Abstract
Background: The human ultraviolet-B (UVB) experimental pain model induces cutaneous neurogenic inflammation, involves hyperalgesia, and is widely used as a pharmacological screening pain model. Aim: To estimate the test-retest reliability of the UVB pain model by application of a comprehensive set of vasomotor and quantitative sensory assessment methods and to estimate sample sizes required for parallel or crossover pharmacological screening studies when this model is considered to be applied. Methods: The upper arms of 15 healthy male volunteers were UVB irradiated with three times the minimal erythema dose. Neurogenic inflammation was assessed by measuring erythema index, superficial blood flow and skin temperature at baseline, 1 day, 2 days and 3 days post irradiation. Sensory changes were assessed by brush stroke, von Frey hairs, pressure algometry, heat-evoked pain, stimulus response function to weight calibrated pin-prick stimulation, and the area of secondary hyperalgesia. The experiment was repeated with a two-week interval. Systematic bias, Coefficient of variation (CV), and intra-class correlation (ICC) were calculated within and between UVB irradiations. The sample sizes for parallel and crossover studies were calculated. Results: Neurogenic inflammation (erythema index) and primary hyperalgesia (pin-prick stimulation) remained significant for 3 days, and were highly reproducible within and between the UVB irradiations resulting of low sample sizes (4-26) in both parallel and crossover studies. Conclusion: Based on sample size calculations, it is recommended to use the erythema index to assess neurogenic inflammation, and pin-prick stimulation for primary hyperalgesia for both parallel and crossover pharmacological screening studies.
Keywords: Ultraviolet-B, quantitative sensory test, reproducibility, sample size, primary hyperalgesia, secondary hyperalgesia
Introduction
The UVB model is a human [1-6] and animal [7,8] experimental pain model of a local cutaneous hyperalgesia and inflammation. The model is widely used for assessing efficacy and mode-of-action of analgesic and anti-inflammatory drugs in clinical trials [1,2,4]. The UVB model induces thermal and mechanical hyperalgesia (primary hyperalgesia) at the site of irradiation [1,3,5,6] and occasionally in the surrounding areas (secondary hyperalgesia) [2,3,6]. The induced inflammatory process, tissue damage, and released cytokines [9,10] cause a characteristic demarked area of erythema (neurogenic inflammation) [5]. The UVB model is in particular useful for pharmacological screening as it can help translating data from animals to humans [7,8]. The UVB induced primary hyperalgesia develops after approx. 24 h and remains for more than 48 h [2,3,6] making the model useful in pharmacological screening studies with repeated dosing or for compounds with long lasting action [3,11-13]. However limited data exist on the reproducibility of this model when more inflammatory and pain biomarkers are included [1-5], which is important for adequate powering of sample size estimations for planning and designing pharmacological studies utilizing a comprehensive test platform.
No studies have so far evaluated the reproducibility and sample size estimations 1) of a broad spectrum of mechanical and thermal stimulus modalities used for assessing the UVB induced hyperalgesia which is important when effects on specific mechanisms are to be evaluated e.g. transient receptor potential vanilloid receptor 1 (TRPV1) antagonists [14] and the 2) different imaging technologies for assessing the vasomotor response associated with neurogenic inflammation.
A recent study assessing primary heat and mechanical hyperalgesia [15] found promising reproducibility, indicating sufficient reliability of the UVB model especially for crossover designs, but unfortunately sample size estimations were not estimated.
In previous clinical drug trials using the UVB model different sample sizes (range 6-45) have been used. A recent meta-analysis of neuroscience literature showed an average power as low as 8% to 31%, probably causing an overestimation of the investigated effect size [16]. Therefore, to rationally plan and design pharmacological screening studies (parallel or crossover) it is important to know the variability of the various parameters both within the same UVB irradiation and between different UVB irradiation to calculate the proper sample size for different study designs.
The aims of the present study were to 1) investigate intra- and inter-individual variation of the UVB model using a variety of quantitative sensory tests and vasomotor assessment methods and 2) to estimate sample sizes for parallel and crossover pharmacological screening studies.
Materials and methods
Subjects
Fifteen healthy, pain free male volunteers (aged between 18 and 27 years) with a body mass index within the normal range (18.5-24.9 kg/m2) participated in this study after giving their written informed consent. The volunteers had been informed about the right to withdraw from the study at any time without giving a reason and that they would be financially compensated for their time spent. The volunteers participated in a screening session where a full medical history was taken and a pre-study examination was performed before any test procedure. The exclusion criteria were: red-haired; presence of an acute pain; skin diseases, or any use of alcohol, caffeine or any analgesic drugs 24 h prior to the sessions or within the sessions.
Study design
The study was conducted at Aalborg University and performed in accordance with the 1996 version of the Declaration of Helsinki. The study was approved by the local ethics committee “Region North Jutland’s Science Ethics Committee” (registration number: N-20100063) and by the Danish Data Protection Agency.
The study consisted of a screening session, a training session with the determination of the minimal erythema dose (MED) and two identical study sessions (session 1 and 2) with a two weeks interval. Each session consisted of visits over four days. The study design is illustrated in Figure 1.
Figure 1.
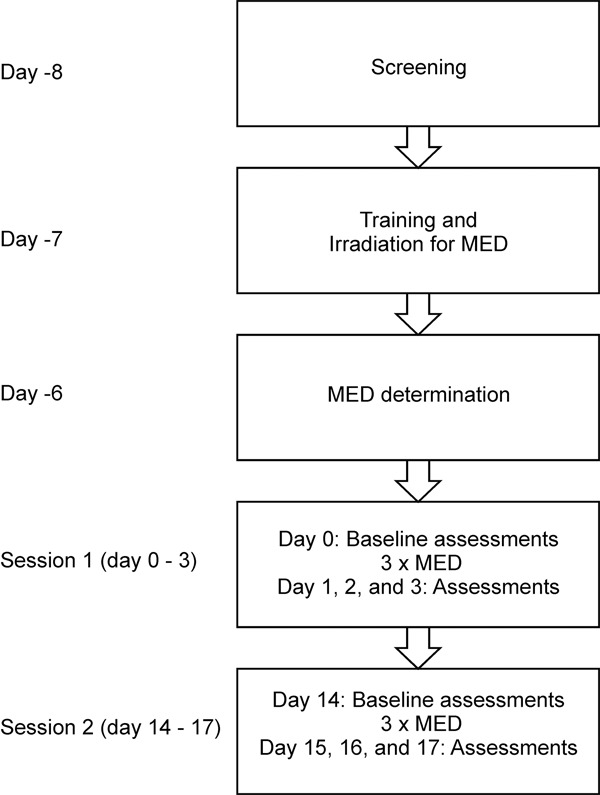
The study design including a screening session, a training session with the UVB irradiation for the determination of the minimal erythema dose (MED) followed by 2 repeated sessions separated by a 2 week’s interval. Assessments were made 1, 2 and 3 days after UVB exposure.
Prior to the study start, the subjects participated in the training session to familiarize with the various test procedures. Assessments of neurogenic inflammation and sensory perception were performed before (baseline) irradiation and 24 h, 48 h and 72 h after irradiation. The baseline assessment of neurogenic inflammation and sensory perception were achieved from the area planned for the UVB irradiation. Subjects were irradiated on the ventro-medial side of the upper arm at a single circular spots with a diameter of 1.5 cm (180 mm2), primary area and with 3 x MED. The between session reliability was assessed by repeating the same procedures on the contralateral upper arm fourteen days after the first irradiation. This allowed an estimation of the reliability between two UVB irradiations.
MED determination
MED was determined using a standardised UVB source (290-320 nm; Saalmann Multitester, SBC LT 400 Herford, Germany). The source was calibrated before each session to ensure the stability of the output. For MED determination, 5 circular spots on the skin with a diameter of 1.5 cm (180 mm2) were exposed with increasing intensities at the volar forearm.
The MED was determined visually 24 h after irradiation using the following grading: 0. No erythema; 1. Very slight erythema (barely perceptible); 2. Well-defined erythema (MED); 3. Moderate to severe erythema; 4. Severe erythema (beet) redness to eschar formation. The circular spots with a grade 2 were defined as the MED of a particular subject.
Neurogenic inflammation
Erythema index
The erythema index (redness) of the skin was measured with a ColorMeter (DSM II Cortex Technology, Hadsund, Denmark). The device provides a read-out of erythema (erythema-index) based on the light absorption characteristics of the skin.
Skin temperature
The skin temperature at the irradiated area was measured with an infrared thermometer (Fluke 561 IR and contact thermometer, Fluke Corporation, Eindhoven, The Netherlands).
Superficial blood flow (sBF)
The sBF was measured with a laser Doppler Imaging (LDI, Moor V5.2 Instrument, Devon, UK). This device scans with a 2-mW helium laser across the skin surface and registers the shifted frequency from the backscattered light. Thereby, the velocity of moving erythrocytes is calculated and presented as a colour coded picture representing a relative measure of perfusion (or flux) in 2 dimensions. The laser head was positioned 30 cm above the skin. An area of 8 x 8 cm was scanned with a resolution of 228 by 230 pixels. The sBF was calculated both in the irradiated (primary) area and the surrounding area (secondary) using relative flux (arbitrary units). The images were analysed using dedicated image-processing software (Moor V5.2 Instruments Ltd.).
Sensory responses
An electronic visual analog scale (eVAS; Aalborg University, Aalborg, Denmark) ranging from 0-10 was used for sensory intensity ratings, where 0 indicated “no sensation”, 5 (midpoint) indicated “pain threshold” and 10 indicated “maximal imaginable pain”. Only the midpoint and ends of the 10 cm sliding scale were marked and the subjects were instructed that the non-painful sensations should be rated below the midpoint and the painful sensations should be rated above the midpoint.
Dynamic mechanical allodynia
Brush evoked dynamic mechanical allodynia was assessed at the site of irradiation (primary area). A standard brush (SENSELab Brush-05, Somedic AB, Hörby, Sweden) was used and manually stroked 3 times along a 2 cm line with an interval of 10 sec. The strokes were applied at an angle of 45° and the subjects were asked to rate the sensation intensity on the eVAS (average of the three subsequent brushes). The subjects were asked to keep their eyes closed during the procedure.
Tactile perception threshold
The tactile perception threshold to thin von Frey filament stimulation was estimated by stimulation with the thinnest von Frey filament (26 mg, 5 g/mm2) and increasing the thickness until the subject perceived the stimulation. Each filament was applied 3 times. The tactile perception threshold was defined as the thinnest filament that a subject perceived at least twice out of 3 stimulations.
Pressure pain threshold (PPT)
The PPT was determined at the irradiated skin with a handheld algometer (Somedic algometer, Somedic AB, Hörby, Sweden). The pressure was applied at a rate of 30 kPa/sec, by using a probe with an area of 0.5 cm2. For determination of the PPT the subjects were instructed to press a response button when the pressure changed from a non-painful to a painful sensation. The average of 3 determinations was used for calculation.
Heat pain threshold (HPT)
HPT was determined by the methods of limits using a thermal sensory testing device (TSA 2001 Medoc TM, Ramat Yishai, Israel). The thermode head (30 x 30 mm) was attached at the site of irradiation using an elastic band. Care was taken to consider upper arm curvatures when placing the probe in order to achieve optimal contact between the probe and the surface of the upper arm. Stimulator temperature range was 32-52°C. Starting temperature was set to 32°C and the temperature was gradually increased at a rate of 0.6°C/s. When the pain threshold was reached, the temperature returned to baseline with a rate of 1.0°C/s. Subjects were instructed to press the stop button as soon as the thermal sensation became painful.
Stimulus-response function to weighted calibrated pin-prick stimulation
The stimulus-response function to weight calibrated pin-prick stimuli was assessed at the irradiated area using weight calibrated pin-prick set (Aalborg University, Denmark) consisting of 8 metallic pins (diameter 0.6 mm) with different weights: 0.8, 1.6, 3.2, 6.4, 12.8, 25.6 and 60.0 g. The subjects were asked to close their eyes and each pin was applied three times (holding the pin for 2 sec.). The subjects rated the average of the three subsequent stimuli on the eVAS.
Determination of the area of secondary hyperalgesia (sArea)
The sArea was quantified with both a von Frey hair and weight calibrated pin-prick stimulation. Based on the findings from previous studies [17], 24 g (122 g/mm2) von Frey hair and the 6.4 g (23 g/mm2) pin were chosen to detect the area of secondary hyperalgesia. The subjects were asked to close their eyes while the stimulation was induced 8 cm away from the area of irradiation and was repeated along a pattern of 8 radial spokes towards the irradiated area. With movement along each spoke at a distance of 5 mm, the subjects were asked to report when the sensation of the pricking changed definitely, i.e. changed from a normal to “different”, “pain”, “burning” or “unpleasant” sensation. This location was marked with a pen, traced on a transparent sheet for later analysis and erased after the measurements to avoid bias during the following measurements. The two areas were determined from these 8 spots by calculating the area of an octagon using the software Vistamatrix (version 1.36.0m, SkillCrest LLC ©).
Statistical analysis
Bias, coefficient of variation (CV), intra-class correlation coefficient (ICC), and sample size estimation were used to estimate the reliability for both parallel and crossover experimental study designs. The post-UVB irradiation measurements were compared between the two UVB irradiations (dominant and non-dominant arms) to estimate the reliability in parallel study designs. The post-UVB irradiation measurements were compared within the same UVB irradiation (1, 2 and 3 days after irradiation) to estimate the reliability in crossover study designs.
Bias
For the assessment of systematic bias, the data were analysed by repeated measures analysis of variance (rmANOVA) with fixed factors of time (four levels; baseline, 1 day, 2 days and 3 days) and session (two levels; session 1 and session 2) and the random subjects factor (SPSS 20, IBM). Secondary hyperalgesia is not present in naïve skin i.e. at baseline, thus, the time factor only included the post treatment measures. The time-by-session interaction was also included in the model to test whether the temporal development of the UVB model was different between sessions. Greenhouse-Geisser correction was used to correct for lack of sphericity often occurring when time is included as a factor. Post hoc Bonferroni multiple corrections were used and p-values less than 0.05 was considered statistically significant.
ICC
The ICC (2,1) model [18] was used to estimate the relative reliability both within and between sessions. The ICC is the between subjects variation divided by the total variation:
ICC = σs 2/(σs 2+σe 2)
and it relates to the consistency of the subjects rank or position in the test relative to the retest [19].
CV
The absolute reliability was estimated by calculating the CV:
CV = SD/μ,
where SD is the standard deviation and μ is the mean of the measurements for each individual. CV is therefore an absolute measure of reliability describing the typical error divided by the mean [20].
Sample size estimation
The minimal samples sizes needed to detect a clinically relevant effect of a potential pharmaceutical screening were calculated for a parallel and a crossover study. The desired significance level (α) was set to 0.05 by convention and the desired power (1-β) was set to 0.9. The clinically relevant effect (E) was estimated as 30% of the difference between baseline measurements and post-UVB irradiation measurements. For a parallel study design the sample size (nparallel) was estimated as:
nparallel = 21.01σ 2/E 2,
where σ is the standard deviation of the post-UVB irradiation measurements [21]. For a crossover study design the sample size (ncorss-over) was estimated as
ncorss-over = 21.01σ 2(1-ρ)/E 2,
where ρ is Pearsons correlation coefficient estimated between measurements at 24 h and 78 h [21].
Results
Fifteen subjects completed the study. None of the subjects reported pain during the UVB irradiation and no blister or skin damage appeared on the irradiated skin. The MEDs were between 55-160 mJ/cm2.
Neurogenic inflammation
Erythema index
The erythema index was significantly increased after UVB irradiation (p < 0.001) and there was no significant difference between the 3 post UVB measurements and no statistically significant difference between sessions (Figure 2).
Figure 2.
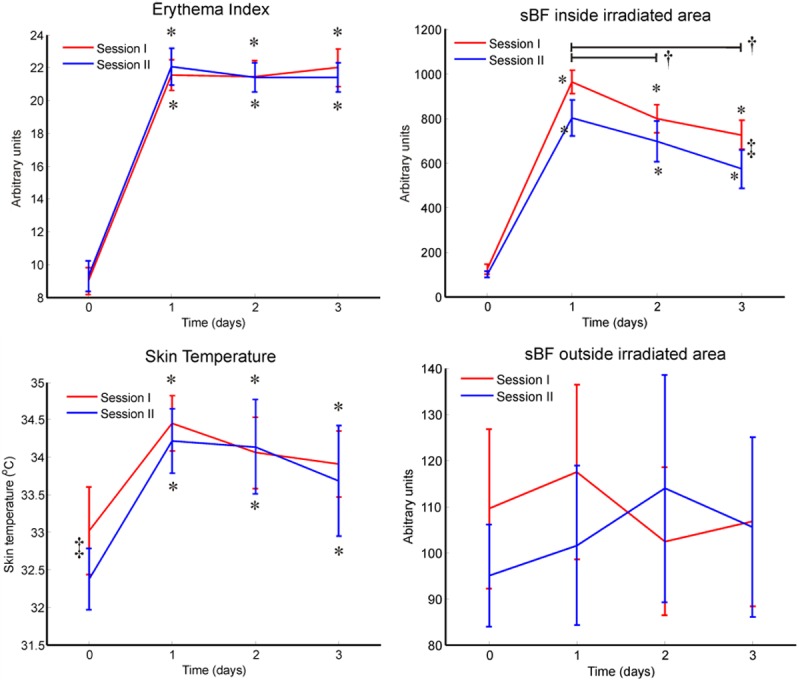
Neurogenic inflammation of the UVB model. The neurogenic inflammatory response to UVB treatment was observed as an increased erythema, skin temperature, and superficial blood flow (sBF) inside the irradiated area (p < 0.001, rmANOVA, Bonferroni), but not sBF outside the irradiated area. Difference between post UVB treatment and baseline is indicated by *, difference between sessions is indicated by ‡, and a black line spans differences within post UVB treatment assessments is indicated by †.
Skin temperature
The skin temperature was significantly increased after UVB irradiation (p < 0.001) and there was no significant difference between the 3 post-UVB measurements. Baseline skin temperature was 0.6°C higher in session 1 than in session 2 (p < 0.011; Figure 2).
sBF inside the irradiated area
The sBF inside the irradiated area was significantly increased after UVB irradiation (p < 0.001), and decreased during the 3 post treatment days (p < 0.04). The sBF inside the irradiated area was higher after the first UVB irradiation than the second UVB irradiation at the first and the third days after UVB irradiation (p < 0.01, Figure 2).
sBF outside the irradiated area
The sBF surrounding the irradiated area was not significantly increased after UVB irradiation and there were no statistically significantly differences between the SBF outside the irradiated area generated by the two UVB sessions (Figure 2).
Reliability and sample size estimation
The reliability measures and sample size estimations of the neurogenic inflammation assessments are shown in Table 1.
Table 1.
Reliability and sample size estimation of neurogenic inflammation assessment methods in the human UVB model. The intra-class correlation coefficient (ICC) and coefficient of variation (CV) were calculated within the 3 consecutive days after one UVB irradiation as well as between two UVB irradiations separated by two weeks. The sample size was estimated for crossover and parallel designed drug studies. Neurogenic inflammation was assessed as erythema, skin temperature and superficial blood flow (sBF) inside and outside the irradiated area
| Within UVB irradiation | Between UVB irradiation | Estimated Sample Size | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| ICC | CV (%) | ICC | CV (%) | Crossover | Parallel | |
| Erythema index | 0.50 | 6 | 0.63 | 4 | 4 | 6 |
| Skin Temperature | 0.43 | 2 | 0.38 | 2 | 73 | 134 |
| sBF inside irradiated area | 0.29 | 20 | 0.35 | 19 | 15 | 20 |
| sBF outside irradiated area | 0.31 | 22 | -0.05 | 23 | 6850 | 10230 |
Primary sensory responses
Dynamic mechanical allodynia
The sensation evoked by brush stroking was increased at day 2 after UVB irradiation compared to baseline (p < 0.022), whereas no significant changes from baseline was observed at days 1 and 3 (Figure 3). There were no differences between the two sessions and there were no interactions between session and time factors.
Figure 3.
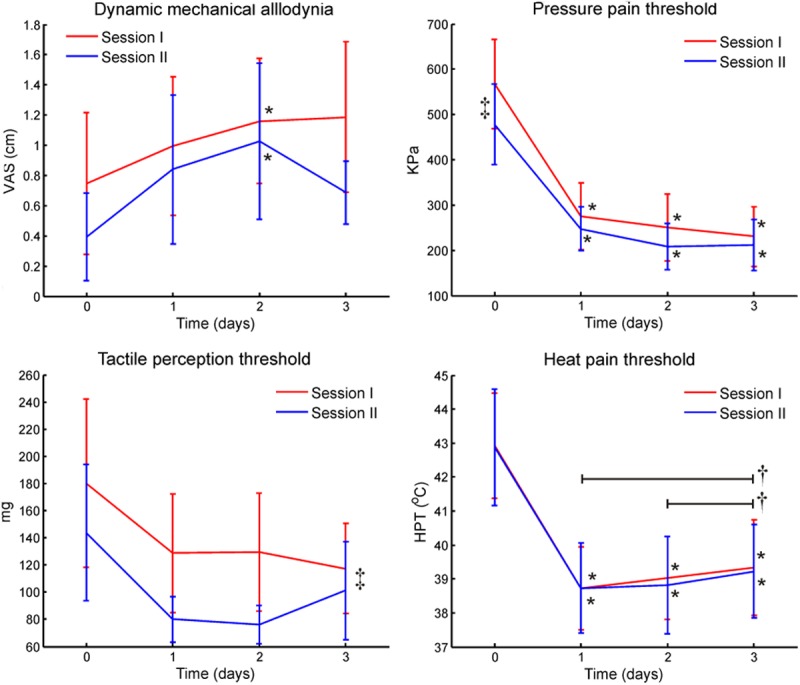
Primary sensory changes of the UVB model. Primary sensory changes to UVB treatment were assessed for dynamic mechanical allodynia to brush stroking, tactile perception threshold to von Frey stimulation, pressure pain threshold, and heat pain threshold. Difference between post UVB treatment and baseline is indicated by *, difference between sessions is indicated by ‡, and a black line spans differences within post UVB treatment assessments is indicated by †.
Tactile perception threshold
The tactile sensation threshold to von Frey hairs was different between sessions (p < 0.047; Figure 3). There was also a significant time effect (p < 0.046) but pairwise Bonferroni corrected comparisons did not indicate any significance. No interaction between the session and time factors was found.
Stimulus-response function to weighted calibrated pin-prick stimulation
The stimulus response functions of the weight calibrated pin-prick stimulation showed that the VAS increased as the pin-prick weights were increased (p < 0.001). The VAS was increased by the UVB irradiation at all three post UVB irradiation days as compared to baseline (p < 0.001). The post UVB irradiation VAS scores were not statistically different from each other. Furthermore, there was no statistically difference between the two UVB irradiation sessions (Figure 4).
Figure 4.
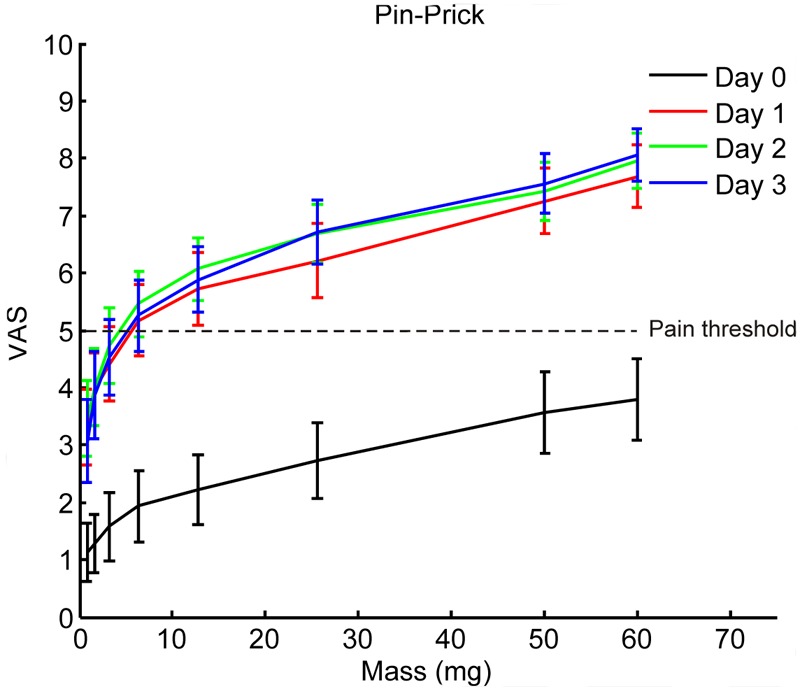
Stimulus response functions of weight calibrated pin prick stimulation before (Day 0) and up to three days (Day 1-3) after UVB irradiation. There were no statistical significant differences between the two UVB irradiation sessions therefore the data from the two sessions are plotted together (rmANOVA, p > 0.05). The VAS increased as the intensities increased (rmANOVA, Bonferonni, p < 0.001). The VAS assessed at baseline was increased by UVB irradiation assessed at all three days (rmANOVA, Bonferonni, p < 0.001).
The reliability analysis showed better reliability and lower estimated sample sizes for heavier pin-prick stimulation compared to lighter pin-prick stimulation (Table 2).
Table 2.
Reliability and sample size estimation of primary sensory responses in the human UVB model. The intra-class correlation coefficient (ICC) and coefficient of variation (CV) were calculated within the 3 consecutive days after one UVB irradiation as well as between two UVB irradiations separated by two weeks. The sample size was estimated for crossover and parallel designed drug studies. Primary sensory responses were assessed as dynamic mechanical allodynia by brush stroking, tactile perception threshold to von Frey stimulation, pressure pain threshold, heat pain threshold, and the sensation to pin-prick stimulation with calibrated weights at 12.8 g and 60.0 g
| Within UVB irradiation | Between UVB irradiation | Estimated Sample Size | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| ICC | CV (%) | ICC | CV (%) | Crossover | Parallel | |
| Dynamic mechanical allodynia | 0.74 | 40 | 0.25 | 47 | 352 | 1043 |
| Tactile perception threshold | 0.62 | 26 | 0.08 | 33 | 180 | 333 |
| Pressure pain threshold | 0.74 | 21 | 0.59 | 23 | 15 | 42 |
| Heat pain threshold | 0.94 | 1 | 0.89 | 2 | 6 | 100 |
| Pin-prick, 12.8 g | 0.68 | 16 | 0.64 | 15 | 19 | 46 |
| Pin-prick, 60.0 g | 0.76 | 8 | 0.54 | 9 | 7 | 26 |
Pressure pain threshold (PPT)
The PPT was significantly decreased after both UVB treatments at all 3 days (p < 0.006), and there were no significant differences between the post UVB irradiation days. The PPT was different between the two UVB sessions (p < 0.020, Figure 3). No interaction between the session and time factors was found.
Heat pain threshold (HPT)
The HPT was significantly decreased after UVB irradiation (p < 0.001), where all 3 post UVB radiation days were lower than baseline (p < 0.001), and the HPT at the third day was increased compared to the first and the second day after UVB irradiation (p < 0.040). There were no significant differences between the two UVB sessions and there were no interactions between the session and time factors (Figure 3).
sArea
Von Frey hair evoked sArea
The sArea assessed by von Frey hair was larger than zero at all three post UVB radiation days, but not larger than the irradiated area (180 mm2). Time (p < 0.002), session (p < 0.001) and interaction (p < 0.046) effects were observed between post-UVB treatments assessments. The sArea was the largest after the first UVB session assessed at both the first (p < 0.005) and the second (p < 0.002) days. The sArea decreased from the first to the third day (p < 0.02) after the first UVB irradiation, but no differences were observed between days after the second UVB irradiation (Figure 5).
Figure 5.
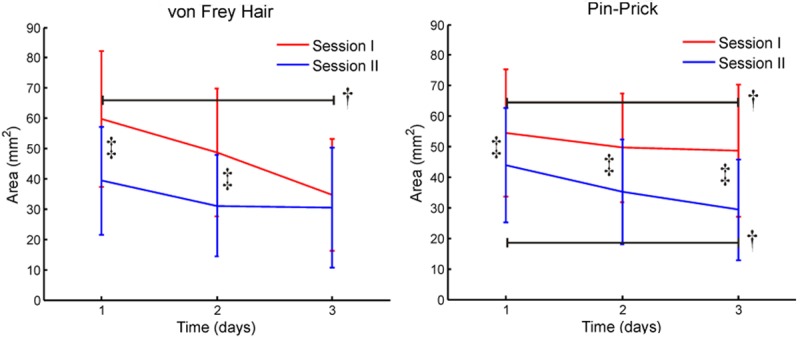
The area of secondary hyperalgesia in the UVB model. The area of secondary hyperalgesia after UVB treatment was assessed as sensory changes when stimulated by von Fray filaments and weight calibrated pin prick stimulation. Difference between sessions is indicated by ‡, and a black line spans differences within post UVB treatment assessments is indicated by †.
Pin-prick evoked sArea
The sArea assessed by pin-prick stimulation was larger than zero at all three post UVB radiation days, but not larger than the irradiated area (180 mm2). The sArea was larger after the first session than after the second session (p < 0.002), and the sArea decreased from the first to the third day UVB (p < 0.031, Figure 5).
The reliability measures and sample size estimations of the sArea assessments are shown in Table 3.
Table 3.
Reliability and sample size estimation of the area of secondary hyperalgesia in the human UVB model. The intra-class correlation coefficient (ICC) and coefficient of variation (CV) were calculated within the 3 consecutive days after one UVB irradiation as well as between two UVB irradiations separated by two weeks. The sample size was estimated for crossover and parallel designed drug studies. The area of secondary hyperalgesia was assessed as the area of sensory changes when stimulated by von Fray filaments and weight calibrated pin-prick stimulation
| Within UVB irradiation | Between UVB irradiation | Estimated Sample Size | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| ICC | CV (%) | ICC | CV (%) | Crossover | Parallel | |
| Von Frey | 0.82 | 50 | 0.82 | 45 | 48 | 212 |
| Pin-Prick | 0.90 | 39 | 0.82 | 44 | 21 | 171 |
Discussion
The present study for the first time 1) investigated the effect size and reproducibility of a comprehensive set of measures for UVB induced neurogenic inflammation and pain sensitization and 2) estimated the number of volunteers to be in included in crossover and parallel pharmacological studies to detect a 30% recovery (drug effect) of the UVB induced changes.
The sample size estimation is derived from both the variation of measurements under fixed conditions, the effect size caused by UVB irradiation, and for crossover studies also the correlation of the assessments between test and re-test. Furthermore, we reported the sample size estimation needed to detect a 30% recovery by the tested drug. It should be noted that if smaller effect size are expected, more subjects must be added to the study to detect the possible effect of the tested drug.
Sample size estimation is needed for designing basic and pharmacological studies using the UVB inflammatory pain model. Recently, analysis of the biomedical literature has indicated a surprisingly low power of studies in the biomedical research [16,22] and hence adequate sample sizes and reproducible outcome measures are pivotal for study designs and in particular for studies where the mode-of-mechanism is assessed for new analgesic compounds under development.
Neurogenic inflammatory assessments
UVB induced neurogenic inflammation was highly reproducible providing low numbers of participants as well in crossover as in parallel group studies with acceptable ranging from 4-15 volunteers and 6-20 volunteers, respectively.
Erythema
The quantitative and objective assessment of the erythema index showed a significant development over the first 24 hours and remained stable up to 72 hours which adds quantitative data to the previous studies where erythema has been assessed by visual inspection [1,2,3,5,14,23-26]. Assessing erythema in a quantitative and objective way is recommended as it is much more reliable than visual scores of redness as done in many studies. Objective assessment of erythema provides a reliable assessment tool for allowing low sample sizes for both crossover (n = 4) and parallel (n = 6) study designs.
Superficial blood flow (sBF)
An eight-fold increase of sBF in the irradiated area was observed after 24 h as previously reported using the same laser Doppler imaging technology [24] and we found a slight decline at the day 2 and 3 assessments. Hoffmann and Schmelz [23] found a slightly earlier peak in blood flow at 12 h and a significant elevation up to 4 days after the irradiation. Like assessing erythema index, assessment of the sBF provides an objective and reliable assessment method with slightly larger sample sizes for both crossover (n = 15) and parallel (n = 20) designs.
Modulation of UVB induced neurogenic inflammation
The use of different techniques for assessment of neurogenic inflammation has been suggested by Zachariae et al. [27] as they found a significant effect of hypnotic suggestions on cutaneous blood flow but no effect on erythema indicating separate regulatory mechanisms behind central nervous system influence on UVB induced erythema and skin blood flow.
Reliable assessment of the UVB induced neurogenic inflammation is becoming increasingly important as new compounds under development such as TRPV1-antagonists have shown to modulate neurogenic inflammation significantly [14] and hence it is recommended to include this parameter in the drug screening platform. Drugs interacting with the peripheral neuronal network via specific receptor systems may modulate differently the different component of the neurogenic inflammation and hence provide important mechanistic information. Both temperature and erythema in the primary area have previously been shown to be modulated by a topical non-steroidal anti-inflammatory drug patch [28,29]. Botulinum toxin A is found to have no effect on UVB induced neurogenic inflammation [26] whereas the toxin caused significant inhibition of capsaicin-induced neurogenic inflammation [30] indicating the importance of incorporating and utilising different techniques in the provocation and analysis of neurogenic inflammation. The neurogenic inflammation induced by UVB and capsaicin are fundamentally very different where the UVB erythema is restricted to the area of damage/irradiation and the capsaicin causes a substantial spread outside the area affected.
Primary allodynia and hyperalgesia
The present study detected significant dynamic mechanical allodynia within the irradiated area at day 2 after UVB irradiation, in accordance with the study by Sycha et al. [26]. The static primary pin-prick hyperalgesia assessed with the highest fixed stimulus intensity showed the most reproducible sensory changes. Bishop et al. [5] assessed the primary hyperalgesia with an electronic von Frey 6 h, 1 day, 2 days, 3 days, and 4 days after UVB irradiation and detected a significant decrease in the mechanical pain threshold for all time points with a peak of effect at day 1 but the study did not assess the reliability of the test. In the current study the pin-prick hyperalgesia remained stable for 3 days providing a good opportunity to follow drug effects over days using only one induction of primary hyperalgesia. As compared with e.g. topical capsaicin the primary hyperalgesia is only stable few hours after induction.
Stimulus-response function to weight calibrated pin-prick stimulation
The present study showed an upward shift of the stimulus-response function to weight calibrated pin-prick stimulation assessed in the area of primary hyperalgesia. The shift remained for the 3-day period of the study. This finding was similar to the results from Sycha et al. [26] who investigated hyperalgesia to pin-prick 1 day after the irradiation. This study also found that the intensity of hyperalgesia was proportionally increased with an increase in pin-prick weight [26]. Based on the results obtained from the present study the pin-prick test is a more reliable method for the detection of primary hyperalgesia in this model, compared to the tactile sensation threshold obtained by von Frey hairs. Furthermore, the most reliable results were obtained with the heaviest pin-pricks. Therefore, the pin-prick method is a reliable indicator for the inflammatory activation of Aδ- fibers in the area of primary hyperalgesia.
Pressure pain threshold (PPT)
The present study detected a significant decrease in the PPT, but the degree of decrease differs between the two sessions. The PPT was at baseline lower in session 2 (left arm) in comparison to the session 1 (right arm). The issue might be a matter of arm dominance [31], as the non-dominant side is more sensitive to pain than the dominant side and thirteen of the fifteen subjects in this study were right-handed. Increased PPT both inside and outside the UVB irradiated area has previously been shown [32], however it is still not clear if the pressure is activating cutaneous nerve fibers or if the response from deep nociceptors is being sensitized. Although the sample sizes are reasonable (15 and 42), additional studies on the PPT in the model should be performed before PPT assessments can be recommended for pharmacological screening studies.
Heat pain threshold (HPT)
A decrease in the HPT following the UVB irradiation was detected with the lowest thresholds at day 1. The HPT gradually returned towards baseline, but remained below baseline for 3 days. Several studies have investigated the HPT in this model and have detected a significant decrease in the HPT 1 day after irradiation [2,4,5,23,25,26]. Two other studies have also investigated the time-course of the HPT with similar results as seen in the present study [5,23], but these studies did not investigate the reliability of this test. The reproducibility test showed that UVB irradiation induced sensory changes can be assessed by HPT in crossover studies with 6 subject to include, but not in parallel studies (n = 100). This pronounced difference in sample sizes is caused by the high relative reliability of HPT assessments.
Secondary hyperalgesia
In the present study, an area of altered sensation was observed to both von Frey hair and pin-prick stimuli. The area decreased during the 3-day study period, but remained significantly different from zero. The area was not larger than the irradiated area and it is questionable if the changed sensation reflects a secondary phenomenon or rather a primary sensory change. Although both methods were able to detect an area of altered sensation, the pin-prick method required fewer subjects compared to the von Frey filament method to detect a 30% return to baseline in the UVB model. Based on these results, both tests seem to be reliable within the same subject, but the variation between subjects was large. The size of area of altered sensation was larger during session 1 compared to session 2, which indicates that the volunteers were more familiar with the process and thereby had a smaller reaction, or that there were differences between the dominant and non-dominant arms. In the present study, the mean sBF outside the irradiated area was investigated, but no change was detected after the irradiation.
The UVB induced sArea has been investigated in several other studies [1,2,3,5,26], but different sites of irradiation were chosen and not all of the studies succeeded in the detection of the sArea. One of the studies [5] investigated the sArea on the forearm of the volunteers, but secondary hyperalgesia could not be detected. A similar result was observed in a preliminary pilot study performed prior to the present study, where the forearms of four volunteers were irradiated, but no sArea was detected (data not shown). These findings indicate a possible relation between the development of a sArea and the site of irradiation but the issue needs to be further addressed. Another noticeable factor is the weight of the von Frey hair used for the detection of the sArea. The studies applying 150 g von Frey [1-3] were able to detect the presence of a sArea, while the study applying the 10 g von Frey filament did not detect sArea i.e. Bishop et al [5]. A 10 g von Frey was probably not a suitable pressure for the detection of any sArea.
The UVB model as a pharmaceutical screening tool
The primary pin-prick hyperalgesia has been shown to be UVB dose-dependent in animals [7] and human studies [5] and hence this parameter seems to translate from animal to humans. In addition primary pin-prick hyperalgesia is modulated by different compounds e.g. tramadol [12] and a lidocaine patch [13].
Primary heat hyperalgesia after UVB is a very consistent finding across studies with a very high reproducibility and sufficient sensitivity to detect even an analgesic effect of weak anti-inflammatory drugs [4], weak analgesics such as paracetamol-ketorolac [15], but also capable of profiling new analgesic compounds such as TRPV1 antagonists [14].
The development of secondary pin-prick hyperalgesia after UVB is controversial and hence 2 different methods (von Frey hair and pin-prick) were applied in the present study. These methods were not able to show reliable secondary hyperalgesia. However, Sycha et al. [3] were able to detect an effect of a COX-2 inhibitor on both primary and secondary hyperalgesia and in another study the opioid remifentanil but not the anti-convulsant gabapentin had significant effect on the secondary hyperalgesic area [2]. Topical application of opioids did not have any significant effect on the development of hyperalgesia [33].
Conclusion
The present study showed that in the UVB model induced neurogenic inflammation and hyperalgesia can be reliably tested for a period of 3 days after UVB irradiation and between two UVB sessions 2 weeks apart. Assessment of the erythema index is highly stable and recommended for assessment of neurogenic inflammation in both crossover and parallel drug studies. Primary hyperalgesia was most reliable assessed by a 60 g pin-prick stimulation in both crossover and parallel drug studies. Assessment of the heat pain threshold is only recommended for crossover study designs. When choosing assessment tools and experimental designs, in proof-of-mechanism drug trials it is important to select the mechanistic tools adequate for the drug action but only those with good reproducibility can be selected. Otherwise other pain models should be considered.
Acknowledgements
The authors are grateful for statistical discussions with José Alberto Biurrun Manresa.
Disclosure of conflict of interest
None.
References
- 1.Gustorff B, Anzenhofer S, Sycha T, Lehr S, Kress HG. The Sunburn Pain Model: The Stability of Primary and Secondary Hyperalgesia over 10 Hours in a Crossover Setting. Anesth Analg. 2004;98:173–177. doi: 10.1213/01.ANE.0000093224.77281.A5. [DOI] [PubMed] [Google Scholar]
- 2.Gustorff B, Hoechtl K, Sycha T, Felouzis E, Lehr S, Kress HG. The Effects of Remifentanil and Gabapentin on Hyperalgesia in a New Extended Inflammatory Skin Pain Model in Healthy Volunteers. Anesth Analg. 2004;98:401–407. doi: 10.1213/01.ANE.0000095150.76735.5D. [DOI] [PubMed] [Google Scholar]
- 3.Sycha T, Anzenhofer S, Lehr S, Schmetterer L, Chizh B, Eichler HG, Gustorff B. Rofecoxib attenuates both primary and secondary inflammatory hyperalgesia: A randomized, double blinded, placebo controlled crossover trial in the UV-B pain model. Pain. 2005;113:316–322. doi: 10.1016/j.pain.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Sycha T, Gustorff B, Lehr S, Tanew A, Eichlerl HG, Schmetterer L. A simple pain model for the evaluation of analgesic effects of NSAIDs in healthy subjects. Br J Clin Pharmacol. 2003;56:165–172. doi: 10.1046/j.0306-5251.2003.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bishop T, Ballard A, Holmes H, Young AR, McMahon SB. Ultraviolet-B induced inflammation of human skin: Characterisation and comparison with traditional models of hyperlagesia. EJP. 2009;13:524–532. doi: 10.1016/j.ejpain.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Gustorff B, Sycha T, Lieba-Samal D, Rolke R, Treede RD, Magerl W. The pattern and time course of somatosensory changes in the human UVB sunburn model reveal the presence of peripheral and central sensitization. Pain. 2013;154:586–597. doi: 10.1016/j.pain.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 7.Bishop T, Hewson DW, Yip PK, Fahey MS, Dawbarn D, Young AR, McMahon SB. Characterisation of ultraviolet-B-induced inflammation as a model of hyperalgesia in the rat. Pain. 2007;131:70–82. doi: 10.1016/j.pain.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Bishop T, Marchand F, Young AR, Lewin GR, McMahon SB. Ultraviolet-B-induced mechanical hyperalgesia: A role for peripheral sensitisation. Pain. 2010;150:141–152. doi: 10.1016/j.pain.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Dawes JM, Calvo M, Perkins JR, Paterson KJ, Kiesewetter H, Hobbs C, Kaan TKY, Orengo C, Bennett DLH, McMahon SB. CXCL5 mediates UVB irradiation-induced pain. Sci Transl Med. 2011;3:90ra60. doi: 10.1126/scitranslmed.3002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angst MS, Clark JD, Carvalho B, Tingle M, Schmelz M, Yeomans DC. Cytokine profile in human skin in response to experimental inflammation, noxious stimulation, and administration of a COX-inhibitor: A microdialysis study. Pain. 2008;139:15–27. doi: 10.1016/j.pain.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 11.Rother M, Rother I. Placebo controlled, crossover validation study of oral ibuprofen and topical hydrocortisone-21-acetate for a model of ultraviolet B radiation (UVR)-induced pain and inflammation. J Pain Res. 2011;4:357–363. doi: 10.2147/JPR.S24821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ortner CM, Steiner I, Margeta K, Schulz M, Gustorff B. Dose response of tramadol and its combination with paracetamol in UVB induced hyperalgesia. EJP. 2012;16:562–573. doi: 10.1016/j.ejpain.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Gustorff B, Hauer D, Thaler J, Seis A, Draxler J. Antihyperalgesic efficacy of 5% lidocaine medicated plaster in capsaicin and sunburn pain models two randomized, double-blinded, placebo-controlled crossover trials in healthy volunteers. Expert Opin Pharmacother. 2011;12:2781–2790. doi: 10.1517/14656566.2011.601868. [DOI] [PubMed] [Google Scholar]
- 14.Chizh BA, O’Donnell MB, Napolitano A, Wang J, Brooke AC, Aylott MC, Bullman JN, Gray EJ, Lai RY, Williams PM, Appleby JM. The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain. 2007;132:132–141. doi: 10.1016/j.pain.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Lorenzini KI, Besson M, Daali Y, Salomon D, Dayer P, Desmeules J. Validation of the simplified UVB model to assess the pharmacodynamics of analgesics in healthy human volunteers. Chimia. 2012;66:296–299. doi: 10.2533/chimia.2012.296. [DOI] [PubMed] [Google Scholar]
- 16.Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, Munafó MR. Power failure: Why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 17.Gustafsson H, Åkesson J, Lau CL, Williams D, Miller L, Yap S, Rolan P. A comparison of two formulations of intradermal capsaicin as models of neuropathic pain in healthy volunteers. Br J Clin Pharmacol. 2009;68:511–517. doi: 10.1111/j.1365-2125.2009.03489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 19.Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res. 2005;19:231–240. doi: 10.1519/15184.1. [DOI] [PubMed] [Google Scholar]
- 20.Atkinson G, Nevill AM. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 1998;26:217–238. doi: 10.2165/00007256-199826040-00002. [DOI] [PubMed] [Google Scholar]
- 21.Julious SA. Tutorial in biostatistics: Sample sizes for clinical trials with Normal data. Stat Med. 2004;23:1921–1986. doi: 10.1002/sim.1783. [DOI] [PubMed] [Google Scholar]
- 22.Ioannidis JPA. Why most published research findings are false. PLoS Med. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann RT, Schmelz M. Time course of UVA- and UVB induced inflammation and hyperalgesia in human skin. EJP. 1999;3:131–139. doi: 10.1053/eujp.1998.0106. [DOI] [PubMed] [Google Scholar]
- 24.Bickel A, Dorfs S, Schmelz M, Forster C, Uhl W, Handwerker HO. Effects of antihyperalgesic drugs on experimentally induced hyperalgesia in man. Pain. 1998;76:317–325. doi: 10.1016/S0304-3959(98)00062-1. [DOI] [PubMed] [Google Scholar]
- 25.Koppert W, Likar R, Geisslinger G, Zeck S, Schmelz M, Sittl R. Peripheral antihyperalgesic effect of morphine to heat, but not mechanical, stimulation in healthy volunteers after ultraviolet-B irradiation. Anesth Analg. 1999;88:117–122. [PubMed] [Google Scholar]
- 26.Sycha T, Samal D, Chizh B, Lehr S, Gustorff B, Schnider P, Auff E. A lack of antinociceptive or antiinflammatory effect of botulinum toxin A in an inflammatory human pain model. Anesth Analg. 2006;102:509–516. doi: 10.1213/01.ane.0000194447.46763.73. [DOI] [PubMed] [Google Scholar]
- 27.Zachariae R, Oster H, Bjerring P. Effects of hypnotic suggestions on ultraviolet B radiation-induced erythema and skin blood flow. Photodermatol Photoimmunol Photomed. 1994;10:154–160. [PubMed] [Google Scholar]
- 28.Kienzler JL, Magnette J, Queille-Roussel C, Sanchez-Ponton A, Ortonne JP. Diclofenac-Na gel is effective in reducing the pain and inflammation associated with exposure to ultraviolet light - Results of two clinical studies. Skin Pharmacol Physiol. 2005;18:144–152. doi: 10.1159/000084912. [DOI] [PubMed] [Google Scholar]
- 29.Andersen PH, Abrams K, Maibach H. Ultraviolet B dose-dependent inflammation in humans: A reflectance spectroscopic and laser Doppler flowmetric study using topical pharmacologic antagonists on irradiated skin. Photodermatol Photoimmunol Photomed. 1992;9:17–23. [PubMed] [Google Scholar]
- 30.Gazerani P, Staahl C, Drewes AM, Arendt-Nielsen L. The effects of Botulinum Toxin type A on capsaicin-evoked pain, flare, and secondary hyperalgesia in an experimental human model of trigeminal sensitization. Pain. 2006;122:315–325. doi: 10.1016/j.pain.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 31.Newton AV, Mumford JM. Lateral dominance, pain perception, and pain tolerance. J Dent Res. 1972;51:940–942. doi: 10.1177/00220345720510043501. [DOI] [PubMed] [Google Scholar]
- 32.Lo Vecchio S, da Silva LB, Gazerani P, Graven-Nielsen T, Petersen L, Arendt-Nielsen L. Heat rekindling of UV-B-irradiated skin: a human experimental model of peripheral and central sensitisation. Abstracts of the 14th World Congress on Pain, International Association for the Study of Pain. 2012:No. PT 298. [Google Scholar]
- 33.Draxler J, Schuch M, Paul A, Sycha T, Valenta C, Likar R, Gustorff B. Topical application of morphine and buprenorphine gel has no effect in the sunburn model. Schmerz. 2008;22:571–574. doi: 10.1007/s00482-008-0660-x. [DOI] [PubMed] [Google Scholar]


