of aggressive lymphoma histologies.
PATIENTS AND METHODS
Study Design and Objectives
The primary end point of this multicenter phase II trial was response rate in patients with relapsed or refractory aggressive NHL after administration of alisertib. Secondary end points included safety, duration of response, progression-free survival (PFS), and correlative pharmacokinetic and pharmacodynamic exploratory studies. Institutional review boards approved the protocol at all participating sites, and informed written consent was obtained from all patients before enrollment. The study was registered before enrollment of patients.
Key Eligibility Criteria
Patients age ≥ 18 years were eligible if they had a diagnosis (WHO classification) of DLBCL, MCL, transformed follicular lymphoma with ≥ 50% diffuse large-cell component, Burkitt's lymphoma, precursor B-lymphoblastic leukemia/lymphoma, or T-cell lymphoma (excluding primary cutaneous T-cell lymphoma).12 Patients must have received at least one prior chemotherapy regimen for lymphoma and have measurable disease. Baseline laboratory parameters included absolute neutrophil count ≥ 1,250 cells/μL, platelet count ≥ 75,000 cells/μL, and adequate renal and hepatic function. Patients with other malignancies requiring treatment, active CNS lymphoma, HIV positivity, or other active infection were excluded.
Baseline Evaluation and Treatment
Baseline evaluation included history and physical examination, positron emission tomography and computed tomography scans, routine laboratory studies, bone marrow evaluation, and ECG. Treatment cycles were 21 days, and initially, up to 1 year of therapy was allowed; a subsequent amendment to the protocol allowed for continuous therapy until progression. Alisertib (supplied by Millennium Pharmaceuticals) was orally administered at a dose of 50 mg every 12 hours with one cup of water for 7 consecutive days. Patients were instructed to take nothing by mouth except water and prescribed medications for 2 hours before and 1 hour after each dose.
To begin a cycle of therapy, patients must have had absolute neutrophil count ≥ 1,000/μL and platelet count ≥ 75,000/μL, unless cytopenias had resulted from bone marrow infiltration. In addition, any toxicity related to alisertib (except alopecia) must have resolved to grade ≤ 1 as determined by Common Terminology Criteria for Adverse Events (version 3.0). Delays up to 1 week were allowed; if therapy was delayed between 1 and 2 weeks, dose adjustment for subsequent cycles was mandated. If treatment was delayed > 2 weeks, patients were removed from the protocol.
Dose adjustments were also mandated for grade 4 neutropenia or thrombocytopenia lasting > 7 days, neutropenic fever, grade 3 thrombocytopenia with clinically significant bleeding, or platelet count < 10,000/μL at any time. Any grade ≥ 3 nonhematologic toxicity related to the drug, except arthralgias/myalgias, nausea, diarrhea, or fatigue, mandated dose adjustments. For these events, doses were adjusted to 40 mg every 12 hours after the first episode and to 30 mg every 12 hours after a second episode. Growth factor support was only permitted after either a cycle delay or neutropenic fever. Concomitant therapy with proton-pump inhibitors was not permitted, and use of benzodiazepines during therapy was discouraged because of pharmacologic similarity to alisertib. For diarrhea, patients were instructed to take loperamide at occurrence of the first loose stool, then every 2 hours until diarrhea free.
Criteria for Response and Toxicity
Assessment of response was performed with computed tomography scans between days 14 and 21 of even-numbered cycles, and positron emission tomography scans were performed after cycles two and six. Bone marrow biopsy was performed only if needed to confirm a complete response (CR). Response was defined using the International Working Group NHL criteria.13 National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0) were used to grade toxicities.
PFS was calculated from the first dose of study drug to the first documentation of disease progression, death regardless of cause, or change in therapy as a result of disease progression, whichever occurred first. Patients who were alive and progression free at the time of final data analysis were censored at the time of last assessment. If disease progression did not occur by the end of treatment, patients were evaluated every 3 months until progression with physical examination, laboratory, and imaging studies.
Pharmacokinetics
Blood samples were collected from patients on day 8 of cycle one for measurement of alisertib trough plasma concentrations. Plasma concentrations of alisertib were measured at Tandem Labs (West Trenton, NJ) using a solid-phase extraction from human plasma (K2EDTA) samples, followed by quantitative analysis using liquid chromatography with tandem mass spectral detection.
Chromatography consisted of gradient separation across a Sunfire C8 2.1- × 50-mm, 5-[um]m analytic column (Waters, Milford, MA). Mass spectrometric detection was performed using an API 4000 Sciex triple quadruple mass spectrometer (AB Sciex, Framingham, MA).
Measurement of AAK Expression and Copy Number in Archived Tumor Tissue Samples
Banked tumor tissue samples (submitted slides or formalin-fixed paraffin-embedded blocks) were evaluated for AAK and phosphohistone H3 (Ser10; marker of mitotic cells) protein expression by semiquantitative immunohistochemistry (IHC) at Mosaic Laboratories (Lake Forest, CA) and for AAK gene copy number by fluorescence in situ hybridization (FISH) at PhenoPath Laboratories (Seattle, WA). The AAK antibody was purchased from Novocastra Laboratories (Newcastle on Tyne, United Kingdom), and staining was developed using Vulcan Red chromogen (Biocare Medical, Concord, CA). The phosphohistone H3 antibody was purchased from Upstate (Billerica, MA). AAK copy number in parrafin tumor sections was validated using a dual-probe FISH assay (Kreatech Diagnostics, Amsterdam, the Netherlands) containing an AAK-specific probe (20q13) and a control probe to chromosome 20q11.
Cytokine Evaluation
We used the RayBio Human Cytokine Antibody Array G Series 2000 kit (Norcross, GA) with accessories for simultaneous detection of 174 cytokines in eight samples on three different slides. Serum was collected on days 1 (pretreatment) and 8 (post-treatment). Fluorescence was detected with a GenePix scanner (Axon, Sunnyvale, CA) using the Cy3 channel. Signal intensity was obtained using the GenePix scanner and scanning software.
Statistical Methods
The trial used a Simon optimal two-stage design. In the first stage, 21 evaluable patients were enrolled, and with ≥ two observed responses, 20 additional evaluable patients were enrolled in the second stage. Sample size was determined with a one-sided test at significance of α = 0.05, power of 90%, null hypothesis of response rate ≤ 5%, and alternative hypothesis of response rate ≥ 20%. Statistical analysis was primarily descriptive and graphical in nature. Summary tabulations are presented displaying number of observations; mean, standard deviation, median, minimum, and maximum for continuous variables; and number and percentage per category for categorical data. Time-to-event data were analyzed using the Kaplan-Meier life test method, and results were summarized by 25th, 50th (median), and 75th percentiles, with associated two-sided 95% CIs as well as percentage of censored observations.
RESULTS
Patient Characteristics
Between February 2009 and August 2010, 48 patients were enrolled. Baseline clinical characteristics and prior therapies are listed in Table 1. Seventy-three percent were men, and median age was 68 years. Histologies included DLBCL (n = 21), MCL (n = 13), transformed follicular lymphoma (n = 5), T-cell lymphoma (n = 8), and Burkitt's lymphoma (n = 1). The median number of prior therapies was three, and these included high-dose chemotherapy and autologous stem-cell transplantation (n = 11), platinum-containing salvage regimens (n = 31), and bendamustine (n = 7).
Table 1.
Baseline Patient Demographic and Clinical Characteristics (N = 48)
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| Median | 67.5 | |
| Range | 32 to 85 | |
| Female sex | 13 | 27 |
| White race | 47 | 98 |
| Weight, kg | ||
| Median | 86 | |
| Range | 42 to 175 | |
| ECOG performance status | ||
| 0 | 35 | |
| 1 | 52 | |
| 2 | 13 | |
| Ann Arbor disease stage | ||
| II | 1 | 2 |
| III | 12 | 25 |
| IV | 23 | 48 |
| B-cell lymphoma histology | ||
| DLBCL | 21 | 44 |
| MCL | 13 | 27 |
| Transformed FL | 5 | 10 |
| Burkitt's lymphoma | 1 | 2 |
| Aggressive T-cell lymphoma | 8 | 17 |
| No. of prior regimens | ||
| Median | 3 | |
| Range | 1 to 9 | |
| ≥ Three prior lines of therapy | 26 | 54 |
| Prior radiation therapy | 17 | 35 |
| Second-line regimen type | ||
| ASCT | 11 | 23 |
| Platinum containing | 31 | 65 |
| Bendamustine | 7 | 15 |
Abbreviations: ASCT, autologous stem-cell transplantation; DLBCL, diffuse large B-cell lymphoma; ECOG, Eastern Cooperative Oncology Group; FL, follicular lymphoma; MCL, mantle-cell lymphoma.
Safety
Adverse events (AEs) are listed in Table 2. Common drug-related grade ≥ 3 AEs included neutropenia (63%), leukopenia (54%), anemia (35%), thrombocytopenia (33%), stomatitis (15%), and fatigue (6%). There were six cases of febrile neutropenia that were considered treatment related. Four patients died during cycle one of the study, two as a result of rapidly progressive DLBCL; there was one septic event related to study drug in a patient with DLBCL on day 14, and one patient with T-cell lymphoma and known cardiac history experienced sudden death (cause unknown).
Table 2.
Drug-Related AEs ≥ 10% (all grade) or Grade ≥ 3 in ≥ Two Patients (N = 48)
| AE | Any Grade |
Grade ≥ 3* |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Any drug-related AE | 48 | 100 | 44 | 92 |
| Neutropenia | 38 | 79 | 30 | 63 |
| Leukopenia | 34 | 71 | 26 | 54 |
| Anemia | 29 | 60 | 17 | 35 |
| Fatigue | 28 | 58 | 3 | 6 |
| Diarrhea | 27 | 56 | 1 | 2 |
| Thrombocytopenia | 24 | 50 | 16 | 33 |
| Alopecia | 23 | 48 | — | — |
| Somnolence | 21 | 44 | 1 | 2 |
| Stomatitis | 17 | 35 | 7 | 15 |
| Nausea | 13 | 27 | 1 | 2 |
| Vomiting | 9 | 19 | — | — |
| Asthenia | 9 | 19 | 1 | 2 |
| Decreased appetite | 8 | 17 | 1 | 2 |
| Dehydration | 7 | 15 | 2 | 4 |
| Febrile neutropenia | 6 | 13 | 6 | 13 |
| Dizziness | 6 | 13 | — | — |
| Memory impairment | 6 | 13 | — | — |
| Insomnia | 6 | 13 | — | — |
| Abdominal pain | 5 | 10 | — | — |
| Pyrexia | 5 | 10 | — | — |
| Confusion | 5 | 10 | 1 | 2 |
| Pneumonia | 4 | 8 | 3 | 6 |
| Pancytopenia | 3 | 6 | 3 | 6 |
| Lymphopenia | 2 | 4 | 2 | 4 |
Abbreviation: AE, adverse event.
Grade ≥ 3 AEs occurring in one patient each (2%) and not included in table were: ischaemic colitis, dysphagia, decreased ejection fraction, activated partial thromboplastin time prolonged, multiorgan failure, mucosal hemorrhage, oropharyngeal candidiasis, sepsis, syncope, intracranial hemorrhage, balance disorder, acidosis, dyspnea epistaxis, atrial fibrillation, and rhabdomyolysis.
Alopecia occurred in approximately half of patients. Other common grade 1 and 2 drug-related toxicities included somnolence, nausea and vomiting, and fever. Twenty-five patients required dose reduction to 40 mg twice daily; in 17 of these patients, the dose reduction occurred in cycle two, as a result of delayed recovery or AE during cycle one. Six patients required a second dose reduction to 30 mg twice daily. One patient with angioimmunoblastic T-cell NHL developed myelodysplastic syndrome after 16 months of alisertib treatment; previous therapy included ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine), ICE (ifosfamide, carboplatin, etoposide), and cyclosporine.
Efficacy
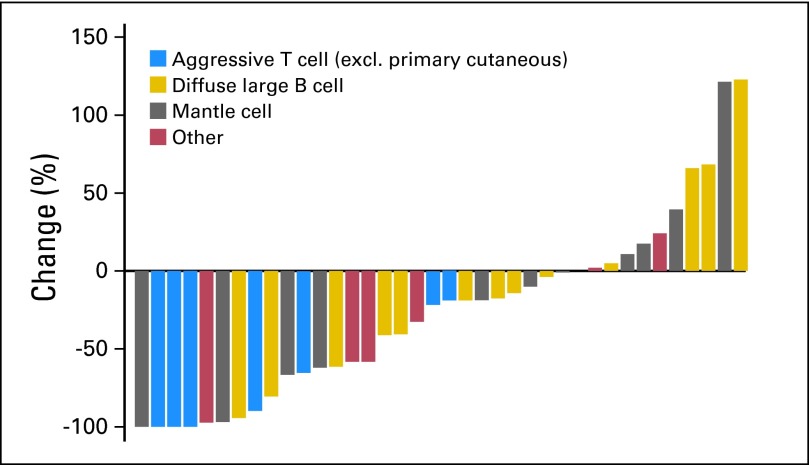
At the end of the first stage, five of 21 patients experienced a response, including one CR and four partial responses (PRs), which met the criteria for continuing the trial. The overall response rate for all treated patients (CR plus PR) was 27% (13 of 48 patients). In all 48 patients, this included 10% CRs and 17% PRs; 33% of patients achieved stable disease. The adjusted median response rate (for 13 of 41 patients) was 22% (95% CI, 8% to 38%).14 Response rates by histology were: DLBCL, three of 21 patients; MCL, three of 13; Burkitt's lymphoma, one of one; transformed follicular lymphoma, two of five; and T-cell lymphoma, four of eight. Seven patients were not evaluable for response because of early death (n = 2), early protocol withdrawal as a result of AE or patient choice (n = 4), and inadequate imaging to assess response (n = 1). Response rate was 32% for the response-evaluable population only (Fig 1).
Fig 1.
Waterfall plot of responses by histology. excl., excluding.
Kaplan-Meier estimates of PFS for all patients and for patients with T-cell lymphoma are shown in Figure 2. Duration of response is shown in Figure 3. Three patients with T-cell lymphoma—two CRs and one PR—continued therapy beyond 1 year. One patient (ALK-negative anaplastic large-cell lymphoma) received > 40 cycles of study drug.
Fig 2.
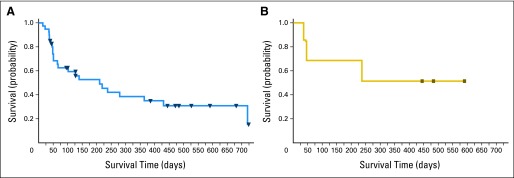
Progression-free survival in (A) all response-evaluable patients and (B) patients with aggressive T-cell lymphoma.
Fig 3.
Duration of response by histology. excl., excluding.
Pharmacokinetics and AE-Related Dose Reduction
Steady-state trough alisertib concentration was determined in cycle one for 25 patients. The geometric mean of alisertib steady-state trough concentration was 1.8 μmol/L (coefficient of variation [CV], 47%; n = 25), which was above the 1-μmol/L steady-state plasma concentration associated with saturating levels and antitumor activity in preclinical xenograft models. A trend was observed (Figs 4A and B) for a higher incidence of AE-related dose reductions in cycle two, with higher trough concentrations (geometric mean, 2,375 nmol/L [CV, 54%; n = 10] v 1,504 nmol/L [CV, 35%; n = 15]).
Fig 4.
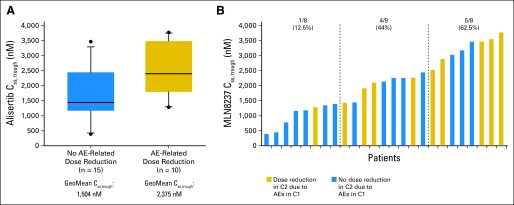
Correlation of trough concentrations of alisertib with adverse events (AEs). (A) Box plots of steady-state trough concentrations (Css, trough) by AE-related dose reductions. (B) Css, trough versus AE-related dose reductions, indicating increased frequency of dose reductions were required in patients with the highest trough concentrations of alisertib. C1, cycle one; C2, cycle two; GeoMean, geometric mean.
Correlative Studies
To determine whether AAK expression in tumors affected response to therapy, FISH analysis for AAK gene amplification was performed on baseline samples (n = 35) and did not reveal differences among histologies. When total AAK protein was evaluated by IHC, marked variability, in both the proportion of tumor cells expressing AAK as well as the intensity of protein staining, was observed; there was no correlation between AAK protein expression or intensity and clinical response.
As an exploratory pharmacodynamic assessment, cytokine profiles were determined on days 1 and 8 in 16 patients. Of the 174 nonredundant cytokines, only three cytokines were significantly elevated on day 8 in all 16 patients: FLT3L, eotaxin-2, and GRO (CXCL1). In contrast, six cytokines were decreased: MMP-9, SDF-1, TRAIL-R3, HGF, TECK, and VEGF-D. Five cytokines were significantly elevated only in patients who responded to therapy: eotaxin-2, b-NGF, M-CSFR, PDGFR, and IL-21R, whereas six cytokines were decreased in patients who responded to therapy: MMP-9, EGF, SDF-1, activin A, amphiregulin, and TGF3. Statistics were not applied to these observations because of the small sample size and exploratory nature of the work.
DISCUSSION
In this multicenter study, we demonstrated clinical activity of alisertib in a variety of aggressive B- and T-cell histologies of lymphoma. Overall, the agent was generally well tolerated, with the most common toxicities being cytopenias. The toxicity profile was similar to that reported in early-phase trials of patients with solid tumors, in which neutropenia and stomatitis were the most commonly observed toxicities with a similar dose and schedule.10,11,15 Half of patients in our study required dose reductions, and our limited pharmacokinetic analyses suggested higher drug trough levels in these patients. Hematopoietic growth factors were not permitted during cycle one of our study, and dose-intensity might have been better maintained if prophylactic growth factors had been used. Despite this, some patients in our study were able to tolerate therapy with alisertib > 1 year. However, patients receiving prolonged dosing with alisertib should be carefully observed; a study of AAK knockout mice suggests a high risk of hematologic neoplasm development.16 In this regard, it is notable that a single heavily pretreated patient developed myelodysplastic syndrome while receiving alisertib, although a causal relationship of the event with alisertib or any specific prior therapy was not possible to ascertain. Although no other patients have reported this event in the compound database, with > 800 patients administered alisertib, additional follow-up will be required.
In HTLV-1–infected T cells, accumulation of AAK results in increased nuclear factor (NF) –κB activity, increasing growth and survival in vitro.17 Additional laboratory studies have demonstrated sensitivity of gamma-delta T-cell lymphoma, natural killer–cell leukemia/lymphoma, and HTLV-associated T-cell lymphoma to alisertib.18–20 Such in vitro findings are consistent with our clinical observation of alisertib activity in these diseases. On the basis of this activity, SWOG and the US Intergroup are accruing patients with relapsed/refractory noncutaneous T-cell NHL to a study of single-agent alisertib (clinicaltrials.gov NCT01466881). In addition, there is a global randomized phase III study comparing alisertib with investigator-choice therapy in a similar patient population (clinicaltrials.gov NCT01482962).
Romidepsin, a histone deacytelase inhibitor, was recently approved for the treatment of relapsed peripheral T-cell lymphoma based on a pivotal trial demonstrating a response rate of 25%, with some durable responses.21 Laboratory studies suggest that histone deacetylase inhibition of lymphoma cells can lead to transcriptional and post-transcriptional changes that sensitize such cells to mitosis-specific agents such as AAK inhibitors.22 These studies suggest that alisertib in combination with romidepsin in T-cell lymphoma may be a rational combination for future study, if the overlapping toxicities can be managed.
In our study, activity was also observed in aggressive B-cell lymphoma. AAK has been shown to phosphorylate I-κB, an antagonist of NF-κB.23 Because increased activity of NF-κB has been shown in activated B-cell DLBCL to possibly affect chemotherapy resistance, it is interesting to hypothesize that AAK inhibition may be particularly relevant in this high-risk subgroup of patients.24 Moreover, aurora kinases are upregulated by MYC and seem to be required for maintenance of MYC-driven B-cell lymphoma. Increased levels of aurora kinases A and B paralleled levels of MYC transcripts in studies of pediatric Burkitt's lymphoma, and increased AAK expression is recognized as a hallmark of MYC-driven B-cell lymphoma.25 Both in vitro and murine studies have suggested that MYC-driven lymphoma cells are highly susceptible to AAK and aurora B inhibition.25,26 Taken together, AAK inhibitors may be of particular benefit in MYC-driven DLBCL, which represents perhaps the largest unmet clinical need in aggressive lymphoma.27–29 In our study, one patient with Burkitt's lymphoma and one with transformed follicular lymphoma with double-hit IHC features responded to treatment. Future studies should evaluate MYC as a candidate biomarker of response to AAK inhibition.
In B-cell NHL, preclinical data suggest several rational combinations to pursue with alisertib. Alisertib as a single agent had only modest activity in a mouse xenograft model of MCL; however, synergy was observed when combined with another microtubule targeting agent—docetaxel.7 Subsequent in vitro and in vivo laboratory studies in hematopoietic neoplasms demonstrated synergy when AAK inhibitors were combined with vincristine,30–32 and there are promising preclinical data on combining alisertib with rituximab.33 On the basis of these studies, a phase I/II study combining alisertib with vincristine and rituximab in a variety of B-cell lymphoma histologies is currently enrolling patients (clinicaltrials.gov NCT01397825).
Marked heterogeneity in AAK gene expression and amplification were present in tumor samples from our patients, and we were not able to demonstrate a correlation between AAK expression and clinical benefit from alisertib. One preclinical study has suggested that inhibition of AAK may act positively or negatively during tumor development in a p53-dependent manner.34 Future clinical trials should explore whether underlying p53 status of the tumor may predict response to alisertib.
In conclusion, data from this phase II study suggest that alisertib is clinically active in both B- and T-cell aggressive lymphomas, presenting an opportunity for a novel therapeutic strategy of AAK inhibition in these indications. On the basis of these results, several confirmatory single-agent and combination studies have been initiated using alisertib and other inhibitors of aurora kinases in lymphoma.16
Acknowledgment
We thank the following clinicians for their efforts in enrolling patients onto this study: James Lee, MD (Hematology Oncology Associates of South Jersey, Mount Holly, NJ), Jeffrey Neidhardt, MD (San Juan Oncology Associates, Farmington, NM), Robert Rifkin, MD (Rocky Mountain Cancer Centers, Denver, CO), Ali Khojasteh, MD (Columbia Comprehensive Cancer Center, Jefferson City, MO), Jeffrey Letzer, DO (Kalamazoo Hematology and Oncology, Kalamazoo, MI), Luis Meza, MD (Southwest Oncology Associates, Lafayette, LA), Swaminathan Padmanabhan, MD (University of Texas Science Center at San Antonio, San Antonio, TX), Peter Rosen, MD (Tower Cancer Research Foundation, Beverly Hills, CA), and Michael Scola, MD (Hematology Oncology Associates of Northern New Jersey, Morristown, NJ). We also thank the patients who participated in this study and their families.
Footnotes
See accompanying article on page 57
Supported by Millennium Pharmaceuticals; by Rochester-Arizona SPORE Grant No. CA-130805 in Lymphoma (J.W.F., D.M., E.C., D.P., A.G.A.); and by a Scholar in Clinical Research grant from the Leukemia and Lymphoma Society (J.W.F.).
Presented in part at the 11th International Congress on Malignant Lymphoma, Lugano, Switzerland, June 15-18, 2011, and 53rd Annual Meeting of the American Society of Hematology, San Diego, CA, December 10-13, 2011.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00807495.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: E. Jane Leonard, Millennium Pharmaceuticals (C); Hadi Danaee, Millennium Pharmaceuticals (C); Xiaofei Zhou, Millennium Pharmaceuticals (C); Howard Fingert, Millennium Pharmaceuticals (C) Consultant or Advisory Role: None Stock Ownership: E. Jane Leonard, Takeda Pharmaceuticals; Hadi Danaee, Takeda Pharmaceuticals Honoraria: Steven H. Bernstein, Millennium Pharmaceuticals Research Funding: Jonathan W. Friedberg, Millennium Pharmaceuticals; Daruka Mahadevan, Millennium Pharmaceuticals; Steven H. Bernstein, Millennium Pharmaceuticals Expert Testimony: None Patents: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Jonathan W. Friedberg, Daruka Mahadevan, E. Jane Leonard, Howard Fingert, Steven H. Bernstein
Provision of study materials or patients: Jonathan W. Friedberg, Daniel Persky, Izidore S. Lossos
Collection and assembly of data: Jonathan W. Friedberg, Daruka Mahadevan, Erin Cebula, Amit G. Agarwal, E. Jane Leonard, Hadi Danaee, Xiaofei Zhou, Steven H. Bernstein
Data analysis and interpretation: Jonathan W. Friedberg, Daruka Mahadevan, JungAh Jung, Daniel Persky, Izidore S. Lossos, Amit G. Agarwal, Richard Burack, E. Jane Leonard, Xiaofei Zhou, Steven H. Bernstein
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027–5033. doi: 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 2.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ikezoe T, Takeuchi T, Yang J, et al. Analysis of aurora B kinase in non-Hodgkin lymphoma. Lab Invest. 2009;89:1364–1373. doi: 10.1038/labinvest.2009.106. [DOI] [PubMed] [Google Scholar]
- 4.Camacho E, Beà S, Salaverria I, et al. Analysis of aurora-A and hMPS1 mitotic kinases in mantle cell lymphoma. Int J Cancer. 2006;118:357–363. doi: 10.1002/ijc.21370. [DOI] [PubMed] [Google Scholar]
- 5.Yakushijin Y, Hamada M, Yasukawa M. The expression of the aurora-A gene and its significance with tumorgenesis in non-Hodgkin's lymphoma. Leuk Lymphoma. 2004;45:1741–1746. doi: 10.1080/10428190410001683615. [DOI] [PubMed] [Google Scholar]
- 6.Hamada M, Yakushijin Y, Ohtsuka M, et al. Aurora2/BTAK/STK15 is involved in cell cycle checkpoint and cell survival of aggressive non-Hodgkin's lymphoma. Br J Haematol. 2003;121:439–447. doi: 10.1046/j.1365-2141.2003.04311.x. [DOI] [PubMed] [Google Scholar]
- 7.Qi W, Cooke LS, Liu X, et al. Aurora inhibitor MLN8237 in combination with docetaxel enhances apoptosis and anti-tumor activity in mantle cell lymphoma. Biochem Pharmacol. 2011;81:881–890. doi: 10.1016/j.bcp.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asteriti IA, Giubettini M, Lavia P, et al. Aurora-A inactivation causes mitotic spindle pole fragmentation by unbalancing microtubule-generated forces. Mol Cancer. 2011;10:131. doi: 10.1186/1476-4598-10-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manfredi MG, Ecsedy JA, Chakravarty A, et al. Characterization of alisertib (MLN8237), an investigational small-molecule inhibitor of aurora A kinase using novel in vivo pharmacodynamic assays. Clin Cancer Res. 2011;17:7614–7624. doi: 10.1158/1078-0432.CCR-11-1536. [DOI] [PubMed] [Google Scholar]
- 10.Cervantes A, Elez E, Roda D, et al. Phase I pharmacokinetic/pharmacodynamic study of MLN8237, an investigational, oral, selective aurora A kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2012;18:4764–4774. doi: 10.1158/1078-0432.CCR-12-0571. [DOI] [PubMed] [Google Scholar]
- 11.Dees EC, Cohen RB, von Mehren M, et al. Phase I study of aurora A kinase inhibitor MLN8237 in advanced solid tumors: Safety, pharmacokinetics, pharmacodynamics, and bioavailability of two oral formulations. Clin Cancer Res. 2012;18:4775–4784. doi: 10.1158/1078-0432.CCR-12-0589. [DOI] [PubMed] [Google Scholar]
- 12.Harris NL, Swerdlow S, Campo E, et al. The World Health Organization classification of lymphoid neoplasms: What's new? Ann Oncol. 2008;19:iv119. [Google Scholar]
- 13.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 14.Koyama T, Chen H. Proper inference from Simon's two-stage designs. Stat Med. 2008;27:3145–3154. doi: 10.1002/sim.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matulonis UA, Sharma S, Ghamande S, et al. Phase II study of MLN8237 (alisertib), an investigational aurora A kinase inhibitor, in patients with platinum-resistant or -refractory epithelial ovarian, fallopian tube, or primary peritoneal carcinoma. Gynecol Oncol. 2012;127:63–69. doi: 10.1016/j.ygyno.2012.06.040. [DOI] [PubMed] [Google Scholar]
- 16.Farag SS. The potential role of aurora kinase inhibitors in haematological malignancies. Br J Haematol. 2011;155:561–579. doi: 10.1111/j.1365-2141.2011.08898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomita M, Toyota M, Ishikawa C, et al. Overexpression of aurora A by loss of CHFR gene expression increases the growth and survival of HTLV-1-infected T cells through enhanced NF-kappaB activity. Int J Cancer. 2009;124:2607–2615. doi: 10.1002/ijc.24257. [DOI] [PubMed] [Google Scholar]
- 18.Tomita M, Mori N. Aurora A selective inhibitor MLN8237 suppresses the growth and survival of HTLV-1-infected T-cells in vitro. Cancer Sci. 2010;101:1204–1211. doi: 10.1111/j.1349-7006.2010.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maris JM, Morton CL, Gorlick R, et al. Initial testing of the aurora kinase A inhibitor MLN8237 by the Pediatric Preclinical Testing Program (PPTP) Pediatr Blood Cancer. 2010;55:26–34. doi: 10.1002/pbc.22430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iqbal J, Weisenburger DD, Chowdhury A, et al. Natural killer cell lymphoma shares strikingly similar molecular features with a group of non-hepatosplenic gammadelta T-cell lymphoma and is highly sensitive to a novel aurora kinase A inhibitor in vitro. Leukemia. 2011;25:348–358. doi: 10.1038/leu.2010.255. [DOI] [PubMed] [Google Scholar]
- 21.Coiffier B, Pro B, Prince HM, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J ClinOncol. 2012;30:631–636. doi: 10.1200/JCO.2011.37.4223. [DOI] [PubMed] [Google Scholar]
- 22.Kretzner L, Scuto A, Dino PM, et al. Combining histone deacetylase inhibitor vorinostat with aurora kinase inhibitors enhances lymphoma cell killing with repression of c-Myc, hTERT, and microRNA levels. Cancer Res. 2011;71:3912–3920. doi: 10.1158/0008-5472.CAN-10-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun C, Chan F, Briassouli P, et al. Aurora kinase inhibition downregulates NF-kappaB and sensitises tumour cells to chemotherapeutic agents. Biochem Biophys Res Commun. 2007;352:220–225. doi: 10.1016/j.bbrc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 25.den Hollander J, Rimpi S, Doherty JR, et al. Aurora kinases A and B are up-regulated by Myc and are essential for maintenance of the malignant state. Blood. 2010;116:1498–1505. doi: 10.1182/blood-2009-11-251074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang D, Liu H, Goga A, et al. Therapeutic potential of a synthetic lethal interaction between the MYC proto-oncogene and inhibition of aurora-B kinase. Proc Natl Acad Sci U S A. 2010;107:13836–13841. doi: 10.1073/pnas.1008366107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedberg JW. Double-hit diffuse large B-cell lymphoma. J Clin Oncol. 2012;30:3439–3443. doi: 10.1200/JCO.2012.43.5800. [DOI] [PubMed] [Google Scholar]
- 28.Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3452–3459. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green TM, Young KH, Visco C, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3460–3467. doi: 10.1200/JCO.2011.41.4342. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida K, Nagai T, Ohmine K, et al. Vincristine potentiates the anti-proliferative effect of an aurora kinase inhibitor, VE-465, in myeloid leukemia cells. Biochem Pharmacol. 2011;82:1884–1890. doi: 10.1016/j.bcp.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Lentini L, Amato A, Schillaci T, et al. Aurora-A transcriptional silencing and vincristine treatment show a synergistic effect in human tumor cells. Oncol Res. 2008;17:115–125. doi: 10.3727/096504008785055521. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Ikezoe T, Nishioka C, et al. AZD1152, a novel and selective aurora B kinase inhibitor, induces growth arrest, apoptosis, and sensitization for tubulin depolymerizing agent or topoisomerase II inhibitor in human acute leukemia cells in vitro and in vivo. Blood. 2007;110:2034–2040. doi: 10.1182/blood-2007-02-073700. [DOI] [PubMed] [Google Scholar]
- 33.Mahadevan D, Stejskal A, Cooke LS, et al. Aurora A inhibitor (MLN8237) plus vincristine plus rituximab is synthetic lethal and a potential curative therapy in aggressive B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2012;18:2210–2219. doi: 10.1158/1078-0432.CCR-11-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao JH, Wu D, Perez-Losada J, et al. Crosstalk between aurora-A and p53: Frequent deletion or downregulation of aurora-A in tumors from p53 null mice. Cancer Cell. 2007;11:161–173. doi: 10.1016/j.ccr.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]