Abstract
The implication of molecular biology in crop improvement is now more than three decades old. Not surprisingly, technology has moved on, and there are a number of new techniques that may or may not come under the genetically modified (GM) banner and, therefore, GM regulations. In cisgenic technology, cisgenes from crossable plants are used and it is a single procedure of gene introduction whereby the problem of linkage drag of other genes is overcome. The gene used in cisgenic approach is similar compared with classical breeding and cisgenic plant should be treated equally as classically bred plant and differently from transgenic plants. Therefore, it offers a sturdy reference to treat cisgenic plants similarly as classically bred plants, by exemption of cisgenesis from the current GMO legislations. This review covers the implications of cisgenesis towards the sustainable development in the genetic improvement of crops and considers the prospects for the technology.
Keywords: Cisgenic, Transgenic, Intragene, Linkage drag, Sexually compatible.
INTRODUCTION
Genetic modification of plants factually involves the introduction of foreign genes into the plant genomic background. Currently, genetically modified plants give a promising impact to various crop improvement programmes. The foremost outcome is the development of varieties resistance against various biotic and abiotic stresses. Genetically engineered traits comprise priceless alternatives from the conventional breeding, but, there arise a public issue on consumption of transgenic plants. This unlocks a new vista for engineering crop plants using the DNA from a sexually compatible donor plant [1]. Ample varieties of plant genes having agronomically desirable traits have been identified due to the advancement of plant molecular biology resulting in divergence of imperative gene sources, from prokaryotes to plants and will eventually improve the gene discovery process by ongoing genomic research [2]. For example, an herbicide tolerance plant has been developed by inducing the native target genes with point mutation. The plants in which acetolactate synthase (ALS) genes were modified showed the same level of sulfonylurea tolerance comparing to those plants containing bacterial transgenes for ALS tolerance [3].
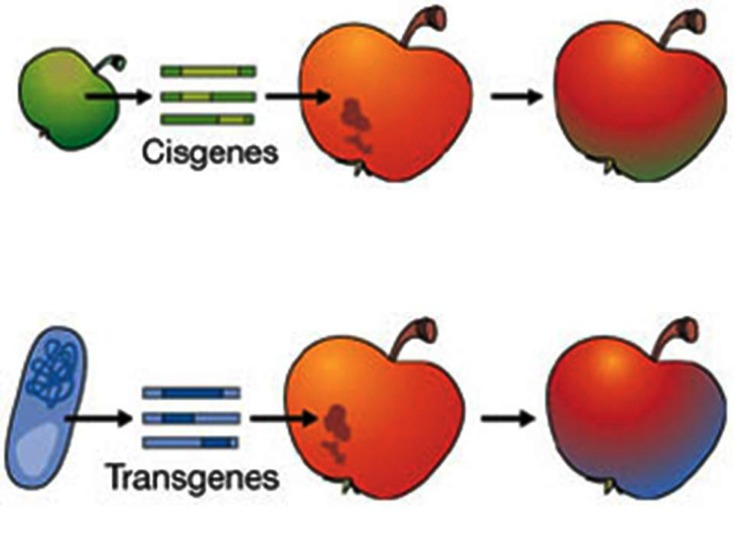
The application of genome sequencing in crop plants like rice, maize, potato, and the development of efficient gene isolation techniques like map- based cloning and allele mining brought a new-fangled part of research in plant breeding by utilizing the cloned native genes [4]. During the last few decades, a variety of indigenous genes, coding for valuable traits like disease resistance and quality, from crop plants and their wild relatives have been isolated, characterized ably and introduced into the genetic background of elite germplasm. These native genes, isolated from the crop plant itself or from other cross compatible species, are currently referred as cisgenes to distinguish such group of genes from the transgenes (Fig. 1) [5]. In cisgenic approach as there is no introduction of new gene class from cross incompatible species, hence the existing genetic variation symbolize the one which are applied in conventional breeding programme which have been safely used since decades.
Fig. (1).
Comparison between Cisgene and transgene technology (Source: Schouten et al. [5]).
DEFINITION
It simply refers to genetic modification using one of the techniques of recombinant DNA technology, but using no “foreign” DNA; in other words, the manipulation is done using DNA entirely from the same species as the host plant, or a species that is closely related enough to be sexually compatible. Therefore, it is not really a new technique. The use of the term is an attempt to distinguish GM plants or other organisms produced in this way from transgenics that is GM plants that contain DNA from unrelated organisms.
Schouten et al. [5] introduced the term cisgenesis and defined cisgenesis as the modification in the genetic background of a recipient plant by a naturally derived gene from a cross compatible species including its introns and its native promoter and terminator flanked in the normal sense orientation. Since cisgenes shared a common gene pool available for traditional breeding the final cisgenic plant should be devoid of any kind of foreign DNA viz., selection markers and vector- backbone sequences. Sometimes the word cisgenesis is also referred to as Agrobacterium-mediated gene transfer from a sexually compatible plant where only the T-DNA borders may be present in the recipient organism after transformation [6].
Cisgenesis is the genetic modification of a recipient plant with a natural gene from a crossable—sexually compatible—plant
Transgenesis is the genetic modification of a recipient plant with one or more genes from any non-plant organism, or from a donor plant that is sexually incompatible with the recipient plant.
Why Cisgenesis?
The worthiness of GM techniques for developing highly reliable and good quality food supply to the world has been set off by public worries about the security of the derived food and their resulting products. Most particularly, the controversy has spotlight on the probable unpredictable hazards arising from the agglomeration of certain new substances in crop plants that confers toxicity, allergy and genetic threats in the human nutrition [7].
Cisgenic plants are presumably considered safer than those produced through conventionally breeded plants because of the lack of linkage drag. In cisgenesis, only the desired genes are introduced without the undesirable genes. Cisgenesis furnishes no unnecessary hazard compared to induced translocation or mutation breeding. Therefore, cisgenesis precludes linkage drag, and hence, prevents hazards from unidentified hitch hiking genes [8]. Due to this reason, cisgenesis is normally safe than traditional breeding programmes and various biotic and abiotic stress resistance genes can be pyramided to provide wider and long lasting forms of resistance.
There are also legitimate public reasons that brought the obligation to clearly differentiate cisgenes from the transgenes. The notion towards transgenic technology often brought annoying circumstances to many people, followed by their firm regulation worldwide. Common people are also found to be much satisfied with cis/intragenic crop than transgenic crops. In Mississippi, ananalysis revealed that 81% of public favored to eat cisgenic vegetables while only 14 – 23% for transgenic vegetables [9].
According to the Sustainability Council of New Zealand [10], interest in cisgenics also has been stimulated by:
The idea that cisgenic GMOs will avoid the market confrontation that has overwhelmed other types of GM foods. Several researchers believe they will be able to induce consumers that the GM industry has rehabilitated, by listening cautiously to public concerns about using GM in the food chain. The argument that their GMOs will not cross the species hurdle is offered as a confirmation of that modification.
The optimism that cisgenic GMOs will not be subject to the same regulatory examination as GMOs made by current techniques, and lobbying efforts by developers are in progress in New Zealand to secure the regulatory discount.
The assumption that GMOs developed from this technique may prove difficult to identify and could thus be undetectable to regulators and consumers.
However, the faith of cisgenic GM foods to be invisible to consumer is against their demand upon transparency about the use of GM foods. On the other hand, letting off cisgenic foods from the GMO regulation will facade the public’s rights to know about the introduction of newly developed foods by new technologies to the food chain. Therefore, such primary tension highlights the incoherent nature of the cisgenics as a commercial approach.
CISGENESIS VERSUS CONVENTIONAL BREEDING
Advantages of Cisgenesis Over Conventional Breeding
Conquer the setback of linkage drag. Introgression of innovative traits into the cultivated varieties by conventional methods comprises wide crosses and widespread backcrossing. However, these traits are constantly linked within a large share of unwanted chromosomes, the so-called linkage drag. Some of these genes affect the normal features of the crop as they may engage in the production of diverse kinds of toxins or allergens. In vegetatively propagated crops like potatoes and apples, their heterozygous nature further brought impediment in successful transfer of traits of interest [11]. Hence, direct transfer of desired genes through cisgenesis into an existing variety without altering any of the properties enviable for the consumers can be accomplished. An ample amount of marker- free transformants where single T-DNA was arbitrarily inserted, and produced acceptable expression of the cloned cisgene in the beneficiary species. It is followed by the selection of plants in the growth chamber then glasshouse and field. Selection of the best performing plants with realistic gene insertions and least negative side effects is made in the field where linkage drag with unwanted gene is deficient. Plant breeding techniques with the objective to introduce durable resistance to the potato-late-blight-caused by Phytophthora infestans involve stacking of resistance genes from various resistant wild species including Solanum demissum and S. bulbocastanum. Introgression of resistance gene from the new donor S. bulbocastanumbegan in the early 1970s, but the accomplishment of the technique was hindered by linkage drag. For the time being diverse native resistance genes have been screened and isolated from the donor plants along with S. demissum [12, 13] which would allow stacking of cloned resistance genes to the susceptible elite potato cultivars by cisgenesis.
Maintains original genetic make-up of plant variety. In a hybridization method, the genetic makeup of the progeny plant varies from its parents because it has been a mixture of both the parental genomes. In spite of this, there is a necessity to conserve some part of the genome which revealed certain constructive traits. Through conventional plant breeding such an approach is not possible entirely due to self incompatibility among the vegetatively propagated plants like grape, potato, apple etc. When crossing is done in a prominent grape variety Merlot or Cabernet sauvignon with a disease resistant variety the genetic constitution of the progeny plants will not at all be similar to the parent plants. Hence, traditional breeding programme will no longer confer disease and pest resistance to the notable parent cultivars [6]. In a Dutch project called DURPh (Durable Resistance against Phytophthora), which has been going ahead since 2006 under considerable public support, cisgenic breeding tools are used in order to get up to four different resistance genes into one variety without changing other original traits of the modified variety [14]. In this way, it must be probable that multiple R genes can supplement to a more durable resistance against late blight [15].
Reduction in pesticide application. The key purpose of cisgenesis is to transfer disease resistance genes to susceptible varieties. The vital goal here is to lessen substantial pesticide application. As a result, there is decline in the input costs of the farmers and decreased pesticide leftovers on the plants and also in their products, which is mostly favored by the consumers. This reduced the environmental pollution by pesticides and in turn helped in sustainable agricultural development. On the other hand, if cisgenic comes under the current GMO regulation, then this novel technique will be held back [16]. Potato is susceptible to different pests and diseases. Most noteworthy between them is the late blight, induced by the fungal pathogen Phytophthora infestans, causing maximum damage potential world-wide. As an outcome breeding efforts are massive in order to get less susceptible and resistant new varieties, respectively, and new technologies are used especially in this breeding sector. Approximately 200 wild Solanum species with potential resistance genes are known in Middle and South America. Only a small percentage of them has been explored for use in breeding programmes up to now [17]. The availability of resistant varieties would lead to enormous reduction of pesticides input for plant protection measures as well as of the yield loss.
Time Saving. In conventional hybridization programmes, there is linkage drag, where there is inheritance of thousands of unwanted genes to the progeny. Several backcrossed generations are required to get rid of such kind of undesired genes. Cisgenesis overcomes the problem of linkage drag and only the gene of interest is introduced into the genome of the recipient plant within a short period of time. Thus, this saves a lot of time. For example in apple-breeding, integration of a disease resistance gene takes about 40 years through traditional methods. The transfer of apple scab resistance gene Vf, which has been cloned of late [18], into the novel cultivars using cisgenic technique could give rise to better results within a short period of time. The comparatively long period of tree breeding, which may last decades via traditional techniques, makes the genetic modification of trees a striking target [19]. Cisgenesis could be employed for the rapid introduction of desired traits into commercially successful cultivars without changing their constructive characteristics through introgression by traditional methods. In general, gene transfer technologies may successfully curtail the juvenile period of fruit trees [20].
DISPARITY BETWEEN TRANSGENESIS AND CISGENECIS
Each pertinent technique used in transgenesis can be employed to produce cisgenic plant. However, the key disparity lies in the source from where the gene of interest is obtained and largelyit is discussed below.
Transgenesis
✓ The gene which is introduced in transgenic technology is generally acquired from an unfamiliar species that is not at all familiar to the beneficial plant or sexually compatible species. Therefore, transgenic technology doesn’t admire species barriers.
✓ Transgenic technology can widen the genetic resource of the recipient plants. Such type of unique gene favors the expression of certain unique trait in the recipient plant which never expresses naturally or cannot be induced by conventional breeding techniques.
✓ Such new gene might be involved in modifying the vigor of the recipient plant in different directions; the modification in vigor may disseminate as a result of gene influx from its wild relatives, possibly generating a change in the natural vegetation.
✓ As a result, lawmakers and the competent authorities began to consider much on the safety for thoughtful delivery of transgenic crops into the environment and have mounted under the frame of biosafety regulations to control the possible supposition.
Cisgenesis
✓ As cisgenesis allows the transfer of the gene of interest along with its promoter, they will be present in the genome of the species or in the cross compatible relatives for many generations. Consequently, cisgenesis respects species barriers.
✓ Cisgenesis does not make any change in the gene pool of the target plant and add any supplementary characters.
✓ There is no change in the vigor that would otherwise take place in case of conventional breeding programme. Therefore, cisgenesis does not harm nontarget species or environmental hazards and potential allergens associated with GM food and feed. Here lies the significant distinction between cisgenic and transgenic technology.
✓ Therefore, the vigilant introduction and release of cisgenic plants to reach the consumers provide equal security as those plants produced by traditional methods. In this concern of food security, the authorities should consider cisgenic plants equally as traditionally bred plants.
Limitations of Cisgenesis
Although cisgenics technology is exhibiting considerable advantages over the transgenic counterpart, but still there are a few limitations associated with this technology. Compared to transgenesis, one of the disadvantages shared by cisgenesis is that characters outside the sexually compatible gene pool cannot be introduced. Furthermore, development of cisgenic crops involves extraordinary proficiency and time compared to transgenic crops. Therefore, the required genes or fragments of genes may not be readily accessible but have to be isolated from the sexually compatible gene pool [21]. The author further elaborated few issues, firstly, the production of marker free plants usually requires the development of innovative protocols, since such protocols may not be readily available for the crop in question. Secondly, since 20 – 80% of the transformants contain vector-backbone sequences, many transgenic lines have to be removed. Therefore, substantial hard work has to be done, particularly on crops with low transformation efficiencies to create large number of transformants.
Cisgenesis and Sustainable Crop Improvement
Traditional plant breeding played a vital role in the crop improvement programme during the early days including introgressive hybridization, induced mutation and somatic hybridization. These techniques randomly change the plant genetic composition and thus create genetic diversity [22]. Although, all these techniques have certain demerits like the problem of linkage drag, require long period to release a variety, still, the resulting plant can be introduced into the food chain without any regulation. Generally, they are regarded as safe and have been consumed securely without any complaints from old days and consumers didn’t have any kind of objection regarding the products.
Suppose when cisgenic plants are grown in the fields, their pollen grains may disperse and fertilization will take place with the wild relatives in the adjacent vegetation. As most of the cisgenes are from their wild relatives and have been present in the natural vegetation from past days, cisgenesis solves the current biosafety problems. Furthermore such kind of genes may have been employed earlier in traditional breeding. Hence, concurrence of cisgenic crops and non-GM crops will not create problem, as there is no invasion of unknown genes from the cisgenic cultivar to the non-GM cultivar. It is doubtful to envisage the blending point of the cisgene into the plant genome as it is a random process, similar to traditional induced translocation breeding [5].
Genes within the same gene pool could have been transferred into novel varieties in a one step process without the transfer of undesirable genes and, above all, without the integration of any alien genes. Therefore, such plants obtained are non-transgenic, inspite of using the methods of molecular biology and plant genetic engineering. This perception gives a choice to the present genetic engineering methods where the genes used for integration are derived primarily from bacterial origin. The genetic constitution of the derived intragenic plants can be assumed as a small dislocation of endogenous genes within the species. Such changes did not vary from the spontaneous revolution that takes place automatically as a result of micro- translocations in plant genomes or due to induced mutation [23].
Upcoming plant breeding efforts, together with transgenic approaches, will focus on breeding varieties with enhanced consumer traits having a direct advantage for the consumer, including functional, healthy and tasty foods [24]. Quality traits (e.g. the accumulation of beneficial nutrients) are usually influenced by a plant’s metabolic network, and thus regularly governed by enzymes. The manipulation of key enzymes may be used to attain a desired effect. This may, depending on the activity of the promoter, lead to major alterations in a plant’s general metabolism. Chiefly, when the plant’s metabolism needs to be targeted by GM, unforeseen effects potentially occur due to various interactions within the metabolic network.
It is apparent that the sequence organization of the cisgene will stay intact in contrast with the donor mother plant, therefore, genotypic or phenotypic outcomes of the cisgenic or intragenic plants can be assumed equivalent to the donor plant. Hence, it can be expected that perhaps ample knowledge about the determination of food and feed safety has been provided for achievement of some peculiar task for the evaluation of risk factor linked with these crops [25].
For successful implementation of cisgenics technology in crop improvement, genes related with the requisite trait should be well defined. Molecular markers may assist in their identification, especially as they have become important tools of traditional plant breeding methods [26]. The identification and isolation of these genes are to a great extent facilitated by constant achievements in plant genome sequencing. Progressively updated databases are useful tools for in silico research.Subsequently, at least theoretically, the number of genes accessible for cisgenic or intragenic modification is mounting. Their association with precise functions may be based on sequence similarities. The approach to identify sequences of putatively alike function through database searches has been exploited for the recognition of plant-derived DNAs (P-DNAs, used as alternative for conventional T-DNAs in Agrobacterium-mediated transformation) in a number of plant genomes [27]. However, a significant requirement for the proficient use of recognized genes is their comprehensive investigational characterization, which is cost- and time-consuming.
Whether cisgenesis will be a substantial technology or not depends on the means how the current competent authorities look upon cisgenic plant [28]; public preferences on the said products; the conclusion on the labeling of these plants and their derivative products as GM; and patents given on the GM technologies and genes. Despite the fact that controlling the patents and consumer preferences is exclusively unfeasible for the authorities, then, apparently it is a good verdict to grade cisgenic and transgenic plants in a different way.
GM technology, on the other hand, if applied for improving traits other than that for food and feed, then there may not arise any objection from the public regarding the transgenic crops. The advantage of this technology has been dispaired with public debates on the probable unpredictable outcomes and the social beliefs that people have in their mind like GM technology is unnatural, genetic erosion of indigenous varieties and also some division of individuals don’t like the plan of incorporating a bacterial or animal gene (s) into the plant genomes. All these reasons have obsessed the suggestion for development of crops that bestow no risks to the public and the product is environmentally, economically sustainable and socially tolerable. Hence, cisgenesis will be a better option in these regards and will overlay the way towards sustainable crop improvement programmes.
A Turning Point for Cisgenesis
Cisgenesis denotes a next knockfavoring a new era of GM organisms. Absence of marker genes may be antibiotic or herbicide resistance genes in the final product and also the introgressed gene(s) are derived from cross compatible species to the future species will lessen environmental worries and increase the consumer’s preferences. The first scientific statement of bringing forth a true plant obtained by cisgenic approach was reported in apple through the insertion of the internal scab resistance gene HcrVf2 influenced by their own regulatory genes into the cultivar Gala, a scab susceptible cultivar [29].
“Cisgenic” is a registered trademark of a New Zealand-based company, which has adopted this method to engineer pasture species/fodder crops (e.g. ryegrass and clover). The company defines its approach as intermediate between cisgenesis and intragenesis, e.g. allowing the omission of introns in sequences to be introduced [30]. Cisgenesis has been tested in the Netherlands, Germany, Switzerland and Italy, in particular in fruit trees. However, some forest trees (e.g. poplars) that are used for wood or energy production have also been improved through cisgenic approaches [31].
On 16 February 2012, European Food Safety Authority [6] reported the detail study concerning the safety aspects of cisgenic plants and validated that cisgenic plants are secure to be used in terms of environment, food and feed, similar to the traditionally bred plants. However, the present GMO regulation keeps the cisgenic micro-organisms out from its supervision. The resulting outcomes from these micro- organisms are extensively employed all over the Food and Feed industry during the last 15 years. Application of cisgenesis in micro-organisms is termed as ‘self-cloning’. While the techniques of self-cloning have been debarred from the supervision of GMO, entire team of the European Working Group towards New Breeding Techniques [32] reported that it would be a wise decision to consider cisgenic crops separately from the current GMO Regulation.
As cisgenesis doesn’t confess the establishment of foreign genes which is related with the intricacy of linkage drag in traditional plant breeding, it debars the addition of undesirable features together with the risk attached with these genes. It is also found to be related to the effects of introduced gene obtained from the cross compatible plant in cisgenesis compared to traditionally bred plants. But, when such native genes are used in case of intragenesis a blend of certain new genetic material occurswhich is absent in cisgenic and traditionally bred plants and such genes may symbolze curious exhilarating traits and the risk associated with them.The sequences which are identical with the short T DNA borders present in the recipient plant after transformation in cisgenesis with T DNA borders could also be present in different plant cultivars. Hence, the risk connected with these sequences will be analogous to those obtained from traditionally bred plants [33]. Taking into account the similar outcomes of cisgenesis and conventional plant breeding along with mutation breeding, there is a proposition of excluding cisgenic crops from the current GMO regulations [34]. Cisgenic crops need to be viewed at the same level with the conventionally bred plants including those from hybridization, in vitro fertilization, induced polyploidy, protoplast fusion and induced mutagenesis. Gaskell [35] reported that according to latest Eurobarometer, the consent of consumers for purchasing cisgenic apples was considerably more than transgenic apples among the total representative states.
Mielby [36], had carried out a sociological survey to find out the preference level of bread prepared from the cisgenic derived flour, among the target section of people in Denmark and found that exclusively about 25% was against the product. Gaskell et al. [37] also conducted a study from the specimen representing 32 European countries regarding the consumer preference of cisgenic and transgenic technology adopting apple sacb resistance, mildew, and canker, from this study it was revealed that in the whole of the European Union (EU), 55% people sustained cisgenic apples and 33% towards transgenic apples. Around 85% of German respondents do not want animals to be fed with GM feed, according to a survey conducted by market research company Forsa. In a further study by Forsa, 75% of German consumers want to see food producers and retailers make wider use of Germany’s voluntary “GM-free” labeling scheme and would opt for products labeled “GM-free” if available [38].
Cisgenesis has also been recognized as a potentially useful strategy to enhance the biomass of trees suitable for bioenergy production [19]. An example is the attempt towards cisgenic modification of the gibberellic acid pathway in poplar [39].
CISGENESIS AND CROP IMPROVEMENT
Stress tolerance and disease and pest resistance (plant incorporated protection, PIP) are currently major goals of plant breeders and researchers working on the development of cisgenic crops. Also, quality aspects may be improved by incorporating additional copies of a given gene. The improvement of quality traits in plants is a major goal in plant breeding programmes, indicated also by trends in the pipelines of biotech-companies that currently employ transgenic methods. The targeted traits include fatty acid composition (omega-3 fatty acids, reduced saturated and increased unsaturated fatty acids contents, elimination of trans fats), enhanced flavor, fiber quality, improved shelf life, and also optimization for the use as food, feed, biofuel or industrial uses [41, 42].
Fruit trees (Rosaceae) and vegetatively propagated crops like potatoes are currently the primary target for cisgenic modification. The possibility to develop a marketable product depends, inter alia, on the trait of interest (monogenic, oligogenic) and the availability of the gene (or several genes) responsible for its manifestation. In a first step, monogenic traits may be targeted. However, gene pyramiding is also feasible.Trees, in general, are an attractive target for cisgenic modifications. The major reason may be seen in the decreased time needed for the development of a new cultivar that will be successful in the market [20]. The current scientific peer-reviewed literature claiming to present “cisgenic approaches do not necessarily satisfy the definition of cisgenesis sensu stricto. Currently, only two articles – Vanblaere et al. [29] and Holme et al. [43] – are likely to fit the definition of cisgenesis as coined by Schouten et al. [5].
Cisgenic Apple lines cv. “Gala” were produced by Vanblaere et al. [29]. They employed the ORF of the HcrVf2 genomic region from the wild relative Malus floribunda, including 242-bp from its 5’ UTR and 220-bp from its 3’ UTR and conferring scab resistance. The segment between the recombination sites that contains the nptII gene for kanamycin selection was removed through dexamethasone-induced recombination and thus resulted in marker-free lines. Presence of HcrVf2, absence of trfA (responsible for initiation of replication) and nptIII as part of the backbone, and the fusion marker gene nptII/codA were demonstrated by PCR.
“Cisgenic barley with improved phytase activity” was demonstrated by Holme et al. [43]. They achieved the marker-free status of the cisgenic plants by using the pClean dual binary vector system that uses hygromycin resistance for selection [44]. The genomic region belongs to HvPAPhy_a gene comprised of 5208-bp and was amplified by PCR. With the introgression of supplementary copies of the HvPAPhy gene, the accumulation of phytase levels in the mature barley grain will be extremely useful for both the bioavailability of phosphate in the grain and regarding the environmental aspects.
Kamrani et al. [45] published a paper called “Cisgenic inhibition of the potato cold induced phosphorylase L gene expression and decrease in sugar contents”. However, in their approach they used an RNA silencing construct, under the influence of 35S promoter and the OCS terminator sites, as well asselected putative transgenic shoots on kanamycin-containing medium. Removal of the selection marker was not reported.
Lutken et al. [46] described an approach towards cisgenic modification of Kalanchoë that would replace the application of growth regulators. They stated that the chemicals are potentially damaging to human health and the environment and thus will be banned in the EU in the near future. For this, they identified KNOX genes involved in vegetative vivipary and overexpressed two of them (KxhKN4 and KxhKN5) by introducing the complete cDNAs governed by the 35S promoter and NOS terminator sites, respectively. They also used a post-transcriptional gene silencing (PTGS) complex that contained a 326-bp fragment of KxhKN5.A detailed abstract was published by Kichey et al. [47]. The authors reported the production of barley with enhanced nitrogen use efficiency (NUE). Their cisgenic approach used the genomic sequence of TIP2 (3532-bp), including promoter (1999-bp upstream) and terminator (564-bp downstream), and the GS1 gene (GS1a isoform) which consisted of a 5.2-kb gene fragment, including 1.5-kb promoter and 491-bp terminator. Kuhl et al. [48] presented “a partially cisgenic event” in potato, which was accomplished by introducing an 8.59-kb fragment of the RB gene conferring late blight resistance (including 2.5-kb upstream of the start ATG and 2.48-kb downstream of the stop codon). As the selectable marker nptII was retained in the transformants they referred to them, by definition correctly, as “transgenic”.In strawberries, cisgenic disease resistance against Botrytis cinerea was investigated by Schaart [49] using the endogenous strawberry gene encoding for polygalacturonase inhibiting protein PGIP, observing the strict use of strawberry-own DNA sequences as target gene and as promoter and applying a selectable marker removal method for the elimination of marker genes.Han et al. [50] examined the impact of the introduction of five cisgenes PtGA20ox7, PtGA2ox2,Pt RGL1_1, PtRGL1_2 and PtGAI1 associated with gibberellin metabolism from the genome sequenced clone Nisqually-1 of Populus trichocarpa and were transferred into the clone INRA 717-1B4 of Populus tremula × alba. The growth performance, morphology and xylem cell size were identified under the greenhouse. The genes employed in this studywere expressed in the xylem and phloem and identified by microarray expression data. They recorded a huge variation in a large number of independent events they analyzed. The successful introduction of these cisgenes was confirmed using PCR primers specific to the flanking T-DNA region which was missing in their wild relatives.
CONCLUSION
Application of cisgenic techniques enhances the possibility to introgress the preferred genes into the novel cultivars (mostly single gene in the first step), without disturbing their favorable characteristics. Therefore, the most compelling contribution of cisgenesis may be anticipated for the development of monogenic resistance traits. But, the application of gene pyramiding will also accomplish a more durable resistance. Major advantages could be expected in breeding of plants with long life spans such as trees. Traits such as abiotic stress tolerance are usually complex (e.g. due to polygenic traits). The introgression of one gene or QTL is usually not sufficient to engineer stress-tolerant lines [62]. Gene pyramiding will be necessary in this aspect, implying that the sequences and functions of genes are well characterized.The information regarding cisgenesis is very rare and there is only certain frivolous information given in the seminars and conference proceedings. Thus, if we expand our area of research towards cisgenic approach and if it has been exempted from the regulatory framework of GM technology it is anticipated that cisgenesis may wipe out the likely uncertain outcomes and the social beliefs that public have in their mind regarding GM technology. Therefore, cisgenesis will be playing an important role in sustainable crop improvement.
Table 1.
Percentage of Consumers in US and France Indicat-ing They would Eat a Vegetable with a Variety of Characteristics
| Characteristics of Vegetables | US(%) | France(%) |
|---|---|---|
| An extra gene from the same vegetable | 77.3 | 37.5 |
| An extra gene from a different vegetable | 61.7 | 21.0 |
| Several extra genes from a different vegetable | 52.7 | 17.5 |
| An extra gene from a different bacterium | 25.3 | 7.0 |
| An extra gene from a different fungus | 25.7 | 12.5 |
| An extra gene from a different virus | 17.3 | 3.0 |
| An extra gene from a different animal | 23.7 | 4.5 |
| Number of Observations | 501 | 200 |
Source: Lusk & Rosan [40].
Table 2.
Engineering Crop Plants through Cisgenesis
| Species | Trait | Gene | Donator | Reference |
|---|---|---|---|---|
| Apple | Apple Scab Resistance | HcrVf2 | Malus floribunda | [29] |
| Apple | Induces anthocyanin accumulation/red apple fruit color | MdMYB10 | Malus domestica | [51] |
| Barley | Phytase activity | HvPAPhy_a | _ | [42] |
| Barley | Nitrogen Use Efficiency (NUE) | gTIP2 and gGS1a | _ | [46] |
| Rye-grass | Drought tolerance | Lpvp1 | Lolium perenne | [52] |
| Poplar | Gibberellin metabolism | PtGA20ox7, PtGA2ox2,Pt RGL1_1, PtRGL1_2 and PtGAI1 | Populus trichocarpa clone Nisqually-1 | [49] |
| Potato | Late blight resistance | Rpi-blb1, Rpi-blb2, Rpi-blb3 |
Solanum bulbocastanum | [53-55] |
| Potato | Late blight resistance | Rpi-vnt1 | Solanum venturi | [55] |
| Potato | Late blight resistance | R2 , R3a, R3b, R5, R6, R7, R8, R9, R10 ,R11 | Solanum demissum | [55-58] |
| Potato | Late blight resistance | Rpi-pra1 | Solanum papita | [55] |
| Potato | Late blight resistance | Rpi-sto1 | Solanum stoloferum | [55] |
| Potato | Late blight resistance | Rpi-ber1 | Solanum berthaultii | [59] |
| Potato | Late blight resistance | Rpi-pnt1 | Solanum pinnatisectum | [60] |
| Potato | Nematode resistance (G. rostochiensis) | Gro1-4 | Solanum tuberosum | [61] |
| Strawberry | Fruit rot (Botrytis cinerea) | PGIP | _ | [48] |
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Caius Rommens M. All-native DNA transformation: a new approach to plant genetic engineering. Trends in Plant. Sci. 2004;9 (9):457–464. doi: 10.1016/j.tplants.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Holtorf H, Guitton M C, Reski R. Plant functional genomics. Naturwissenschaften. 2002;89:235–249. doi: 10.1007/s00114-002-0321-3. [DOI] [PubMed] [Google Scholar]
- 3.Bernasconi P, Alison Woodworth R, Barbara Rosen A, Mani Subramanian V, Daniel Siehl L. A naturally occurring point mutation confers broad range tolerance to herbicides that target acetolactate synthase. J. Biol. Chem. 1995;270:17381–17385. doi: 10.1074/jbc.270.29.17381. [DOI] [PubMed] [Google Scholar]
- 4.Jacobsen E, Karaba N. Nataraja.Cisgenics - Facilitating the second green revolution in India by improved traditional plant breeding. Curr. Sci. 2008;94(11): 1365–1366. [Google Scholar]
- 5.Schouten HJ, Krens FA, Jacobsen E. Cisgenic plants are similar to traditionally bred plants: international regulations for genetically modified organisms should be altered to exempt cisgenesis. EMBO Rep. 2006;7:750–753. doi: 10.1038/sj.embor.7400769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.EFSA Sciientific opinon addressing the safety assessment of plants developed through cisgenesis and intragenesis. EFSA Journal. 2012;10(2 )(2561): 1–33. [Google Scholar]
- 7.Anthony J, Conner J, Jacobs ME. Genetic engineering of crops as potential source of genetic hazard in the human diet. Mut-234. doi: 10.1016/s1383-5742(99)00020-4. [DOI] [PubMed] [Google Scholar]
- 8.Schouten HJ, Jacobsen E. Cisgenesis and intragenesis, sisters in innovative plant breeding. Trends Plant Sci. 2008;13:260–261. doi: 10.1016/j.tplants.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Venkatesh V, Steven H. Strauss.Modifying Plant Growth the Cisgenic Way. ISB News Rep September. 2010 [Google Scholar]
- 10.Sustainability Council of New Zealand Hide and Seek - Developers Skirt Around Detectability of Cisgenic GMOs Sustainability Council of New Zealand. http://www.sustainabilitynz.org. (Accessed June 2011) . 2011a:4– 15. [Google Scholar]
- 11.Jacobsen E, Schouten HJ. Cisgenesis strongly improves introgression breeding and induced translocation breeding of plants. Trends Biotechnol. 2007;25:219–223. doi: 10.1016/j.tibtech.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Huang S, van der Vossen EA, Kuang H, Vleeshouwers VG, Zhang N, Borm TJ, van Eck HJ, Baker B, Jacobsen E, Visser RG. Comparative genomics enabled the isolation of the R3? late blight resistance gene in potato. Plant J. 2005;42:251–261. doi: 10.1111/j.1365-313X.2005.02365.x. [DOI] [PubMed] [Google Scholar]
- 13.van der Vossen EA, Gros J, Sikkema A, Muskens M, Wouters D, Wolters P, Pereira A, Allefs S. The Rpi-blb2 gene from Solanum bulbocastanum is an Mi-1 gene homolog conferring broad-spectrum late blight resistance in potato. Plant J. 2005;44:208–222. doi: 10.1111/j.1365-313X.2005.02527.x. [DOI] [PubMed] [Google Scholar]
- 14.Haverkort A, Struik P, Visser R, Jacobsen E. Applied Biotechnology to Combat Late Blight in Potato Caused by Phytophthora Infestans. Potato Res. 2009;52(3):249–264. [Google Scholar]
- 15.Zhu SS, Paek YG, Kim TK, Visser RGF, Jacobsen E. In: Strategies to produce cisgenic transformants in potato. Proceedings of The 18th triennial conference of the European association for potato research in Oulu Finland. 2011 [Google Scholar]
- 16.Lusser M, Parisi C, Plan D, Rodriguez-Cerezo E. New plant breeding techniques.State-of-the-art and prospects for commercial development. JRC European Commission. 2011 :19–55. [Google Scholar]
- 17.Jansky S. Overcoming hybridization barriers in potato. Plant Breed. 2006;125:1–12. [Google Scholar]
- 18.Belfanti E, Silfverberg-Dilworth E, Tartarini S, Patocchi A, Barbieri M, Zhu J, Vinatzer BA, Gianfranceschi L, Gessler C, Sansavini S. The HcrVf2 gene from a wild apple confers scab resistance to a transgenic cultivated variety. Proc. Natl. Acad. Sci USA. 2004;101:886–890. doi: 10.1073/pnas.0304808101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harfouche A, Meilan R, Altman A. Tree genetic engineering and applications to sustainable forestry and biomass production. Trends in Biotechnol. 2011;29(1):9–17. doi: 10.1016/j.tibtech.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Flachowsky H, Le Roux PM, Peil A, Patocchi A, Richter K, Hanke MV. Application of a high-speed breeding technology to apple (Malus × domestica) based on transgenic early flowering plants and marker-assisted selection. New Phytologist. 2011;192(2): 364–377. doi: 10.1111/j.1469-8137.2011.03813.x. [DOI] [PubMed] [Google Scholar]
- 21.Holme I B, Wendt T, Holm PB. Current developments of intragenic and cisgenic crops. ISB News Report. 2013:1–5. [Google Scholar]
- 22.Rommens CM, Haring MA, Swords K, Davies HV, Belknap WR. The intragenic approach as a new extension to traditional plant breeding. Trends Plant Sci. 2007;12(9): 397–403. doi: 10.1016/j.tplants.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 23.van Harten AM, editor. Cambridge : University res sambridge; 1998. In Mutation breeding: theory and practical applications. pp. 12–233. [Google Scholar]
- 24.Kok EJ, Keijer J, Kleter GA, Kuiper HA. Comparative safety assessment of plant-derived foods. Regul. Toxicol. Pharmacol. 2008;50 (1): 98–113. doi: 10.1016/j.yrtph.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Prins TW, Kok EJ. Food and feed safety aspects of cisgenic crop plant varieties. RIKILT Rep. 2010:1–23. [Google Scholar]
- 26.Collard BC, Mackill DJ. Marker-assisted selection: an approach for precision plant breeding in the twenty-first century. Philosophical Transactions of the Royal Society Biological Sciences. 2008;363 (1491): 557–572. doi: 10.1098/rstb.2007.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rommens CM, Bougri O, Yan H, Humara JM, Owen J, Swords K, Ye JS. Plant-derived transfer DNAs. Plant Physiol. 2005;139:1338–1349. doi: 10.1104/pp.105.068692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradford KJ, Van Deynze A, Gutterson N, Parrott W, Strauss SH. Regulating transgenic crops sensibly: lessons from plant breeding, biotechnology and genomics. Nat. Biotechnol. 2005;23:439–444. doi: 10.1038/nbt1084. [DOI] [PubMed] [Google Scholar]
- 29.Vanblaere T, Szankowski I, Schaart J, Schouten H, Flachowsky H, Broggini GAL, Gessler C. The development of a cisgenic apple plant. J. Biotech. 2011;154:304– 311. doi: 10.1016/j.jbiotec.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Sustainability Council of New Zealand.Semantically Engineered Grasses - New Zealand Developers' New Spin on GM Sustainability Council of New Zealand. http://www.sustainabilitynz.org 2011b pp Accessed June. 2011:3–20. [Google Scholar]
- 31.Strauss SH, Brunner AM, Busov VB, Ma CP, Meilan R. Ten lessons from 15 years of transgenic Populus research. Forestry. 2004;77:455–465. [Google Scholar]
- 32. Final report working group on new breeding techniques. 2012:69. [Google Scholar]
- 33.Filipecki M, Malepszy S. Unintended consequences of plant transformation: a molecular insight. J. Applied Genet. 2006;47(4): 277–286. doi: 10.1007/BF03194637. [DOI] [PubMed] [Google Scholar]
- 34.Schouten HJ, Krens FA, Jacobsen E. Do cisgenic plants warrant less stringent oversight?. Nature Biotech. . 006b;24:753. doi: 10.1038/nbt0706-753. [DOI] [PubMed] [Google Scholar]
- 35.Gaskell G. Europeans and biotechnology in 2010.inds of change?. A report to the European Commission's DG Research. 2010:1–176. [Google Scholar]
- 36.Mielby H. Public attitudes to cisgenic crops PhD Thesis Department of Human Nutrition Faculty of Life Sciences. University of Copenhagen: Denmark. 2011 [Google Scholar]
- 37.Gaskell G, Allansdottir A, Allum N, Castro P, Esmer Y, Fischler C, Jackson J, Kronberger N, Hampel J, Mejlgaard N, Quintanilha A, Rammer A, Revuelta G, Stares S, Torgersen H, Wager W. The 2010 Eurobarometer on the life sciences. Nat. Biotechnol. 2011;29:113–114. doi: 10.1038/nbt.1771. [DOI] [PubMed] [Google Scholar]
- 38.Sustainability Market Intelligence Report. Quarterly Report. http://bit.ly/lMnwbG Accessed July. 2009 [Google Scholar]
- 39.ISB News Report. http://www.isb.vt.edu/news/2009/nov09.pdf. Accessed April 04 . 2012 [Google Scholar]
- 40.Lusk J L, Rosan A. Consumer acceptance of ingenic food. Biotechnol J. 2006;1(12): 1433–4. doi: 10.1002/biot.200600187. [DOI] [PubMed] [Google Scholar]
- 41.Dunwell JM. Crop biotechnology: prospects and opportunities. J. Agr. Sci. 2011;149(S1): 17–29. [Google Scholar]
- 42.Stein AJ, Rodríguez-Cerezo E. International trade and the global pipeline of new GM crops. Nat. Biotech. 2010;28:23–25. doi: 10.1038/nbt0110-23b. [DOI] [PubMed] [Google Scholar]
- 43.Holme IB, Dionisio G, Brinch-Pedersen H, Wendt T, Madsen CK, Vincze E, Holm PB. Cisgenic barley with improved phytase activity. Plant Biotechnol. J. 2011;10(2):237–247. doi: 10.1111/j.1467-7652.2011.00660.x. [DOI] [PubMed] [Google Scholar]
- 44.Thole V, Worland B, Snape JW, Vain P. The pCLEAN Dual Binary Vector System for Agrobacterium-Mediated Plant Transformation. Plant Physiol. 2007;145:1211–1219. doi: 10.1104/pp.107.108563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamrani M, Kohnehrouz BB, Gholizadeh A. Cisgenic inhibition of the potato cold induced phosphorylase L gene expression and decrease in sugar contents. African J. Biotech. 2011;10(50): 10076–10082. [Google Scholar]
- 46.Lutken H, Laura M, Borghi C, Orgaard M, Allavena A, Rasmussen SK. Expression of KxhKN4 and KxhKN5 genes in Kalanchoe blossfeldiana 'Molly' results in novel compact plant phenotypes: towards a cisgenesis alternative to growth retardants. Plant Cell Rep. 2011;30(12): 2267–2279. doi: 10.1007/s00299-011-1132-9. [DOI] [PubMed] [Google Scholar]
- 47.Kichey T, Holme I, Moller IS, Jahn TP, Holm PB, Schjoerring JK. In: Improving nitrogen use efficiency in barley (Hordeum vulgare L. through the cisgenic approach. Proceedings of the 16th International Plant Nutrition Colloquium. Department of Plant Sciences UC Davis. 2009 [Google Scholar]
- 48.Kuhl JC, Zarka K, Coombs J, Kirk WW, Douches DS. Late Blight Resistance of RB Transgenic Potato Lines. J. American Society for Hortil. Sci. 2007;132(6): 783–789. [Google Scholar]
- 49.Schaart JG, editor. Wageningen Netherlands : Wageningen University. ; 2004. Towards consumer-friendly cisgenic strawberries which are less susceptible to Botrytis cinerea.Ph.D. tesis. [Google Scholar]
- 50.Han KM, Dharmawardhana P, Arias RS, Ma C, Busov V, Strauss SH. Gibberellin-associated cisgenes modify growth, stature and wood properties in Populus. Plant Biotechnol. J. 2010;9(2): 162–178. doi: 10.1111/j.1467-7652.2010.00537.x. [DOI] [PubMed] [Google Scholar]
- 51.Espley RV, Hellens RP, Putterill J, Stevenson DE, Kutty-Amma S, Allan AC. Red colouration in apple fruit is due to the activity of the MYB transcription factor MdMYB10. Plant J. 2007;49(3): 414–427. doi: 10.1111/j.1365-313X.2006.02964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanley Z. GM approaches without GM outcomes Pastoral Genomics Presentation, . http://bit.y/ixguSB. Acccessed May 1 . 2008 [Google Scholar]
- 53.Van der Vossen EAG, Sikkema A, Hekkert B, Gros J, Stevens P, Muskens M, Wouters D, Pereira A, Stiekame W, Allefs S. An ancient R gene from the wild potato species Solanum bulbocastanum confers broad-spectrum resistance to Phytophtora in cultivated potato and tomato. Plant J. 2003;36:867–882. doi: 10.1046/j.1365-313x.2003.01934.x. [DOI] [PubMed] [Google Scholar]
- 54.Park TH, Gros J, Sikkema A, Vleeshouwers VGAA, Muskens M, Allefs S, Jacobsen E, Visser RGF. Van der Vossen, E..G. The late blight resistance locus Rpi-blb3 from Solanum bulbocastanum belongs to a major late blight R Gene cluster on chromosome 4 of potato. Molcular Plant-Microbe Interactions. 2005;18(7): 722–729. doi: 10.1094/MPMI-18-0722. [DOI] [PubMed] [Google Scholar]
- 55.Jacobsen E, Van der Vossen EA, Schaechter M. In Plant disease resistance: breeding and transgenic approaches. Encyclopedia of microbiology Oxford: Elsevier. 2009:597–612. [Google Scholar]
- 56.Huang S, Vleeshouwers VGAA, Werij JS, Hutten RCB, Van Eck HJ, Visser RGF, Jacobsen E. The R3 resistance to Phytophthora infestans in potato is conferred by two closely linked R Genes with distinct specificities. Molecular Plant-Microbe Interactions. 2004;17(4): 428–435. doi: 10.1094/MPMI.2004.17.4.428. [DOI] [PubMed] [Google Scholar]
- 57.Huang S. PhD Thesis. Wageningen University: Wageningen Netherlands; 2005. The discovery and characterisation of the major late blight resistance complex in potato: Genomic structure functional diversity and implications. [Google Scholar]
- 58.El-Kharbotly A, Palomino-Sanchez C. R6 and R7 alleles of potato conferring race-specific resistance to Phytophthora infestans (Mont.) de Bary identified genetic loci clustering with the R3 locus on chromosome. XI Theor Applied Genet. 1996;92:880–884. doi: 10.1007/BF00221901. [DOI] [PubMed] [Google Scholar]
- 59.Ewing EE, Simko I, Smart CD, Bonierbale MW, Misubuti ESG, May GD, Fry WE. Genetic mapping from field of qualitative and quantitative resistance to Phytophthora infestans in a population derived from Solanum tuberosum and Solanum berthaultii. Mol. Breed. 2000;6:25–36. [Google Scholar]
- 60.Kuhl J, Hanneman Jr R, Havey M. Characterization and mapping of Rpi1, a late-blight resistance locus from diploid (1EBN) Mexican Solanum pinnatisectum. Molecular Genet. Genomics. 2001;265:977–985. doi: 10.1007/s004380100490. [DOI] [PubMed] [Google Scholar]
- 61.Paal J, Henselewski H, Muth J, Meksem K, Menendez CM, Salamini F, Ballvora A, Gebhardt C. Molecular cloning of the potato Gro1-4 gene conferring resistance to pathotype Ro1 of the root cyst nematode Globodera rostochiensis based on a candidate gene approach. Plant J. 2004;38:285–297. doi: 10.1111/j.1365-313X.2004.02047.x. [DOI] [PubMed] [Google Scholar]
- 62.Varshney RK, Bansal KC, Aggarwal PK, Datta SK, Craufurd PQ. Agricultural biotechnology for crop improvement in a variable climate: hope or hype?. doi: 10.1016/j.tplants.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 63.Trends Plant Sci. 2011;16(7): 363–371. doi: 10.1016/j.tplants.2011.03.004. [DOI] [PubMed] [Google Scholar]