Abstract
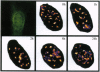
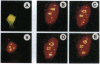
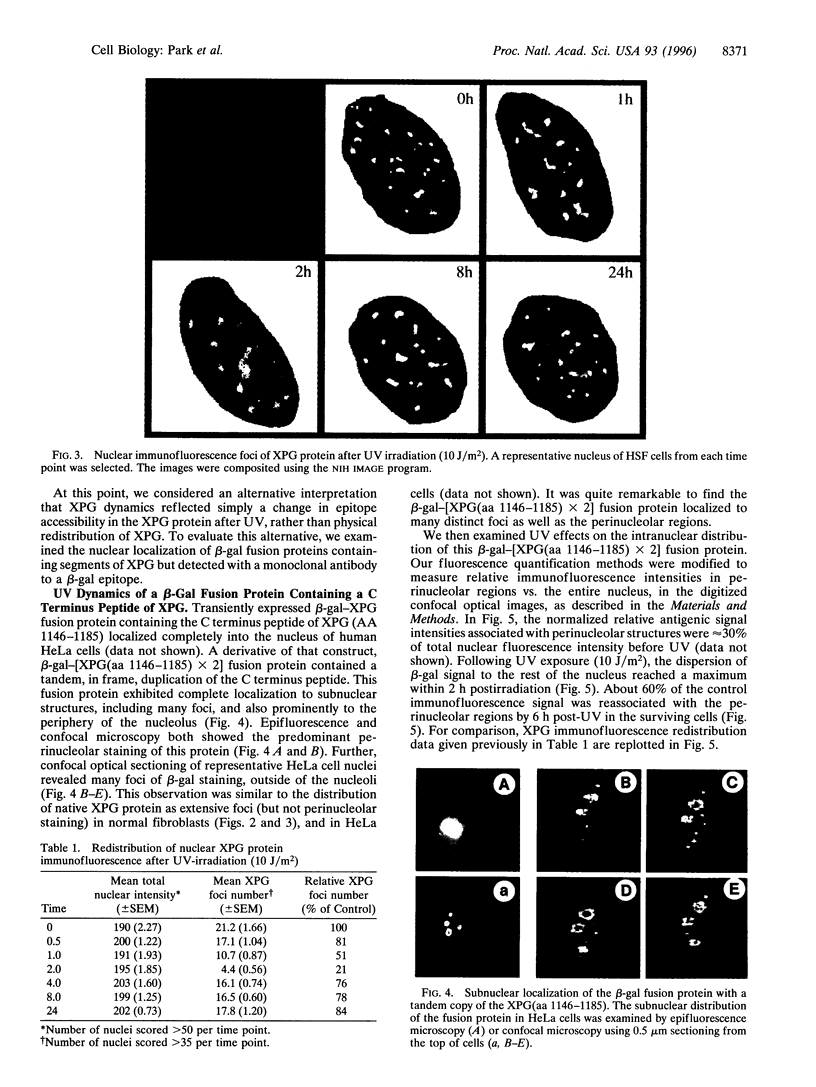
Xeroderma pigmentosum type G (XPG) is a human genetic disease exhibiting extreme sensitivity to sunlight. XPG patients are defective XPG endonuclease, which is an enzyme essential for DNA repair of the major kinds of solar ultraviolet (UV)-induced DNA damages. Here we describe a novel dynamics of this protein within the cell nucleus after UV irradiation of human cells. Using confocal microscopy, we have localized the immunofluorescent, antigenic signal of XPG protein to foci throughout the cell nucleus. Our biochemical studies also established that XPG protein forms a tight association with nuclear structure(s). In human skin fibroblast cells, the number of XPG foci decreased within 2 h after UV irradiation, whereas total nuclear XPG fluorescence intensity remained constant, suggesting redistribution of XPG from a limited number of nuclear foci to the nucleus overall. Within 8 h after UV, most XPG antigenic signal was found as foci. Using beta-galactosidase-XPG fusion constructs (beta-gal-XPG) transfected into HeLa cells, we have identified a single region of XPG that is evidently responsible both for foci formation and for the UV dynamic response. The fusion protein carrying the C terminus of XPG (amino acids 1146-1185) localized beta-gal specific antigenic signal to foci and to the nucleolus regions. After UV irradiation, antigenic beta-gal translocated reversibly from the subnuclear structures to the whole nucleus with kinetics very similar to the movements of XPG protein. These findings lead us to propose a model in which distribution of XPG protein may regulate the rate of DNA repair within transcriptionally active and inactive compartments of the cell nucleus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Bohr V. A., Okumoto D. S., Hanawalt P. C. Survival of UV-irradiated mammalian cells correlates with efficient DNA repair in an essential gene. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3830–3833. doi: 10.1073/pnas.83.11.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr V. A., Phillips D. H., Hanawalt P. C. Heterogeneous DNA damage and repair in the mammalian genome. Cancer Res. 1987 Dec 15;47(24 Pt 1):6426–6436. [PubMed] [Google Scholar]
- Bohr V. A., Smith C. A., Okumoto D. S., Hanawalt P. C. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985 Feb;40(2):359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Carter K. C., Bowman D., Carrington W., Fogarty K., McNeil J. A., Fay F. S., Lawrence J. B. A three-dimensional view of precursor messenger RNA metabolism within the mammalian nucleus. Science. 1993 Feb 26;259(5099):1330–1335. doi: 10.1126/science.8446902. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Madsen P. Increased nuclear cyclin/PCNA antigen staining of non S-phase transformed human amnion cells engaged in nucleotide excision DNA repair. FEBS Lett. 1986 Dec 15;209(2):277–283. doi: 10.1016/0014-5793(86)81127-9. [DOI] [PubMed] [Google Scholar]
- Chen D. J., Strniste G. F., Tokita N. The genotoxicity of alpha particles in human embryonic skin fibroblasts. Radiat Res. 1984 Nov;100(2):321–327. [PubMed] [Google Scholar]
- Ciejek E. M., Tsai M. J., O'Malley B. W. Actively transcribed genes are associated with the nuclear matrix. Nature. 1983 Dec 8;306(5943):607–609. doi: 10.1038/306607a0. [DOI] [PubMed] [Google Scholar]
- Cloud K. G., Shen B., Strniste G. F., Park M. S. XPG protein has a structure-specific endonuclease activity. Mutat Res. 1995 Jul;347(2):55–60. doi: 10.1016/0165-7992(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Cockerill P. N., Garrard W. T. Chromosomal loop anchorage of the kappa immunoglobulin gene occurs next to the enhancer in a region containing topoisomerase II sites. Cell. 1986 Jan 31;44(2):273–282. doi: 10.1016/0092-8674(86)90761-0. [DOI] [PubMed] [Google Scholar]
- Drapkin R., Reardon J. T., Ansari A., Huang J. C., Zawel L., Ahn K., Sancar A., Reinberg D. Dual role of TFIIH in DNA excision repair and in transcription by RNA polymerase II. Nature. 1994 Apr 21;368(6473):769–772. doi: 10.1038/368769a0. [DOI] [PubMed] [Google Scholar]
- Feaver W. J., Svejstrup J. Q., Bardwell L., Bardwell A. J., Buratowski S., Gulyas K. D., Donahue T. F., Friedberg E. C., Kornberg R. D. Dual roles of a multiprotein complex from S. cerevisiae in transcription and DNA repair. Cell. 1993 Dec 31;75(7):1379–1387. doi: 10.1016/0092-8674(93)90624-y. [DOI] [PubMed] [Google Scholar]
- Fey E. G., Wan K. M., Penman S. Epithelial cytoskeletal framework and nuclear matrix-intermediate filament scaffold: three-dimensional organization and protein composition. J Cell Biol. 1984 Jun;98(6):1973–1984. doi: 10.1083/jcb.98.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Habraken Y., Sung P., Prakash L., Prakash S. Human xeroderma pigmentosum group G gene encodes a DNA endonuclease. Nucleic Acids Res. 1994 Aug 25;22(16):3312–3316. doi: 10.1093/nar/22.16.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawalt P. C. Transcription-coupled repair and human disease. Science. 1994 Dec 23;266(5193):1957–1958. doi: 10.1126/science.7801121. [DOI] [PubMed] [Google Scholar]
- Hozák P., Hassan A. B., Jackson D. A., Cook P. R. Visualization of replication factories attached to nucleoskeleton. Cell. 1993 Apr 23;73(2):361–373. doi: 10.1016/0092-8674(93)90235-i. [DOI] [PubMed] [Google Scholar]
- Huang J. C., Svoboda D. L., Reardon J. T., Sancar A. Human nucleotide excision nuclease removes thymine dimers from DNA by incising the 22nd phosphodiester bond 5' and the 6th phosphodiester bond 3' to the photodimer. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3664–3668. doi: 10.1073/pnas.89.8.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. A., Balajee A. S., Mullenders L., Cook P. R. Sites in human nuclei where DNA damaged by ultraviolet light is repaired: visualization and localization relative to the nucleoskeleton. J Cell Sci. 1994 Jul;107(Pt 7):1745–1752. doi: 10.1242/jcs.107.7.1745. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., Hassan A. B., Errington R. J., Cook P. R. Sites in human nuclei where damage induced by ultraviolet light is repaired: localization relative to transcription sites and concentrations of proliferating cell nuclear antigen and the tumour suppressor protein, p53. J Cell Sci. 1994 Jul;107(Pt 7):1753–1760. doi: 10.1242/jcs.107.7.1753. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., Hassan A. B., Errington R. J., Cook P. R. Visualization of focal sites of transcription within human nuclei. EMBO J. 1993 Mar;12(3):1059–1065. doi: 10.1002/j.1460-2075.1993.tb05747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf J. A., Pendergrass S. H., Marrone B. L., Strniste G. F., MacInnes M. A., Park M. S. Multiple nuclear localization signals in XPG nuclease. Mutat Res. 1996 May 15;363(1):67–75. doi: 10.1016/0921-8777(95)00062-3. [DOI] [PubMed] [Google Scholar]
- Ma L., Westbroek A., Jochemsen A. G., Weeda G., Bosch A., Bootsma D., Hoeijmakers J. H., van der Eb A. J. Mutational analysis of ERCC3, which is involved in DNA repair and transcription initiation: identification of domains essential for the DNA repair function. Mol Cell Biol. 1994 Jun;14(6):4126–4134. doi: 10.1128/mcb.14.6.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacInnes M. A., Dickson J. A., Hernandez R. R., Learmonth D., Lin G. Y., Mudgett J. S., Park M. S., Schauer S., Reynolds R. J., Strniste G. F. Human ERCC5 cDNA-cosmid complementation for excision repair and bipartite amino acid domains conserved with RAD proteins of Saccharomyces cerevisiae and Schizosaccharomyces pombe. Mol Cell Biol. 1993 Oct;13(10):6393–6402. doi: 10.1128/mcb.13.10.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon I., Bohr V. A., Smith C. A., Hanawalt P. C. Preferential DNA repair of an active gene in human cells. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8878–8882. doi: 10.1073/pnas.83.23.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. L., Brash D. E., Nairn R. S. Rapid repair kinetics of pyrimidine(6-4)pyrimidone photoproducts in human cells are due to excision rather than conformational change. Nucleic Acids Res. 1990 Feb 25;18(4):963–971. doi: 10.1093/nar/18.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto I., Miura N., Niwa H., Miyazaki J., Tanaka K. Mutational analysis of the structure and function of the xeroderma pigmentosum group A complementing protein. Identification of essential domains for nuclear localization and DNA excision repair. J Biol Chem. 1992 Jun 15;267(17):12182–12187. [PubMed] [Google Scholar]
- Mu D., Park C. H., Matsunaga T., Hsu D. S., Reardon J. T., Sancar A. Reconstitution of human DNA repair excision nuclease in a highly defined system. J Biol Chem. 1995 Feb 10;270(6):2415–2418. doi: 10.1074/jbc.270.6.2415. [DOI] [PubMed] [Google Scholar]
- Mullenders L. H., Van Zeeland A. A., Natarajan A. T. Analysis of the distribution of DNA repair patches in the DNA-nuclear matrix complex from human cells. Biochim Biophys Acta. 1983 Sep 9;740(4):428–435. doi: 10.1016/0167-4781(83)90091-x. [DOI] [PubMed] [Google Scholar]
- Mullenders L. H., van Kesteren van Leeuwen A. C., van Zeeland A. A., Natarajan A. T. Nuclear matrix associated DNA is preferentially repaired in normal human fibroblasts, exposed to a low dose of ultraviolet light but not in Cockayne's syndrome fibroblasts. Nucleic Acids Res. 1988 Nov 25;16(22):10607–10622. doi: 10.1093/nar/16.22.10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullenders L. H., van Kesteren A. C., Bussmann C. J., van Zeeland A. A., Natarajan A. T. Distribution of u.v.-induced repair events in higher-order chromatin loops in human and hamster fibroblasts. Carcinogenesis. 1986 Jun;7(6):995–1002. doi: 10.1093/carcin/7.6.995. [DOI] [PubMed] [Google Scholar]
- O'Donovan A., Davies A. A., Moggs J. G., West S. C., Wood R. D. XPG endonuclease makes the 3' incision in human DNA nucleotide excision repair. Nature. 1994 Sep 29;371(6496):432–435. doi: 10.1038/371432a0. [DOI] [PubMed] [Google Scholar]
- O'Donovan A., Scherly D., Clarkson S. G., Wood R. D. Isolation of active recombinant XPG protein, a human DNA repair endonuclease. J Biol Chem. 1994 Jun 10;269(23):15965–15968. [PubMed] [Google Scholar]
- O'Donovan A., Wood R. D. Identical defects in DNA repair in xeroderma pigmentosum group G and rodent ERCC group 5. Nature. 1993 May 13;363(6425):185–188. doi: 10.1038/363185a0. [DOI] [PubMed] [Google Scholar]
- Razin S. V., Kekelidze M. G., Lukanidin E. M., Scherrer K., Georgiev G. P. Replication origins are attached to the nuclear skeleton. Nucleic Acids Res. 1986 Oct 24;14(20):8189–8207. doi: 10.1093/nar/14.20.8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A. Mechanisms of DNA excision repair. Science. 1994 Dec 23;266(5193):1954–1956. doi: 10.1126/science.7801120. [DOI] [PubMed] [Google Scholar]
- Schaeffer L., Roy R., Humbert S., Moncollin V., Vermeulen W., Hoeijmakers J. H., Chambon P., Egly J. M. DNA repair helicase: a component of BTF2 (TFIIH) basic transcription factor. Science. 1993 Apr 2;260(5104):58–63. doi: 10.1126/science.8465201. [DOI] [PubMed] [Google Scholar]
- Scherly D., Nouspikel T., Corlet J., Ucla C., Bairoch A., Clarkson S. G. Complementation of the DNA repair defect in xeroderma pigmentosum group G cells by a human cDNA related to yeast RAD2. Nature. 1993 May 13;363(6425):182–185. doi: 10.1038/363182a0. [DOI] [PubMed] [Google Scholar]
- Schreiber V., Molinete M., Boeuf H., de Murcia G., Ménissier-de Murcia J. The human poly(ADP-ribose) polymerase nuclear localization signal is a bipartite element functionally separate from DNA binding and catalytic activity. EMBO J. 1992 Sep;11(9):3263–3269. doi: 10.1002/j.1460-2075.1992.tb05404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiomi T., Harada Y., Saito T., Shiomi N., Okuno Y., Yamaizumi M. An ERCC5 gene with homology to yeast RAD2 is involved in group G xeroderma pigmentosum. Mutat Res. 1994 Mar;314(2):167–175. doi: 10.1016/0921-8777(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Svoboda D. L., Taylor J. S., Hearst J. E., Sancar A. DNA repair by eukaryotic nucleotide excision nuclease. Removal of thymine dimer and psoralen monoadduct by HeLa cell-free extract and of thymine dimer by Xenopus laevis oocytes. J Biol Chem. 1993 Jan 25;268(3):1931–1936. [PubMed] [Google Scholar]
- Verheijen R., van Venrooij W., Ramaekers F. The nuclear matrix: structure and composition. J Cell Sci. 1988 May;90(Pt 1):11–36. doi: 10.1242/jcs.90.1.11. [DOI] [PubMed] [Google Scholar]
- Vermeulen W., Jaeken J., Jaspers N. G., Bootsma D., Hoeijmakers J. H. Xeroderma pigmentosum complementation group G associated with Cockayne syndrome. Am J Hum Genet. 1993 Jul;53(1):185–192. [PMC free article] [PubMed] [Google Scholar]
- Xing Y., Johnson C. V., Dobner P. R., Lawrence J. B. Higher level organization of individual gene transcription and RNA splicing. Science. 1993 Feb 26;259(5099):1326–1330. doi: 10.1126/science.8446901. [DOI] [PubMed] [Google Scholar]
- Yan C., Mélèse T. Multiple regions of NSR1 are sufficient for accumulation of a fusion protein within the nucleolus. J Cell Biol. 1993 Dec;123(5):1081–1091. doi: 10.1083/jcb.123.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]