Abstract
Natural killer (NK) cells are cytotoxic cells that are able to rapidly kill viruses, tumor cells, parasites, bacteria, and even cells considered “self”. The activity of NK cells is controlled by a fine balance of inhibitory and activating signals mediated by a complex set of different receptors. However, the function of NK cells is not restricted only to the killing of target cells, NK cells also possess other properties such as the secretion of proangiogenic factors during pregnancy. Here, we demonstrate another unique NK-cell activity, namely the regulation of T-cell mediated allergic responses, which is dependent on the NK-cell specific receptor NKp46 (Ncr1 in mice). Using mice in which the Ncr1 gene has been replaced with a green fluorescent protein, we demonstrate reduced delayed-type hypersensitivity and airway hypersensitivity. Interestingly, we show that this reduction in airway hypersensitivity is due to differences in the stimulation of T cells resulting in an altered cytokine profile.
Keywords: Allergy, Dendritic cells, NK cells, NKp46, Ncr1
Introduction
The activity of natural killer (NK) cells is regulated by complex interactions between inhibitory and activating receptors 1,2; prominent among the latter is NKp46 on human NK cells (named natural cytotoxicity receptor 1 (NCR1) in mice) 3. NKp46/NCR1 is uniquely expressed on NK cells 4 and on a subset of innate lymphoid cells found predominantly in the gut 5,6. Although NKp46 is known to be involved in the killing of various tumor cell lines in vitro 7,8 and in vivo 9, in the recognition of Fusobacterium nucleatum 10 and has also been implicated in the control of type I diabetes 11,12, no cellular ligand has been identified so far for this receptor. NK cells have also been reported to play a role in the regulation not only of innate but also of adaptive immune responses 13,14.
Human NK cells have been shown to be capable of inducing the maturation of dendritic cells (DCs), which is mediated by tumor necrosis factor alpha (TNF-α) and interferon gamma (IFN-γ) 15. DCs are also able to activate NK cells both in vitro 16 and in vivo 17, a process that involves the presentation of interleukin 15 (IL-15) in trans by IL-15R-α. In vitro activated human NK cells can kill immature monocyte-derived DCs in an NKp30-dependent manner while the involvement of NKp46 remains controversial 18,19. In contrast, mature DCs are protected from killing 20.
Several in vivo studies have, furthermore, shown the importance of DC–NK-cell interaction in response to viral infection and this is highlighted by the finding that viruses such as the murine cytomegalovirus (CMV) specifically target the DC–NK-cell axis 21,22.
Since DCs play a central role in the initiation and regulation of immune responses and because NKp46 was shown to interact with DC, we investigated whether the absence of NKp46 influences T-cell-mediated immune responses.
Although NK cells have been shown to play an important role in delayed-type hypersensitivity (DTH) 23,24, their exact role in the initiation and regulation of other allergic responses is poorly understood. Moreover, the NK receptors that are involved in the regulation of a predominantly T-cell-mediated allergic response have not been defined.
Results
The absence of NKp46 influences DTH
To investigate the role of NKp46 in shaping the adaptive immune response, we chose the experimental DTH model because it is one of the most frequently studied models of a T-cell-mediated immune response 25,26, because of the well-studied role of DCs in this model 27, and because NK cells have been shown to interact with DCs 15,19. Mice were sensitized by painting the shaved abdominal skin with a hapten solution on two consecutive days. Three days later, the mice were challenged by painting one ear with the hapten solution while the other ear was treated with the vehicle solution only. The swelling of the 2,4-dinitro-1-fluorbenzene (DNFB) treated ears reached its peak at 36 h after challenge and was significantly reduced in the NCR1gfp/gfp mice as compared to WT (NCR+/+) mice (Fig.1). As the swelling started to recede after 48 h, the difference between WT and NCR1gfp/gfp mice became significantly more pronounced. The differences in the ear swelling reactions were similar in mice of both the C57BL/6 and the 129/Sv background (Fig.1). To exclude that the observed differences were specific for DNFB-induced DTH, we used also oxazolone. Similarly to the DNFB-induced reaction, ear swelling caused by treatment with oxazolone peaked at about 36 h post challenge and was slightly weaker in NCR1gfp/gfp mice than in WT mice. Again, as the swelling receded the differences became more pronounced. This was observed in mice of the C57BL/6 and the 129/Sv backgrounds (Fig.1).
Figure 1.
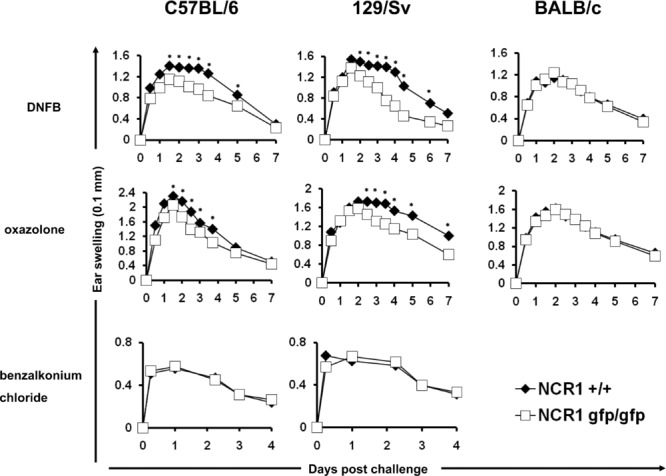
Reduced contact hypersensitivity in the absence of NKp46. Mice were sensitized by painting the shaved abdomen with the indicated contact sensitizer on two consecutive days (top, middle). Ear swelling was elicited on day 5. Shown are mean values (±SD) of one representative experiment out of two performed using 15 or more mice per group per experiment. *p < 0.05, two-tailed Student's t-test. Ear swelling was elicited by painting the ear with the irritant benzalkonium chloride (bottom). Shown are mean values (±SD) of one representative experiment out of two performed using ten mice per group per experiment.
To exclude that the observed differences were due to an altered ability of NCR1gfp/gfp mice to react to an unspecific irritation, the ears of mice were treated with the irritant benzalkonium chloride. Swelling caused by this irritant does not require the induction of a specific immune response 28. Importantly, no differences between WT and NCR1gfp/gfp mice were observed. Finally, we backcrossed the NCR1gfp/gfp mice onto the BALB/c background for more than ten generations, tested the effect of both contact allergens and observed no difference (Fig.1).
NK cells are recruited to inflamed ears at different kinetics than other lymphocyte populations
NK cells have been shown to infiltrate the ears of mice challenged with a contact allergen 23. We therefore investigated the kinetics by which NK, B, and T cells arrive at the site of challenge. Small numbers of CD3+ T cells and B220+ B cells were found to infiltrate the challenged ears already 12 h postchallenge and their numbers peaked at around 36 h and diminished after this (Supporting Information Fig. 1A). This correlates well with the height of the swelling observed that also peaked at 36 h postchallenge. Although not very pronounced, the number of T and B cells found in the ears of NCR1gfp/gfp mice was about 10% lower than in NCR1+/gfp mice (Supporting Information Fig. 1A) or WT mice (not shown), which probably reflects the diminished swelling observed in the NCR1gfp/gfp mice. In contrast, NK cells were first detectable at 24 h postchallenge and the highest numbers of NK cells were observed at 48 h postchallenge and receded more slowly than that of T and B cells (Supporting Information Fig. 1B). This was observed in NCR1gfp/gfp as well as in NCR1+/gfp mice and, using DX5 as a marker for NK cells, also in NCR1+/+ mice (not shown).
Airway hypersensitivity after antigen challenge is reduced in the absence of NKp46
We next examined whether airway hypersensitivity after challenge with ovalbumin (OVA) is altered in the NCR1gfp/gfp mice. C57BL/6 WT and NCR1gfp/gfp mice were immunized intraperitoneally (i.p.) with OVA and aluminum hydroxide as adjuvant on days 0, 7, and 14 and i.n. with OVA in PBS on days 14, 15, 18, and 19. On day 24, mice were challenged i.n., serum was collected (Fig.2A) and OVA-specific immunoglobulin was determined. While no differences in the levels of OVA-specific IgG1 levels were observed, the levels of specific IgG2a were significantly lower in C57BL/6 NCR1gfp/gfp mice (Fig.2B). Importantly, similarly to the DTH responses (Fig.1), no differences in OVA-specific serum IgG levels were observed in the BALB/c mice (Fig.2B).
Figure 2.
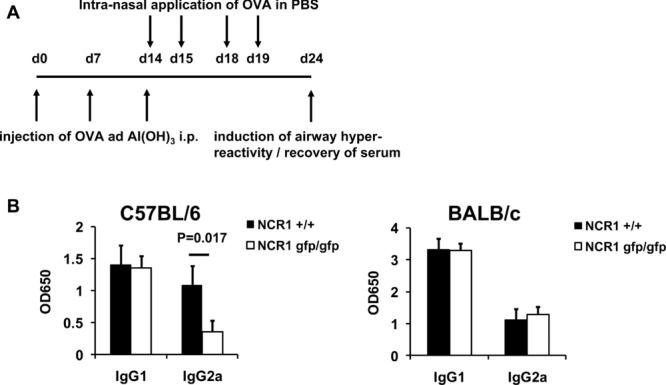
OVA-induced airway hyperreactivity is reduced in the absence of NKp46. (A) Schematic representation of the immunization protocol used. (B) C57BL/6 NCR1+/+ and C57BL/6 NCR1gfp/gfp mice (left) and BALB/c NCR1+/+ and BALB/c NCR1gfp/gfp (right) mice were immunized, challenged using OVA and serum was harvested. OVA-specific IgG was measured using ELISA. Shown are mean values (+SD) of one representative experiment out of three performed using eight or more mice per group per experiment. Statistical analysis was performed using a two-tailed Student's t-test.
To test the reasons accounting for the reduced OVA-specific immunity observed in the NCR1gfp/gfp mice, a histological examination was performed and revealed less infiltration of leukocytes into the lungs of C57BL/6 NCR1gfp/gfp mice, whereas no differences were observed in the BALB/c mice (Fig.3A). To characterize the leukocyte subsets in the inflamed lungs, cells were isolated from explanted lungs directly after rechallenge with OVA and were examined. As shown in Figure3B, the numbers of all cell types investigated (CD3+ T cells, B220+ B cells, CD11c+ cells, and CD49b+ NK cells) were significantly reduced in C57BL/6 NCR1gfp/gfp mice.
Figure 3.
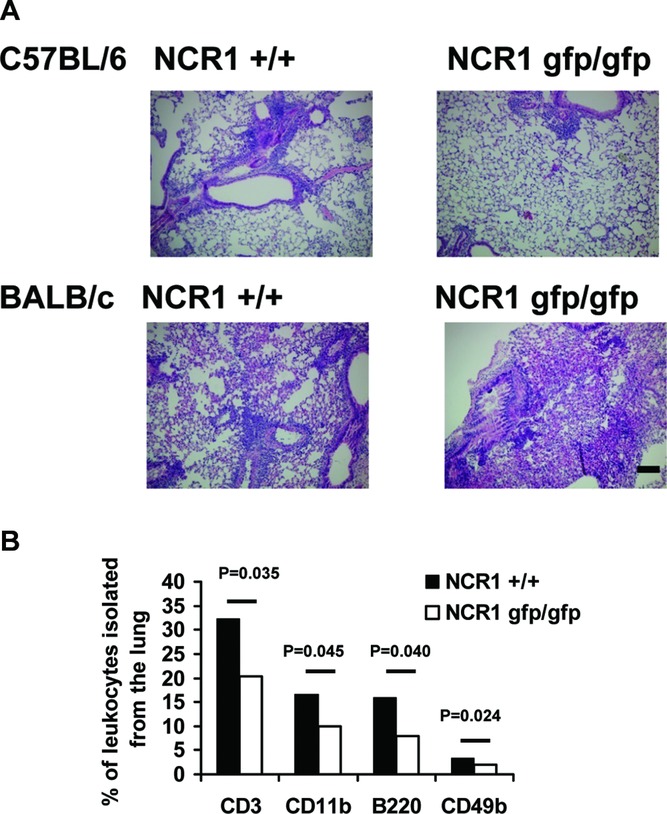
Reduced infiltration of immune cells in the absence of NKp46. (A) C57BL/6 NCR1+/+ and C57BL/6 NCR1gfp/gfp mice (top) and BALB/c NCR1+/+ and BALB/c NCR1gfp/gfp mice (bottom) were immunized and airways were challenged using OVA. Lungs were harvested and tissue was stained using H&E. Shown are representative sections (original magnification 10×) of one representative experiment out of three performed using eight or more mice per group per experiment. Scale bar: 10 μm. (B) C57BL/6 NCR1+/+ and C57L/6 NCR1gfp/gfp mice were immunized and airways were challenged using OVA. Lungs were harvested, and cells were isolated and analyzed by flow cytometry. Shown are mean values (±SD) of one representative experiment out of three performed using four mice per group per experiment. Statistical analysis was performed using a two-tailed Student's t-test.
The number of IFN-γ-producing NK cells is reduced in C57BL/6 NCR1gfp/gfp mice
To investigate if lower numbers of IFN-γ-producing NK cells found in C57BL/6 NCR1gfp/gfp mice after induction of OVA-induced airway hyperreactivity, cells from spleens, lungs, and mediastinal lymph nodes (MLNs), the draining LNs of the lung, were harvested from immunized and rechallenged mice and analyzed for the production of intracellular IFN-γ. The number of IFN-γ-producing CD49b-expressing NK cells was significantly reduced in the spleens and the MLNs of C57BL/6 NCR1gfp/gfp mice (Fig.4A and B). Additionally, the number of IFN-γ-producing CD4+ T cells was significantly reduced in the spleens and the MLNs of C57BL/6 NCR1gfp/gfp mice (Fig.4B). Interestingly, no differences were observed in the lungs (Fig.4B). We also evaluated whether other cytokines would be influenced by the lack of NCR1. NK cells were isolated from the spleens of treated animals and mRNA expression of various cytokines was analyzed by real-time PCR (Fig.4C). As expected, mRNA levels of IFN-γ were significantly lower and interestingly, the mRNA levels of IL-13 were also significantly lower in NK cells isolated from NCR1gfp/gfp mice than those isolated from NCR1+/+ mice. No significant differences were observed for mRNA levels of IL-10, IL-17a, IL-22, TGF-β, and TNF-α.
Figure 4.
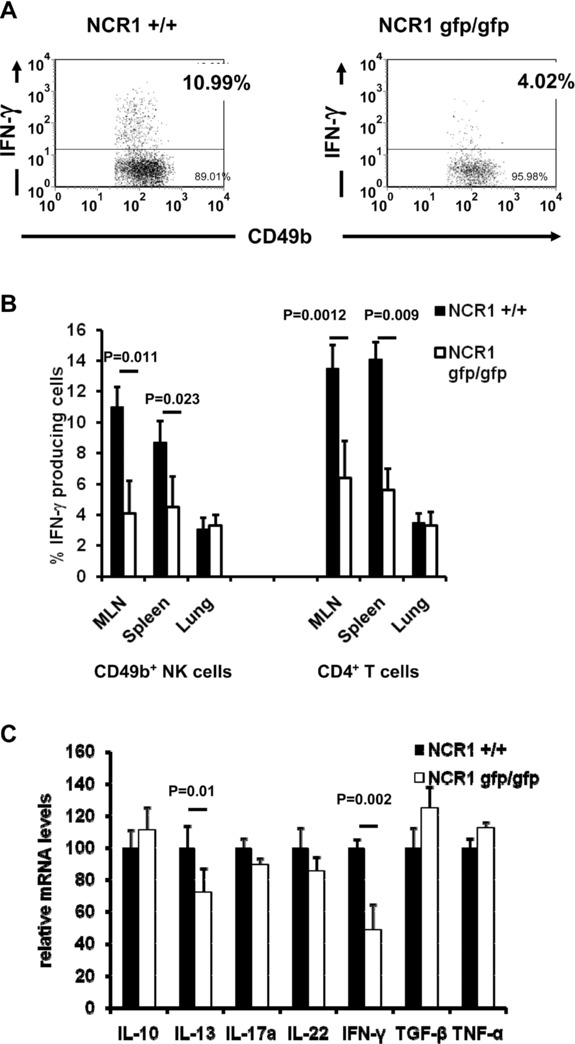
Reduced cytrokine producing NK and CD4+ T cells in the absence of NKp46. C57BL/6 NCR1+/+ and C57BL/6 NCR1gfp/gfp mice were immunized and airways were challenged using OVA. Organs were harvested and cells were analyzed for expression of cytokines. (A) Reduced numbers of IFN-γ-producing NK cells in the MLNs were observed. Cells were analyzed by flow cytometry for expression of IFN-γ and counterstained with CD49b. Shown is one representative experiment out of three performed using four mice per group per experiment. (B) The numbers of IFN-γ-producing NK cells in the spleen, lung, and MLNs are shown. Cells were analyzed by flow cytometry for expression of IFN-γ and were counterstained with CD49b and CD4, respectively. (C) The expression of IFN-γ and IL-13 by NK cells in the absence of NKp46 was also quantified. mRNA was isolated from NK cells isolated from spleens of treated animals by immunomagnetic separation and analyzed by real-time PCR. Relative values obtained from NCR1+/+ mice were set to 100. (B and C) Shown are mean values (+SD) of one representative experiment out of two performed using four mice per group per experiment. Statistical analysis was performed using a two-tailed Student's t-test.
NKp46 is involved in the stimulation of T-cell responses
NK cells have been shown to interact with DCs 29. It was therefore of interest to investigate if the differences in the airway hyperreactivity response to OVA was due to a difference in the stimulation of T cells. To this end, in vivo proliferation assays were performed. CD8+ T cells were isolated from spleens of naïve OT-I mice and CD4+ T cells were isolated from OT-II mice, respectively. The isolated cells were stained with carboxyfluorescein diacetate succinimidyl ester (CFSE) and transferred into C57BL/6 NCR1gfp/gfp and C57BL/6 NCR1+/+ mice, respectively. Mice were challenged i.n. with OVA in PBS on day 2 and day 4 and cells from lungs, spleens, and MLNs were analyzed by flow cytometry for dilution of CFSE on day 5. As expected, when CD8+ T cells from OT-I mice were transferred into C57BL/6 NCR1+/+ mice and then challenged with OVA, a dilution of CFSE, which is indicative of proliferation, was observed (Fig.5A and Supporting Information Fig. 2). In contrast, the proliferative response was much lower when the CD8+ T cells from OT-I mice were transferred into C57BL/6 NCR1gfp/gfp mice (Fig.5A). Similar observations were noted in the MLNs (Fig.5A). Furthermore, a similar reduction of proliferation was seen when CD4+ T cells from OT-II mice were transferred into C57BL/6 NCR1gfp/gfp mice (Fig.5A). As before, the reduction in CFSE dilution was only detected in cells recovered from the spleens and the MLNs but not the lungs indicating that the differences in the stimulation of T cells are restricted to the secondary lymphoid organs.
Figure 5.
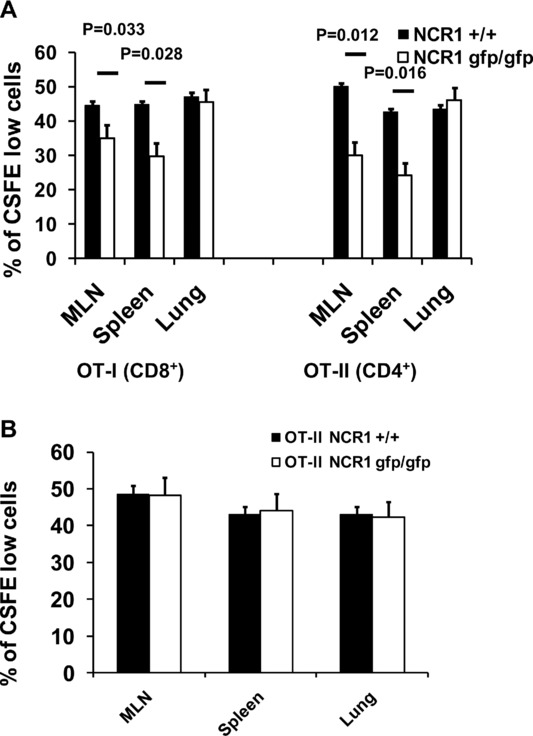
Reduced proliferation of OVA-specific T cells in vivo in the absence of NKp46. (A) CD4+ T cells were isolated from OVA transgenic OT-II mice and CD8+ T cells were isolated from OT-I mice, respectively. Isolated cells were labeled with CFSE and transferred into C57BL/6 NCR1+/+ and C57BL/6 NCR1gfp/gfp mice. Mice were challenged i.n., organs were harvested, and cells were analyzed by flow cytometry for expression of the transgenic T-cell receptor and dilution of CFSE. Shown are representative figures of one representative experiment out of four performed using four mice per group per experiment. (B) NCR1 has no direct effect on the ability of T cells to respond to antigen challenge in vivo. CD4+ T cells were isolated from OT-II C57BL/6 NCR1gfp/gfp and OT-II C57BL/6 NCR1+/+ mice. Isolated cells were labeled with CFSE and challenges with OVA. Shown are mean values (±SD) of one representative experiment out of four performed using four mice per group per experiment.
To exclude the possibility that the differences we observed in WT and C57BL/6 NCR1gfp/gfp mice is due to differences of the ability of T cells to respond to stimulation, C57BL/6 NCR1gfp/gfp were crossed with OT-I and OT-II, respectively. We were not able to detect any differences in the expression of CD69, CD25, or the frequency of CD25+ FoxP3+ cells among all crossed mice (data not shown). To rule out the possibility that the absence of NCR1 has a direct effect on the ability of T cells to respond to antigen challenge in vivo, proliferation assays were performed using transgenic T cells from OT-I C57BL/6 NCR1+/+, OT-I C57BL/6 NCR1gfp/gfp mice, as well as OT-II C57BL/6 NCR1+/+ and OT-II C57BL/6 NCR1gfp/gfp mice with C57BL/6 NCR1+/+ as recipient mice. After i.n. challenge, no differences in the ability of T cells to proliferate were observed, regardless of whether the transferred cells had originated in mice expressing NCR1 or not (Fig.5B and data not shown), indicating that the absence of NKp46 does not influence the ability of T cells to respond to stimulation.
The influence of NKp46 is dependent on the route of application of the immunogen
C57BL/6 NCR1gfp/gfp mice are healthy and fertile under normal conditions and react normally to different pathogens 30. Since the absence of NKp46 seems to have an effect on the stimulation of T cells, it was of interest to investigate if this influence is a general reduction of the immune responses or if immune responses are affected only under specific conditions. We therefore chose a modified immunization protocol in which mice were immunized using OVA and Al(OH)3 as before. However, from day 14 onwards, OVA in PBS was not applied i.n. as before but instead was given i.p (Fig.6A). Importantly, no differences in the levels of specific serum IgG levels were detected between C57BL/6 NCR1+/+ and C57BL/6 NCR1gfp/gfp mice (Fig.6B). To investigate this further, in vivo proliferation assays were performed as above. However this time, mice were challenged i.p. (and not by i.n. administration as before). As expected, no CFSE-labeled cells were recovered from the lungs and the MLNs, respectively, of i.p. challenged mice (Fig.6C). When cells from spleens of i.p. challenged mice were analyzed, no differences in the dilution of CFSE were detected between C57BL/6 NCR1+/+ and C57BL/6 NCR1gfp/gfp mice (Fig.6C). This indicates that the influence of NCR1-expressing NK cells on the regulation of an immune response is dependent on the route of application of the immunogen.
Figure 6.
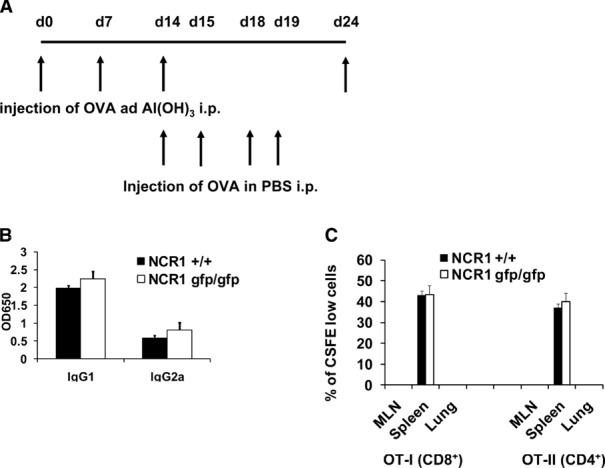
The influence of the absence of NKp46 depends on the route of application of the immunogen. (A) Schematic representation of the modified immunization protocol used. (B) C57BL/6 NCR1+/+ and C57BL/6 NCR1gfp/gfp mice were immunized and challenged i.p. using OVA and serum was harvested. OVA-specific IgG was measured using ELISA. Shown are mean values (+SD) of one representative experiment out of two performed using five mice per group. (C) CD4+ T cells were isolated from OVA transgenic OT-II mice and CD8+ T cells were isolated from OT-I mice, respectively. Isolated cells were labeled with CFSE and transferred into C57BL/6 NCR1+/+ and C57BL/6 NCR1gfp/gfp mice, respectively. Mice were challenged i.p, organs were harvested, and cells were analyzed by flow cytometry for expression of the transgenic T-cell receptor and dilution of CFSE. Shown are representative diagrams of one representative experiment out of two performed using four mice per group per experiment.
Immature BM-DC (imBM-DC) express ligand(s) of NKp46 (NCR1) and NKG2D
Because NK cells have been shown to interact with DCs 29 and since we have demonstrated that the absence of NKp46 affects the T-cell responses in the secondary lymphatic organs, we next investigated whether bone marrow derived DCs (BM-DC) express NK killer ligands by staining them with NCR1-Ig and NKG2D-Ig. As a control, we used a fusion protein consisting of the first extracellular domain of human NKp46 (NKp46D1-Ig), which was shown not to interact with human NKp46 ligands 31. Interestingly, at 8 days of culture, cells stained positive for the ligand of NCR1 and NKG2D (Fig.7A, left). After 10 days of culture, when cells acquire a more mature phenotype, only very weak staining was observed using NCR1-Ig but cells were still stained positive using NKG2D-Ig (Fig.7A, middle). Stimulation of these cells with LPS, which leads to complete maturation of DCs, resulted in the complete downregulation of the ligand of NCR1, whereas expression of ligands of NKG2D remained unchanged (Fig.7A, right).
Figure 7.
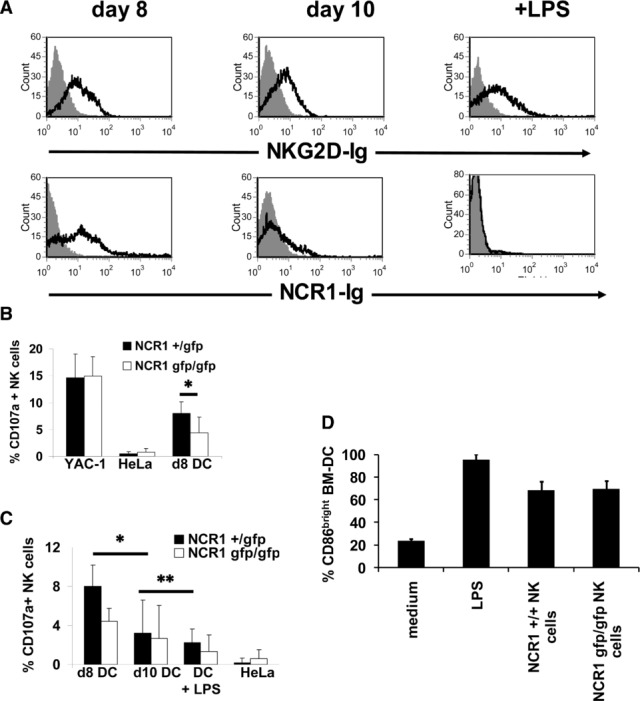
Immature DCs express ligand(s) of NCR1 and NKG2D and are killed by NK cells. Flow cytometry of the expression of ligands for activating NK-cell receptors on bone marrow-derived DCs (BM-DCs). Cells were cultured for the indicated periods of time and stained with the indicated fusion proteins. Staining was visualized using a phycoerythrin- or Cy5-conjugated anti-human IgG antibody. (A) BM-DCs were stained using the indicated fusion protein (open histogram) or the control fusion protein NKp46D1-Ig (filled histogram). Data are representative of at least three independent experiments. (B) NK cells from NCR1+/gfp and NCR1gfp/gfp C57BL/6 mice were incubated with YAC-1, HeLa, and day 8 BM-DCs, and surface mobilization of CD107a was measured by flow cytometry. Shown are mean values (+SD) of three independent experiments performed in duplicate. *p = 0.035, two-tailed Student's t-test. (C) Maturation of BM-DCs leads to reduction in CD107a mobilization. BM-DCs cultured for 8 or 10 days or stimulated with LPS (1 μg/mL) were incubated with NK cells from NCR1+/gfp and NCR1gfp/gfp C57BL mice. Shown are mean values (+SD) of three independent experiments performed in duplicate. *p = 0.037 **p = 0.042, two-tailed Student's t-test. (D) NCR1 is not involved in the induction of maturation of BM-DCs. NK cells from WT C57BL/6 and NCR1gfp/gfp mice, respectively, were co-cultured with imBM-DCs for 48 h and CD86 expression levels were analyzed by flow cytometry. BM-DCs were identified by expression of MHC class II. Shown are mean values of three independent experiments (+SD).
Since the cells in the cultures after 8 days are not a homogenous population and cells vary in their state of maturation, we double stained these 8 days culture cells using antibodies to DCs and maturation markers in combination with the NCR1 fusion protein. Cells stained positive with the NCR1 fusion protein expressed intermediate levels of the DC marker DEC205 and the maturation markers CD86 and MHC class II (Supporting Information Fig. 3A), as is typical for imBM-Dcs 32. Importantly, cells expressing high levels of CD86 and MHC class II did not stain positive for the ligand of NCR1 indicating that this ligand is only expressed by immature, but not mature BM-DCs whereas ligands for NKG2D are expressed by both mature as well as imDCs.
Finally, we assessed whether nonmanipulated DCs would also express NCR1 ligands. To this end, immature DCs were isolated from the skin. These unmanipulated imDCs express significant amounts of the NCR1 ligands and this expression was markedly reduced upon maturation (Supporting Information Fig. 3B and C).
NCR1 is involved in the killing of imBM-DCs
The above results demonstrate that imDCs express a ligand for NCR1. We thus investigated next whether NCR1 is directly involved in the killing of imBM-DCs using a CD107a mobilization assay. As we had knocked out the NCR1 receptor by replacing it with a GFP reporter gene 9, we had generated a mouse model in which all NK cells are labeled with GFP. We isolated NK cells from NCR1+/gfp and NCR1gfp/gfp mice and only GFP expressing cells were gated. As shown in Figure7B, no significant difference in the surface expression of CD107a on NK cells from NCR1+/gfp and NCR1gfp/gfp mice was observed when the classical NK target cells YAC-1 were used. Little or no CD107a mobilization was observed when the control human HeLa cells were the targets. Importantly, the CD107a degranulation was significantly reduced when NK cells from homozygous NCR1gfp/gfp were used (8% versus 4.4% for C57BL/6 and 6% versus 3.4% for 129/Sv mice (not shown), respectively). When more mature, day 10 BM-DCs were used as target cells (Fig.7C), the CD107a mobilization by both hetero- and homozygote NK cells was significantly reduced. This reduction in degranulation was even more pronounced when NK cells were incubated with fully mature LPS-stimulated BM-DCs. Thus, in agreement with the NCR1 recognition of imDCs, NCR1 is involved in the killing of imBM-DCs by activated NK cells in vitro and their ability to kill DCs is dependent on their maturation.
Since BM-DCs also express ligands for NKG2D (Fig.7), anti-NKG2D antibodies were included in the assay (Supporting Information Fig. 4A and data not shown). Interestingly, blocking of NKG2D had only little effect on the degranulation of NK cells from NCR1+/gfp mice. In the absence of NCR1, however, blocking of NKG2D led to a significant reduction of degranulation. Thus, even though ligands for NKG2D and NCR1 are expressed by immature murine BM-DCs, NCR1 is the dominant receptor involved in the killing of imDCs by NK cells.
Human NK cells have been shown to be involved in the regulation of maturation of DCs at higher DC/NK ratios 15. It was therefore of interest to investigate if the maturation state of imDCs changed when co-cultured with NK cells from either WT or NCR1gfp/gfp mice. Interestingly, no differences in the levels of expression of CD86 were observed when DCs from either WT or NCR1gfp/gfp mice were used as target cells (Fig.7D). Moreover, no differences in the secretion of IFN-γ (Supporting Information Fig. 4B) and TNF-α (not shown) were observed, indicating that NCR1 is not involved in the induction of maturation of DCs.
Discussion
We studied here the function of NKp46, a receptor whose expression is restricted to NK cells 8 and to a subset of innate lymphoid cells 5,6, in a model of experimental DTH. We found that the ear swelling response was reduced in the absence of NKp46 and that the NKp46 effect was most pronounced after the swelling had reached its peak. Interestingly, NK cells infiltrate the challenged ears later than other lymphocytes and reach their highest numbers just after the height of the swelling.
It was shown that NK cells are directly involved in contact hypersensitivity and that an ear swelling response is diminished in the absence of NK cells 23. Moreover, transfer of memory-like NK cells from the liver of sensitized mice have been shown to be able to mediate DTH reactions independently of T cells 33,34. However, the NK receptors involved have not been studied.
It cannot formally be ruled out that the diminished ear swelling we observed in NCR1gfp/gfp mice is mediated through a B- and T-cell-independent mechanism. However, this seems unlikely since the NKp46 knockout mice do not show any obvious phenotype under normal conditions and no particular T-cell deficiencies or T-cell overstimulation were observed. Indeed, we did not observe differences in the immune response when OVA was given only i.p. Moreover, we were not able to detect any alterations in the T-cell repertoire and OVA-specific T-cell receptor transgenic T cells from OT-I C57BL/6 NCR1gfp/gfp and C57BL/6 NCR1gfp/gfp mice reacted normally to stimulation in vivo. Finally, although NK cells were shown to directly interact with CD4+ T cells via the Qa-1-NKG2A inhibitory pathway 35 and NK cells kill activated CD4+ T cells in the context of a viral infection such as lymphocytic choriomeningitis virus 36, CD4+ T cells do not appear to express ligand(s) of NKp46 (data not shown and 36).
We therefore suggest that NKp46 influences T-cell responses indirectly, either through the secretion of cytokines or through direct interaction with DCs. Interestingly, reduced NK–DC interaction was shown to effect T-cell polarization and reduced production of proinflammatory cytokines in vitro 37. Moreover, NK cells are known to interact with DCs and we show here that mouse imBM-DCs and immature DCs from the skin express a ligand of NCR1, which is downregulated upon maturation and that imDCs are killed in an NCR1-dependent manner.
Although NKp46 expression was initially described to be exclusive to NK cells, recent studies have identified small subsets of NKp46+ innate lymphoid cells in mice 5,6 and humans 38. It cannot be ruled out that these cells contribute to the reduced allergic responses observed in the NCR1gfp/gfp mice. However, these NKp46 expressing cells are predominantly found in lymphoid tissues associated with the gut and the skin and are very rare in other lymphoid tissues such as the spleen or LNs 6,38,39.
The differences between NKp46-deficient and WT mice were observed only in C57BL/6 but not in BALB/c mice. BALB/c mice were shown to develop significantly greater eosinophilia after repeated i.n. OVA instillation than C57BL/6 mice 40, which could mask the effect of NK cells in this model. Additionally, these two strains are known to produce different amounts of cytokines in various mouse models of disease 41,42. T cells of C57BL/6 mice produce higher levels of Th1 cytokines, while those of BALB/c mice produce higher levels of Th2 cytokines 43. Interestingly, several studies have shown differences in the frequencies of different subsets of plasmacytoid as well as conventional DCs in these mouse strains 44,45 and this could affect the interaction with NK cells and consequently the influence of NK cells on an adaptive immune response.
Differences between NCR1gfp/gfp and NCR1+/+ were only observed when the immunogen was either applied to the skin or i.n. but not when it was given i.p. The skin and the lungs are natural barriers for pathogens and different specialized resident DC subsets have been described 46,47, whereas the main cell types to be recruited to the peritoneal cavity upon injection of OVA are inflammatory monocytes, which have been shown to be direct precursors of DCs 48, and plasmacytoid DCs 49.
A recent report showed an increased, receptor-independent NK-cell reactivity in a mouse in which a mutation of the NCR1 gene had been induced by N-ethyl-N-nitrosourea mutagenesis 50. Some of these findings were also reproduced in another mouse in which codon-improved Cre (iCre) recombinase was inserted in the 3′ UTR of Ncr1 51. The Ncr1gfp/gfp mice and the other mice are substantially different at the DNA, RNA, and protein levels 9,50,51, and this might be the cause of the phenotypic differences observed among the various mice. Regardless of the reason for these differences, it is clear that the Ncr1gfp/gfp mice are the most suitable model for studying NKp46-dependent deficiencies.
Taken together, we show here that allergic immune reactions are impaired in the absence of NCR1 through a mechanism that probably involves interaction of NK cells with DCs.
Materials and methods
Mice
The NCR1gfp/gfp mice on the 129/Sv and C57BL/6 background were described previously 9. All experiments were done in accordance with the guidelines of the ethical committee (MD-07–10885–3). OT-I (C57BL/6) and OT-II (C57BL/6) were a kind gift from S. Jung. NCR1gfp/gfp (C57BL/6) were crossed with OT-I (C57BL/6) and OT-II (C57BL/6), respectively.
Cells
To isolate cells from ears of sensitized mice, the dorsal and the ventral parts of the ears were separated and digested for 75 min with 1 mg/mL type IV collagenase (Sigma, St. Louis, MO, USA) at 37°C. NK cells were isolated from extracted splenocytes using a mouse NK isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) and an AutoMACS instrument according to the manufacturer's instruction. For the in vitro killing assays, mice were injected i.p with 200 μg polyinosinic:polycytidylic acid (poly(I):poly(C)) (Sigma-Aldrich) and splenocytes were removed after 18 h. BM-DCs were generated as described 32.
Flow cytometry
The following antibodies were used for staining: MHC class II I-A/I-E (2G9, BD Pharmingen, San Diego, CA, USA), DEC205 (NLDC-145, FITC conjugated or unconjugated, AbD Serotec, Oxford, UK), CD86 (GL-1, biotin conjugated, BioLegend, San Diego, CA, USA), CD3 (45–2C11, PE conjugated, BD Pharmingen), CD4 (GK1.5, PE conjugated, BioLegend), B220 (RA3–6B2, PE conjugated, BD Pharmingen), Vα2 (B20.1, allophycocyanin conjugated, BioLegend), Vβ5 (MR9–4, PE conjugated, BD Pharmingen), CD11b (LY40, PE conjugated, AbD Serotec), CD49b (DX5, PE or allophycocyanin conjugated, BioLegend), IFN-γ (XMG1.2, allophycocyanin conjugated BioLegend), IL-4 (11B11, PE conjugated, BioLegend), and IL-5 (TRFK5, allophycocyanin conjugated, BD Pharmingen). The NCR1-Ig, NKG2D-Ig (a kind gift of D. Cosman (Amgen)) and the control protein NKp46D1-Ig were produced as described 35,56 and staining of cells was visualized using phycoerythrin- or Cy5-conjugated goat anti-human Ig (Jackson ImmoResearch, West Grove, PA, USA). FcR blocking reagent mouse (Miltenyi Biotech, Bergisch Gladbach, Germany) was used to block Fc receptors before staining.
Staining with unconjugated antibodies was visualized using a phycoerythrin-conjugated anti-rat IgG antibody or staining with biotin-conjugated antibodies was visualized using phycoerythrin- or Cy5-conjugated streptavidin (Jackson ImmoResearch).
For intracellular staining, spleen and LN cells were stimulated with ionomycin (1 μM) (Calbiochem, San Diego, CA, USA) and PMA (10 ng/mL) (Calbiochem) in a 6-well plate at 3 × 106 cells/mL. After 2 h, monensin (2 μM) (Calbiochem) was added for another 2 h of culture. Cells were stained using the FoxP3 staining kit (Miltenyi Biotech).
CD107a mobilization assay
CD107a mobilization assays were performed as described 34. Briefly, 5 × 105 target cells were incubated for 2 h with isolated NK cells at a ratio of 1:1 in the presence of 0.1 μg of an allophycocyanin-conjugated CD107a antibody (1D4B; Southern Biotechnology Associates, Birmingham, AL, USA) before cells were analyzed by flow cytometry. The human cervical adenocarcinoma cell line HeLa and the murine lymphoma cell line YAC-1 were used as controls.
Allergic airway inflammation
Asthma was induced by i.p. injection of 10 μg OVA (Grade III; Sigma-Aldrich) in 3 mg aluminum hydroxide (Al(OH)3) on days 0, 7, and 14. Animals were thereafter challenged with i.n. instillation of 100 μg OVA in 50 μL PBS on days 14, 15, 18, and 19. On day 24, mice were challenged i.n. and bronchio constriction was measured, lungs were harvested and serum was collected.
Allergen challenge assessment
Allergen-induced bronchoconstriction was assessed and performed under continuous airflow conditions as described 52. The Penh value, which is a unitless indicator of changes in airway resistance that correlates well with specific airway resistance 53, was measured using a whole-body plethysmograph connected to a pneumotach.
In vivo proliferation assay
Proliferation of T cells in vivo was assessed as described 54. A total of 1–3 × 106 cells were injected into the tail vein of mice on day 0. On day 2 and 4, mice were challenged with 100 μg of OVA in PBS i.n. or i.p. Control mice were treated with PBS alone. On day 5, mice were sacrificed and their lungs, spleens, and MLNs were collected.
Contact hypersensitivity
To sensitize mice, 17 μL of 0.4% DNFB(Sigma) or 3% 4-ethoxymethylene-2-phenyl-2-oxalin-5-one (oxazolone, Sigma) solution in acetone:olive oil (4:1) was applied to the shaved abdomen on day 0 and 1. On day 5, they were challenged on both sides of the right ear with 8.5 μL 0.4% DNFB and 1% oxazolone solution, respectively. As a control, the left ear was treated with 8.5 μL acetone:olive oil on both sides. Ear thickness was measured using a micrometer (Mitutoyo, Tokyo, Japan) at the indicated time points and specific swelling was calculated by subtracting the measurement of the vehicle-treated ear from that of the hapten-treated ear. For irritant control, mice were challenged with 5% benzalkonium chloride (Sigma) in acetone:olive oil (4:1).
ELISA, histology, and real-time PCR
OVA-specific antibody levels in serum and IFN-γ secretion were performed by standard ELISA 55. Paraffin-embedded sections of organs of treated mice were prepared and stained with H&E as described 55. RNA extraction, RT, and real-time PCR analysis were performed as described 56.
Co-culture and cytokine analysis
A total of 5 × 104 d6 BM-DCs and isolated NK cells were plated in 96-well round-bottom plates at a DC:NK ratio of 5:1. After 48 h, DCs were harvested and analyzed for the expression of CD86. Additionally, supernatants were harvested and IFN-γ secretion was assed using ELISA kit (BD Pharmingen).
Statistical analysis
Statistical analysis of the experimental data was performed using a two-tailed Student's t-test. A p-value <0.05 was considered statistically significant.
Acknowledgments
The authors thank Orchidée Filipe-Santos and Philippe Bousso from the G5 Dynamiques des Réponses Immunes, Institut Pasteur, F-75015, France. This study was supported by The Israeli Science Foundation, The European Consortium (MRTN-CT-2005 and LSCH-CT-2005–518178), The Israeli I-CORE, The ICRF professorship grant by the GIF grant and by the ERC advanced grant. O.M. is a Crown professor of Molecular Immunology. H.G. was supported by the Marie Curie research training network program MRTN-CT-2005-019248.
Glossary
- BM-DC
bone marrow derived DC
- DNFB
2,4-dinitro-1-fluorbenzene
- DTH
delayed-type hypersensitivity
- imBM-DC
immature BM-derived DC
- NCR1
natural cytotoxicity receptor 1
Conflict of interest
HG is currently employed by MedImmune Ltd., Cambridge, UK. However, the data presented here were generated before this employment started and MedImmune had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare no financial or commercial conflict of interest.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher's web-site
Supplementary
References
- 1.Moretta L. Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 2004;23:255–259. doi: 10.1038/sj.emboj.7600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hudspeth K, Silva-Santos B. Mavilio D. Natural cytotoxicity receptors: broader expression patterns and functions in innate and adaptive immune cells. Front. Immunol. 2013;4:69. doi: 10.3389/fimmu.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biassoni R, Pessino A, Bottino C, Pende D, Moretta L. Moretta A. The murine homologue of the human NKp46, a triggering receptor involved in the induction of natural cytotoxicity. Eur. J. Immunol. 1999;29:1014–1020. doi: 10.1002/(SICI)1521-4141(199903)29:03<1014::AID-IMMU1014>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 4.Moretta A, Bottino C, Mingari MC, Biassoni R. Moretta L. What is a natural killer cell? Nat. Immunol. 2002;3:6–8. doi: 10.1038/ni0102-6. [DOI] [PubMed] [Google Scholar]
- 5.Luci C, Reynders A, Ivanov II, Cognet C, Chiche L, Chasson L. Hardwigsen J, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat. Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 6.Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C. Diefenbach A. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat. Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss L, Reich S, Mandelboim O. Slavin S. Murine B-cell leukemia lymphoma (BCL1) cells as a target for NK cell-mediated immunotherapy. Bone Marrow Transplant. 2004;33:1137–1141. doi: 10.1038/sj.bmt.1704475. [DOI] [PubMed] [Google Scholar]
- 8.Sivori S, Pende D, Bottino C, Marcenaro E, Pessino A, Biassoni R, Moretta L, et al. NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human NK cells. Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. Eur. J. Immunol. 1999;29:1656–1666. doi: 10.1002/(SICI)1521-4141(199905)29:05<1656::AID-IMMU1656>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Gazit R, Gruda R, Elboim M, Arnon TI, Katz G, Achdout H, Hanna J, et al. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat. Immunol. 2006;7:517–523. doi: 10.1038/ni1322. [DOI] [PubMed] [Google Scholar]
- 10.Chaushu S, Wilensky A, Gur C, Shapira L, Elboim M, Halftek G, Polak D, et al. Direct recognition of Fusobacterium nucleatum by the NK cell natural cytotoxicity receptor NKp46 aggravates periodontal disease. PLoS Pathog. 2012;8:e1002601. doi: 10.1371/journal.ppat.1002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gur C, Porgador A, Elboim M, Gazit R, Mizrahi S, Stern-Ginossar N, Achdout H, et al. The activating receptor NKp46 is essential for the development of type 1 diabetes. Nat. Immunol. 2011;11:121–128. doi: 10.1038/ni.1834. [DOI] [PubMed] [Google Scholar]
- 12.Gur C, Enk J, Kassem SA, Suissa Y, Magenheim J, Stolovich-Rain M, Nir T, et al. Recognition and killing of human and murine pancreatic beta cells by the NK receptor NKp46. J. Immunol. 2011;187:3096–3103. doi: 10.4049/jimmunol.1101269. [DOI] [PubMed] [Google Scholar]
- 13.Walzer T, Dalod M, Robbins SH, Zitvogel L. Vivier E. Natural-killer cells and dendritic cells: “l'union fait la force. Blood. 2005;106:2252–2258. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- 14.Seidel E, Glasner A. Mandelboim O. Virus-mediated inhibition of natural cytotoxicity receptor recognition. Cell Mol. Life Sci. 2012;69:3911–3920. doi: 10.1007/s00018-012-1001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vitale M, Della Chiesa M, Carlomagno S, Pende D, Arico M, Moretta L. Moretta A. NK-dependent DC maturation is mediated by TNFalpha and IFNgamma released upon engagement of the NKp30 triggering receptor. Blood. 2005;106:566–571. doi: 10.1182/blood-2004-10-4035. [DOI] [PubMed] [Google Scholar]
- 16.Koka R, Burkett P, Chien M, Chai S, Boone DL. Ma A. Cutting edge: murine dendritic cells require IL-15R alpha to prime NK cells. J. Immunol. 2004;173:3594–3598. doi: 10.4049/jimmunol.173.6.3594. [DOI] [PubMed] [Google Scholar]
- 17.Lucas M, Schachterle W, Oberle K, Aichele P. Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spaggiari GM, Carosio R, Pende D, Marcenaro S, Rivera P, Zocchi MR, Moretta L, et al. NK cell-mediated lysis of autologous antigen-presenting cells is triggered by the engagement of the phosphatidylinositol 3-kinase upon ligation of the natural cytotoxicity receptors NKp30 and NKp46. Eur. J. Immunol. 2001;31:1656–1665. doi: 10.1002/1521-4141(200106)31:6<1656::aid-immu1656>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 19.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM. Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J. Exp. Med. 2002;195:343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferlazzo G, Semino C. Melioli G. HLA class I molecule expression is up-regulated during maturation of dendritic cells, protecting them from natural killer cell-mediated lysis. Immunol. Lett. 2001;76:37–41. doi: 10.1016/s0165-2478(00)00323-0. [DOI] [PubMed] [Google Scholar]
- 21.Andrews DM, Andoniou CE, Granucci F, Ricciardi-Castagnoli P. Degli-Esposti MA. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat. Immunol. 2001;2:1077–1084. doi: 10.1038/ni724. [DOI] [PubMed] [Google Scholar]
- 22.Andoniou CE, van Dommelen SL, Voigt V, Andrews DM, Brizard G, Asselin-Paturel C, Delale T, et al. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat. Immunol. 2005;6:1011–1019. doi: 10.1038/ni1244. [DOI] [PubMed] [Google Scholar]
- 23.O'Leary JG, Goodarzi M, Drayton DL. von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 24.Majewska-Szczepanik M, Strzepa A, Drozynska I, Motyl S, Banach T. Szczepanik M. Epicutaneous immunization with hapten-conjugated protein antigen alleviates contact sensitivity mediated by three different types of effector cells. Pharmacol. Rep. 2012;64:919–926. doi: 10.1016/s1734-1140(12)70887-3. [DOI] [PubMed] [Google Scholar]
- 25.Bloch B. The role of idiosyncracy and allergy in dermatology. Arch. Dermat. Syph. 1929;19:175–197. [Google Scholar]
- 26.Asherson GL. Ptak W. Contact and delayed hypersensitivity in the mouse. I. Active sensitization and passive transfer. Immunology. 1968;15:405–416. [PMC free article] [PubMed] [Google Scholar]
- 27.Kissenpfennig A. Malissen B. Langerhans cells–revisiting the paradigm using genetically engineered mice. Trends Immunol. 2006;27:132–139. doi: 10.1016/j.it.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Brasch J, Burgard J. Sterry W. Common pathogenetic pathways in allergic and irritant contact dermatitis. J. Invest. Dermatol. 1992;98:166–170. doi: 10.1111/1523-1747.ep12555804. [DOI] [PubMed] [Google Scholar]
- 29.Wehner R, Dietze K, Bachmann M. Schmitz M. The bidirectional crosstalk between human dendritic cells and natural killer cells. J. Innate Immun. 2011;3:258–263. doi: 10.1159/000323923. [DOI] [PubMed] [Google Scholar]
- 30.Elboim M, Gazit R, Gur C, Ghadially H, Betser-Cohen G. Mandelboim O. Tumor immunoediting by NKp46. J. Immunol. 2010;184:5637–5644. doi: 10.4049/jimmunol.0901644. [DOI] [PubMed] [Google Scholar]
- 31.Arnon TI, Achdout H, Lieberman N, Gazit R, Gonen-Gross T, Katz G, Bar-Ilan A, et al. The mechanisms controlling the recognition of tumor- and virus-infected cells by NKp46. Blood. 2004;103:664–672. doi: 10.1182/blood-2003-05-1716. [DOI] [PubMed] [Google Scholar]
- 32.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N. Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 33.Rouzaire P, Luci C, Blasco E, Bienvenu J, Walzer T, Nicolas JF. Hennino A. Natural killer cells and T cells induce different types of skin reactions during recall responses to haptens. Eur. J. Immunol. 2012;42:80–88. doi: 10.1002/eji.201141820. [DOI] [PubMed] [Google Scholar]
- 34.Min-Oo G, Kamimura Y, Hendricks DW, Nabekura T. Lanier LL. Natural killer cells: walking three paths down memory lane. Trends Immunol. 2013;34:251–258. doi: 10.1016/j.it.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu L, Ikizawa K, Hu D, Werneck MB, Wucherpfennig KW. Cantor H. Regulation of activated CD4+ T cells by NK cells via the Qa-1-NKG2A inhibitory pathway. Immunity. 2007;26:593–604. doi: 10.1016/j.immuni.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waggoner SN, Cornberg M, Selin LK. Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2011;481:394–398. doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Elssen CH, Vanderlocht J, Oth T, Senden-Gijsbers BL, Germeraad WT. Bos GM. Inflammation restraining effects of prostaglandin E2 on natural killer-dendritic cell (NKDC) interaction are imprinted during DC maturation. Blood. 2011;118:2473–2482. doi: 10.1182/blood-2010-09-307835. [DOI] [PubMed] [Google Scholar]
- 38.Tomasello E, Yessaad N, Gregoire E, Hudspeth K, Luci C, Mavilio D, Hardwigsen J, et al. Mapping of NKp46(+) Cells in healthy human lymphoid and non-lymphoid tissues. Front. Immunol. 2012;3:344. doi: 10.3389/fimmu.2012.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reynders A, Yessaad N, Vu Manh TP, Dalod M, Fenis A, Aubry C, Nikitas G, et al. Identity, regulation and in vivo function of gut NKp46+RORgammat+ and NKp46+RORgammat- lymphoid cells. EMBO J. 2011;30:2934–2947. doi: 10.1038/emboj.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shinagawa K. Kojima M. Mouse model of airway remodeling: strain differences. Am. J. Respir. Crit. Care Med. 2003;168:959–967. doi: 10.1164/rccm.200210-1188OC. [DOI] [PubMed] [Google Scholar]
- 41.Gumy A, Louis JA. Launois P. The murine model of infection with Leishmania major and its importance for the deciphering of mechanisms underlying differences in Th cell differentiation in mice from different genetic backgrounds. Int. J. Parasitol. 2004;34:433–444. doi: 10.1016/j.ijpara.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 42.Kodama M, Asano K, Oguma T, Kagawa S, Tomomatsu K, Wakaki M, Takihara T, et al. Strain-specific phenotypes of airway inflammation and bronchial hyperresponsiveness induced by epicutaneous allergen sensitization in BALB/c and C57BL/6 mice. Int. Arch. Allergy Immunol. 2010;152(Suppl 1):67–74. doi: 10.1159/000312128. [DOI] [PubMed] [Google Scholar]
- 43.Mills CD, Kincaid K, Alt JM, Heilman MJ. Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- 44.Asselin-Paturel C, Brizard G, Pin JJ, Briere F. Trinchieri G. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J. Immunol. 2003;171:6466–6477. doi: 10.4049/jimmunol.171.12.6466. [DOI] [PubMed] [Google Scholar]
- 45.Flacher V, Douillard P, Ait-Yahia S, Stoitzner P, Clair-Moninot V, Romani N. Saeland S. Expression of langerin/CD207 reveals dendritic cell heterogeneity between inbred mouse strains. Immunology. 2008;123:339–347. doi: 10.1111/j.1365-2567.2007.02785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Condon TV, Sawyer RT, Fenton MJ. Riches DW. Lung dendritic cells at the innate-adaptive immune interface. J. Leukoc. Biol. 2011;90:883–895. doi: 10.1189/jlb.0311134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toebak MJ, Gibbs S, Bruynzeel DP, Scheper RJ. Rustemeyer T. Dendritic cells: biology of the skin. Contact Dermatitis. 2009;60:2–20. doi: 10.1111/j.1600-0536.2008.01443.x. [DOI] [PubMed] [Google Scholar]
- 48.Randolph GJ, Inaba K, Robbiani DF, Steinman RM. Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753–761. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- 49.Kool M, Soullie T, van Nimwegen M, Willart MA, Muskens F, Jung S, Hoogsteden HC, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J. Exp. Med. 2008;205:869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Narni-Mancinelli E, Jaeger BN, Bernat C, Fenis A, Kung S, De Gassart A, Mahmood S, et al. Tuning of natural killer cell reactivity by NKp46 and Helios calibrates T cell responses. Science. 2012;335:344–348. doi: 10.1126/science.1215621. [DOI] [PubMed] [Google Scholar]
- 51.Eckelhart E, Warsch W, Zebedin E, Simma O, Stoiber D, Kolbe T, Rulicke T, et al. A novel Ncr1-Cre mouse reveals the essential role of STAT5 for NK-cell survival and development. Blood. 2011;117:1565–1573. doi: 10.1182/blood-2010-06-291633. [DOI] [PubMed] [Google Scholar]
- 52.Horani A, Shoseyov D, Doron S, Mruwat R, Amer J, Kerem E. Safadi R. Immune modulation of ovalbumin-induced lung injury in mice using beta-glucosylceramide and a potential role of the liver. Immunobiology. 2011;216:548–557. doi: 10.1016/j.imbio.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG. Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am. J. Respir. Crit. Care Med. 1997;156:766–775. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- 54.Birnberg T, Bar-On L, Sapoznikov A, Caton ML, Cervantes-Barragan L, Makia D, Krauthgamer R, et al. Lack of conventional dendritic cells is compatible with normal development and T cell homeostasis, but causes myeloid proliferative syndrome. Immunity. 2008;29:986–997. doi: 10.1016/j.immuni.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 55.Gur C, Porgador A, Elboim M, Gazit R, Mizrahi S, Stern-Ginossar N, Achdout H, et al. The activating receptor NKp46 is essential for the development of type 1 diabetes. Nat. Immunol. 2010;11:121–128. doi: 10.1038/ni.1834. [DOI] [PubMed] [Google Scholar]
- 56.Stern-Ginossar N, Saleh N, Goldberg MD, Prichard M, Wolf DG. Mandelboim O. Analysis of human cytomegalovirus-encoded microRNA activity during infection. J. Virol. 2009;83:10684–10693. doi: 10.1128/JVI.01292-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary


