Abstract
Hepatitis C is serious health concern worldwide caused by HCV. It causes liver cirrhosis and hepato-cellular carcinoma. Development of prevention solutions is under progress. Meanwhile, the treatment of the viral disease using compounds isolated from natural medicinal plants is promising. The traditional use of photo-chemicals from medicinal plants like Amelanchier alnifolia for viral treatment is hopeful. Therefore, it is of interest to screen for flavonoids from Amelanchier alnifolia against protein targets of HCV. Hence, we assessed the binding of flavonoids to HCV NS3/4A protease and helicase proteins. Results show that Quercitin 3- galactoside and 3-glucosideshowed good binding score with protease and helicase respectively. Their interaction/binding sites are documented in this report. This data provide insights for the consideration of flavonoids as potential inhibitors of HCV/NS3/4A protease and helicase.
Background
Hepatitis C Virus (HCV) is an endemic worldwide problem which affects 170 million people globally and 10 million people in Pakistan [1]. It causes acute and chronic hepatitis and is a main cause of liver cirrhosis and hepatocellular carcinoma [2]. HCV belongs to Flaviviridae family with a positive-sense singlestranded RNA genome which encodes three structural proteins (Core, E1, E2) and six non-structural pmroteins (NS2, NS3, NS4A, NS4B, NS5A & NS5B) [3]. Among all HCV proteins, NS3/4A serine protease and helicase are effective drug targets to develop anti-HCV agents [4]. The basic role of NS3/4A is the proteolytic processing at NS4A/4B, NS4B/5A, and NS5A/5B sites, and it shows a vital role in HCV replication. As it is involved in viral replication, it has worked as a reliable drug target for HCV. Til today, no vaccination is available for treatment of HCV and present standard of care is a combination therapy of Pegylated interferon alpha (PegIFN-α) injections with oral antiviral nucleoside analogue ribavirin (RBV) which leads to treatment of HCV in 50% genotype 1 cases and 80% of genotype 2 cases but this treatment no rapid response and side effects in genotype 1a and 1b patients [5– 7]. Present treatment is expensive. Less effective and has numerous side effects thus, there is a need of developing antiviral agents that are less harmful and hasability to target all genotypes of HCV with the same competence. Recently, two NS3 protease inhibitors have been approved as triple therapy (PEG-IFN- α, ribavirin and Boceprevir or Telaprevir) against HCV [8]. But still there is a strong need to develop specific compounds that can target important factors of the HCV life cycle [9]. Several Medicinal plants are tested and many of them are proved to have antiviral effect in their phytochemicals. Medicinal plants are costeffective, multiple target activities, minor side-effects and thus, preferred over conventional treatment [10–14]. Phytochemicals such as alkaloids, organosulfur compounds, limonoids, lignans, furyl compounds, polyines, thiophenes, proteins, peptides, flavonoids, terpenoids, sulphides, polyphenolics, coumarins, saponins, chlorophyllins have functions like scavenging, antioxidant activities, hindering viral entry, DNA and RNA replication against numerous viruses [15]. Recently, our group reported that phytochemicals Amelanchier alnifolia showed novel inhibition of HCV titer in infected liver cells [16]. Therefore, this study was planned to screen phytochemical of Amelanchier alnifolia against HCV NS3/4A protease and helicase using in silico approaches.
Methodology
This study was designed to dock Amelanchier alnifolia phytochemicals against HCV NS3/4A protein with the following communications. Intel (R) xenon (R) CPU E5620@2.40GHz system having 3.8GB RAM with the open 11.4 (X 86_64) operating platform. Protein-ligand docking was carried out using the MOE (Molecular Operating Environment) software package. Interactions between HCV NS3/4A protease and ligands were imagined using ligPlot feature of MOE software [17].
Ligand preparation:
A literature was searched to find Amelanchier alnifolia phytochemical Structures. Chemical structures of phytochemicals were drew using ChemDraw software (Figure 1) and were transformed into their respective 3D structures. 3D structures were optimized by addion of hydrogens through MOE software package. Energies of selected molecules were minimized with parameters (gradient: 0.05, Force Field: MMFF94X, Chiral Constraint and Current Geometry). Molecules were then saved in .mdb format and were used as input file for MOE-Docking.
Figure 1.
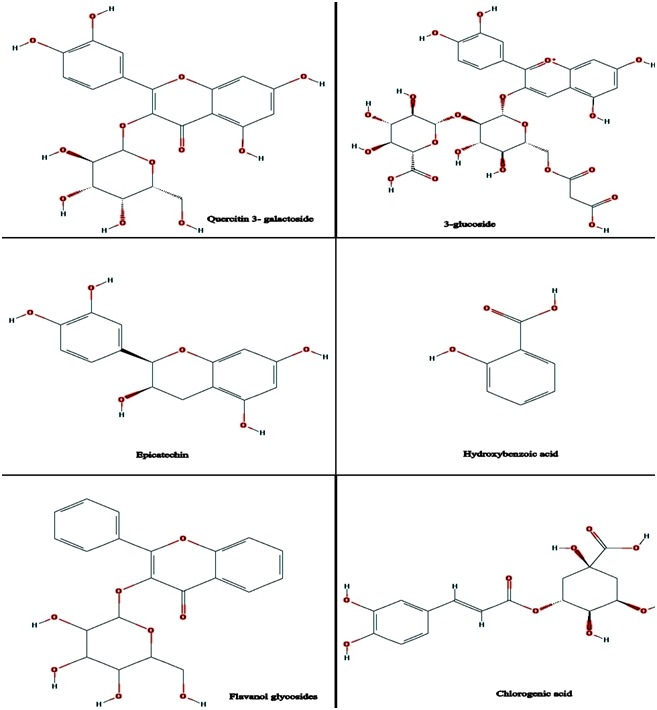
Chemical structures of Amelanchier alnifolia phytochemicals constructed using ChemDraw Software.
Preparation of Receptor Protein:
HCV NS3/4A protease and helicase structures were downloaded from Protein Data Bank using PDB ID: 3P8N and 2fm2 and was optimized by removal of water molecules, 3D protonation and Energy minimization. Energy minimization was carried out using parameters (gradient: 0.05, Force Field: MMFF94X+Solvation and Chiral Constraint: Current Geometry). Energy minimization was stopped when the root mean square gradient falls below the 0.05. The minimized structures were used as the receptor protein for Docking.
Molecular Docking:
MOE docking program with default parameters was used to bind the selected ligands with receptor protein and to find the correct conformation (with the rotation of bonds, structure of molecule is not rigid) of the ligand so as to obtain minimum energy structure. After docking, best conformations were analyzed for hydrogen bonding/π-π interactions.
Results & Discussion
Docking Analysis:
All ligands were docked with the pocket of NS3/4A protease and helicase enzyme and top ranked conformations of each ligand were saved. Compound 01 was ranked as top docked molecule on the basis of S-Score obtained from MOE docking algorithm. Thus, it can be concluded that this compound can serve as competative inhibitor against NS3/4A protease and helicase. All other compounds were having S-Score close to each other. All compounds were studied in detail to obtain their interaction information that can be important for the inhibition of NS3/4A protease and helicase enzyme. Interaction diagrams were obtained by using MOE ligand interaction analysis feature.
Binding interactions of Ligands and Protein:
The most active Compound 01 was ranked as first on the docking score. It is clear from (Figure 2) that this compound was bound deep into the binding cavity of NS3 protease making interactions with the residues His 57, Asp 81 and Asp 168. Similarly, Compound 01 ranked first on the docking score wgich showed deep binding with NS3 helicase cavity making interactions with the residues Ala 157, His 528. On the basis of docking score Compound 02, Compound 03, Compound 04, Compound 05, Compound 06, Compound 07, Compound 08, Compound 09, Compound 10, and Compound 11 were ranked close to each other as shown in Table 1 (see supplementary material). There were some visible interactions observed between the Compounds and the binding site residues Asp 81, His 57 and His 528. The solvent contact was found almost all over the ligand.
Figure 2.
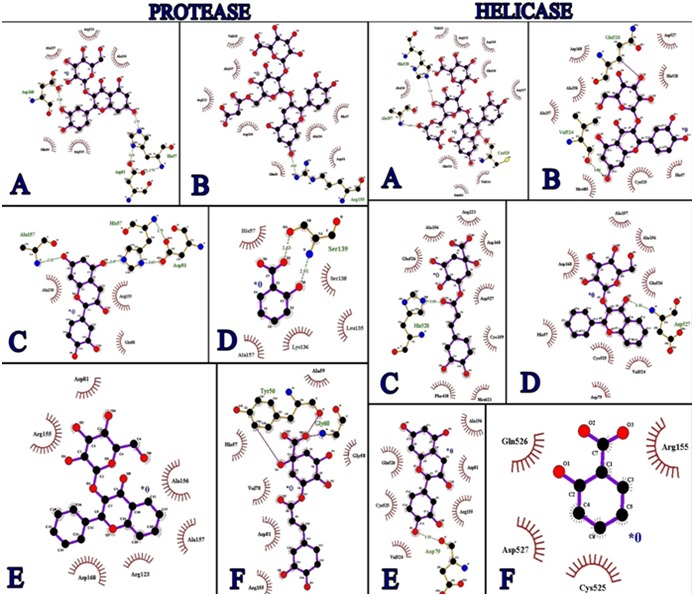
A) Interactions between Compound 01 and active site of NS3/4A protease and helicase; B) Interactions between Compound 02 and active site of NS3/4 protease and helicase, C) Interactions between Compound 03 and active site of NS3/4 protease and helicase; D) Interactions between Compound 04 and active site of NS3/4A protease and helicase; E) Interactions between Compound 05 and active site of NS3/4A protease and helicase; F) Interactions between Compound 06 and active site of NS3/4A protease and helicase.
Conclusion
The design and development of prevention tools for Hepatitis C viralinfection has been a challenge. Therefore, improved strategies for the treatment of Hepatitis C are relevant to the context. The use of extracts from Amelanchier alnifolia containing flavonoids shows promising results intraditional treatment pipelines. Hence, it is interest to document thepotential binding of Amelanchier alnifolia derived flavonoids with HCVNS3/4A protease and helicase. Molecular docking and binding simulations ofsuch flavanoids with HCV target proteins show the good binding ability ofquercitin 3-galactoside and 3- glucoside with protease and helicase,respectively. These observation provide insights to consider Amelanchieralnifolia derived flavonoids as potential inhibitors of HCV target proteins.
Supplementary material
Footnotes
Citation:Khan et al, Bioinformation 9(19): 978-982 (2013)
References
- 1.Naggie S. Top Antivir Med. 2012;20:154. [PMC free article] [PubMed] [Google Scholar]
- 2.Ashfaq UA, et al. Virol J. 2011;8:161. doi: 10.1186/1743-422X-8-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartenschlager R, et al. J Virol. 1994;68:5045. doi: 10.1128/jvi.68.8.5045-5055.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashfaq UA, et al. Genet Vaccines Ther. 2011;9:7. doi: 10.1186/1479-0556-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poynard T, et al. Hepatology. 2003;38:75. doi: 10.1053/jhep.2003.50267. [DOI] [PubMed] [Google Scholar]
- 6.Feld JJ, Hoofnagle JH. Nature. 2005;436:967. doi: 10.1038/nature04082. [DOI] [PubMed] [Google Scholar]
- 7.Raychoudhuri A, et al. J Virol. 2010;84:10991. doi: 10.1128/JVI.00900-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghany MG, et al. Hepatology. 2011;54:1433. doi: 10.1002/hep.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Labrador FX. Recent Pat Antiinfect Drug Discov. 2008;3:157. doi: 10.2174/157489108786242369. [DOI] [PubMed] [Google Scholar]
- 10.Vlietinck AJ, Vanden Berghe DA. J Ethnopharmacol. 1991;32:141. doi: 10.1016/0378-8741(91)90112-q. [DOI] [PubMed] [Google Scholar]
- 11.Cowan MM. Clin Microbiol Rev. 1999;12:564. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Briskin DP. Plant Physiol. 2000;124:507. doi: 10.1104/pp.124.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams JE. Altern Med Rev. 2001;6:567. [PubMed] [Google Scholar]
- 14.Jassim SA, Naji MA. J Appl Microbiol. 2003;95:412. doi: 10.1046/j.1365-2672.2003.02026.x. [DOI] [PubMed] [Google Scholar]
- 15.Naithani R, et al. Mini Rev Med Chem. 2008;8:1106. doi: 10.2174/138955708784567368. [DOI] [PubMed] [Google Scholar]
- 16.Rehman S, et al. Virol J. 2011;8:220. doi: 10.1186/1743-422X-8-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan M, et al. Bioinfo J. 2013;9:710. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


