Abstract
Cutaneous malignant melanoma (CMM) has a high risk of dissemination to regional lymph nodes and visceral organs. Recurrences are most frequently seen within the first 2-3 years after initial treatment, but these patients have a life-long risk of relapse. The prognosis is highly dependent on lymph node involvement and distant metastases, accentuating the importance of close surveillance to identify disease progression at an early stage, and thereby detect recurrences amenable to treatment. Positron emission tomography (PET) has already been proven useful in the staging of CMM, but the utility of PET in follow-up programs for asymptomatic patients in high risk of relapse to detect systemic recurrences has yet to be investigated. We performed a systematic literature search in PUBMED, EMBASE and the Cochrane Controlled Trials Register, and identified 7 original studies on the diagnostic value of FDG-PET in the follow-up of CMM. Sensitivity, specificity, positive and negative predictive values were calculated to examine PET’s diagnostic value in detecting relapse. The mean sensitivity of PET was 96% and the specificity was 92%. The positive and negative predictive values were, respectively, 92% and 95%. Overall, PET has a high diagnostic value and the many advantages of PET indicate utility in the routine follow-up program of CMM. However, the number of prospective studies of high quality is scarce, and as the use of PET and PET/CT is becoming more widespread and the technology is expensive, there is an urgent need for systematic assessment of the diagnostic value.
Keywords: Melanoma, follow-up, PET, PET/CT, cancer, diagnostics, skin cancer, FDG
Introduction
Melanoma incidence and mortality continue to rise significantly on an international scale despite increased focus on melanoma skin cancer, greater knowledge of the disease progression, and numerous preventative campaigns. The United States, together with Denmark and Australia, account for some of the highest age-standardized rates of CMM incidences in the world [1].
Classification of CMM in accord to the American Joint Committee on Cancer’s (AJCC) TNM Staging System [2] includes typical signs of malignancy: change in colour, size, thickness and shape of a mole, and the prognosis is strongly dependent on the pathology as described by the AJCC staging, the offer of treatment, age and sex. There is no predictable pattern in the dissemination of CMM, and thus for staging and follow-up whole body monitoring is necessary.
The 5- and 10-year survival rates drop with higher stages of disease, and this reduction can be attributed to factors such as mitotic rate, ulceration, regional lymph nodes and distant metastases [3]. The recurrence rate of lower stages of CMM were addressed in a study by Mooney et al. who found the recurrence rate of stage IA to be 2% and stage IB to be 15%. Stage IIA-IIB patients had a recurrence rate of 27%, whilst stage IIC patients had a 47% recurrence rate [4]. Thus, due to the high risk of recurrence it is especially important to monitor patients within stages IIC-IIIC.
Treatment of metastasis is surgical resection when possible, hyperthermic isolated limp perfusion (HILP) or radiotherapy, and the use of biological treatment in melanoma is becoming more widespread and shows promising results. Leiter et al. [5] have shown a considerable correlation between early diagnosis of relapse and a longer overall survival in patients where surgery and resection of metastases have been possible. Due to the patients’ high risk of later recurrence, it is important to try to establish “best practice” for follow-up programs to detect disease progression at an early stage.
Melanoma cancer cells have an up-regulated glucose uptake and metabolism, and by intravenously injecting the radioactive glucose tracer, fluorine-18 labelled 2-deoxy-2-fluoro-D-glucose ([18F]FDG), the glucose-analogue will accumulate in potential cancer cells. PET is then used to map the [18F]FDG-distribution in the body, and can identify foci with a large number of cells with increased metabolism and thereby an accumulation of FDG such as malignant tissue or infection [6-8]. When combining PET and CT it is possible to anatomically identify foci with pathological uptake of [18F]FDG, and this combination increases the diagnostic sensitivity and specificity of the test [9].
Many studies have already proven the superiority of PET in staging and restaging of CMM patients [8,10,11], however, few have addressed the value of including PET as a routine surveillance tool in regular follow-up programs of CMM.
At present there is no consensus on the medical algorithm of optimal schedule, frequency of follow-up visits neither on the utility of imaging or blood test in asymptomatic patients with resected primary melanoma. Within studies it is even hard to find a common definition of follow-up. Is follow-up surveillance of asymptomatic patients? Therapy evaluation or further examination of patients presenting with symptoms? The indications for implementing a follow-up program is early detection of recurrent disease, and is based on the assumption that earlier detection of relapse in patients will allow for faster treatment by identifying tumours amenable to surgical resection to improve survival. As part of world-wide recommendations for follow-up in patients with melanoma, yearly repetitive physical examinations with special attention to other suspicious pigmented lesions, tumour satellites, in-transit metastases, regional lymph node and systemic metastases is an absolute minimum [12]. In patients with CMM ultrasound has proven to be a valuable supplement to physical examinations [13], but overall few data are available on the value of routine follow-up and monitoring of cancer patients in terms of beneficial influence on later morbidity, mortality and quality of life.
The present study describes the results of a systematic review involving PET and PET/CT in follow-up after therapy.
Materials and methods
Definition of follow-up and surveillance
This study was conducted to objectively assess the published literature on the diagnostic value of PET as a tool for surveillance, and is a step towards developing a medical algorithm for PET in the regular follow-up program of asymptomatic CMM patients Stage II-III. Accordingly we defined follow-up as surveillance of asymptomatic patients after treatment for malignant melanoma. However, as a reflection of current practise and the lack of an established definition of follow-up of patients with cutaneous malignant melanoma, some of the studies included in this review presented data on both response to therapy and surveillance of symptomatic/asymptomatic patients.
Search strategy and selection criteria
This study was initiated in May 2012 with a comprehensive computer search through the PUBMED and EMBASE databases of the medical literature conducted with the help of the Danish Medical Library. Medical Subjects Headings (MESH) as well as free text were used (melanoma and positron-emission tomography; melanoma and positron-emission tomography and computed tomography; melanoma and neoplasm metastasis and lymphatic metastasis and follow-up studies), and to avoid missing relevant literature, the search was made as broad as possible by evading restrictions on the language or year of publication and by application of all subheadings to the search terms. The search strategy was updated in August 2013 without any additional findings.
The initial sorting was made on a title-basis, and an account was set-up on NCBI to categorize the articles into two groups: choroidal/uveal melanoma and CMM. To get an overview of how many studies were performed on follow-up, it was decided to further divide the CMM-group into the following: Other tracers than FDG; Diagnostic; Staging; and Follow-up. At this point, it was decided to exclude articles on choroidal/uveal melanomas, because the disease, diagnosis and treatment differ substantially from that of CMM. Subsequently, the abstracts of the articles were printed and divided into the following categories: “Original studies: Follow-up, evaluation”; “Reviews: Follow-up, evaluation”; “Other: Follow-up, evaluation”; “Original studies: Diagnosis/staging”; “Reviews: Diagnosis/staging”; “Other tracers than [18F]FDG” and “Not relevant” to separate original studies from reviews and to further exclude irrelevant studies. The reference lists of previous original studies were also searched, adding more studies to the material, and finally the Cochrane Database was searched, without any additional findings.
See Figure 1 for a graphic presentation of the study selection.
Figure 1.
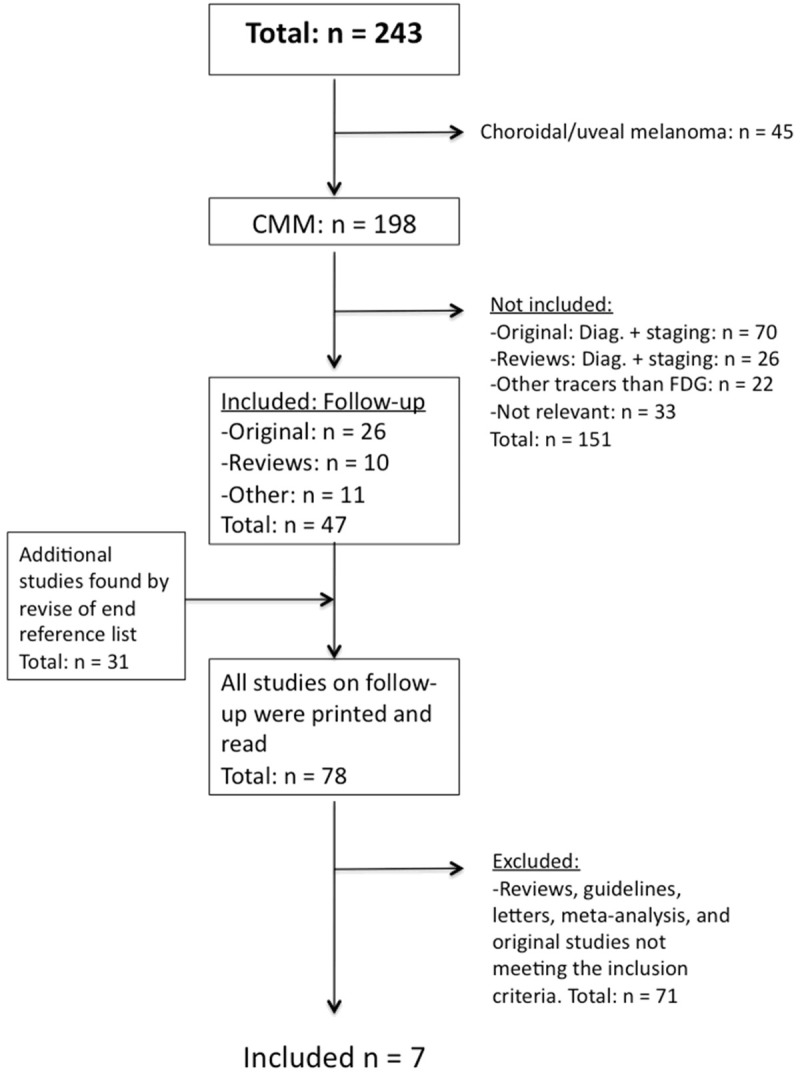
Flowchart for the selection of studies included in the systematic review.
Assessment of the methodological quality
The search strategy retrieved a total 243 references, 198 were relevant for the topic of this study, and were reviewed to assess eligibility for further analysis.
Articles selected for further evaluation (n=47) met the following criteria: Original studies with a comprehensive description of purpose, method and results; patient population ≥30; and use of the PET-tracer [18F]FDG.
Studies in which the definition of follow-up was unclear, when the follow-up regimen was inconsistent or poorly described, studies not reporting detailed results on the diagnostic performance of PET, meta-analyses, reviews, abstracts, letters and case studies were excluded from this systematic review.
Screening of the references cited in the retrieved articles identified 31 additional studies. Accordingly, 78 full-text articles were assessed further, from which seven studies were then finally included in the systematic review.
The following information was extracted from each eligible study (summarized in Table 1): authors’ names; year of publication; description of study population (number of patients); description of imaging procedure; inclusion criteria for study participants (symptomatic or asymptomatic, AJCC-stage); cohort assembly (prospective or retrospective); interpretation of scans; and application of reference test (follow-up, or biopsy).
Table 1.
Overview of the included articles (n=7)
| Studies on follow-up | n | Scanner AJCC Stage Examination | Inclusions criteria | Prospective/retrospective | Interpretation | Reference standard |
|---|---|---|---|---|---|---|
| 2012 | 97 | FDG-PET/CT | Patients undergoing ILI and who had a pre-PET/CT done before ILI and post-PET/CT done 3-monthly following surgery. | Prospective | Visual qualitative | Fine needle aspiration, CT guided biopsy and subsequent surgical resection when feasible. |
| Beasley [17] | Stage IIIB (n=61), IIIC (n=29) and IV (n=3) | 2004-2010 | ||||
| Therapy resp. | Database | |||||
| Follow-up | ||||||
| 2011 | 188 | FDG-PET/CT | Symptomatic patients undergoing ILI and/or HILP. PET/CT done pre and post-treatment (3-monthly for a year and then every 6 months thereafter). | Prospective | NA | Pathological and clinical response to ILI/HILP at 3 months. |
| Raymond [18] | Advanced extremity melanoma | 1995-2010. | ||||
| Stage IIIB, IIIC and IV. | Database | |||||
| Therapy resp. | ||||||
| Follow-up | ||||||
| 2011 | 20 | FDG-PET/CT | First annual surveillance PET/CT performed 12-23 months following diagnosis or development of stage III disease on an asymptomatic adult patient. Annual surveillance PET/CT. | Retrospective | Visual qualitative | NA |
| Abbott [19] | Micro. stage III | May 2008 | ||||
| Follow-up | Database | |||||
| 14 | FDG-PET/CT | |||||
| Macro. stage III | ||||||
| Follow-up | ||||||
| 2011 | 115 | FDG-PET/CT | Symptomatic (n=82) and asymptomatic (n=33) patients with and without increased levels of protein S-100B. | Prospective | Visual evaluation and semiquantitative analysis by measuring SUV max | Fine-needle aspiration cytology, CT, MRI, US, radiological findings, histology report |
| Peric [20] | Stage I (18%), II (41%), III (35%) and IV (4%). | Sept. 2007-feb. 2010 | ||||
| Follow-up | Database | |||||
| 2007 | 30 | FDG-PET | Asymptomatic patients | Prospective | Visual qualitative | Histology, CT and follow-up |
| Koskivuo [15] | Stage IIB-IIIC | March 2004-nov. 2005 | ||||
| Follow-up | Database | |||||
| 2006 | 250 | FDG-PET/CT | Patients referred for: | Retrospective | Visual qualitative | PET, CT and standard references – histological and clinical follow-up (bone scan, MRI and biopsy). |
| Reinhardt [21] | A comparison of PET, CT and PET/CT | Staging n=75, | Nov. 2002-June 2004 | |||
| Therapy resp. | Therapy response n=42 | Database | ||||
| Follow-up | Recurrence staging n=65 | |||||
| Follow-up n=68 | ||||||
| 1999 | 45 | FDG-PET | Patients with high risk of recurrence, clinical suspicion of recurrent disease, or recurrent melanoma. | Retrospective | Visual evaluation and semi quantitative analysis by measuring SUV max | Biopsy or other imaging modality within 6 weeks, or clinical follow-up within 6 months. |
| Nguyen [16] | High risk/recurrent melanoma | 1996-1998 | ||||
| Follow-up | Database |
AJCC, American Joint Committee on Cancer; CMM, Cutaneous malignant melanoma; CT, Computed tomography; FDG, Fluorine-18 labelled 2-deoxy-2-fluoro-D-glucose; US, Ultrasound; HILP, Hyperthermic isolated limb perfusion; ILI, Isolated limb infusion; MRI, Magnetic resonance imaging; NA, not available; PET, Positron Emission Tomography; SUV, Standardized uptake value.
After masking the authors’ names, and year of publication, (Table 2) the methodological quality of the studies was assessed based on the evidence-based criteria from MDRC’s Technology Assessment Program: PET Report, 1996, as described in Table 3.
Table 2.
Characteristics of studies (n=7) on follow-up
| Follow-up | FDG-PET | FDG-PET/CT | ||
|---|---|---|---|---|
|
| ||||
| Characteristics: | Number of studies | % of all studies on FDG-PET | Number of studies | % of all studies on FDG-PET/CT |
| Subjects, n: | ||||
| ≥35 | 1 | 50 | 4 | 80 |
| ≤35 | 1 | 50 | 1 | 20 |
| Design: | ||||
| Randomized | 0 | 0 | 0 | 0 |
| Prospective | 1 | 50 | 3 | 60 |
| Retrospective | 1 | 50 | 2 | 40 |
| Interpretation: | ||||
| SUV | 0 | 0 | 0 | 0 |
| Visual | 1 | 50 | 3 | 60 |
| SUV + visual | 1 | 50 | 1 | 20 |
| NA | 0 | 0 | 1 | 20 |
| Blinding | 0 | 0 | 1 | 20 |
| Not blinded | 2 | 100 | 2 | 40 |
| NA | 0 | 0 | 0 | 40 |
| Sufficient data to make 2x2 table | 1 | 50 | 5 | 100 |
| Not sufficient data to make 2x2 table | 1 | 50 | 0 | 0 |
| Total | 2 | 100 | 5 | 100 |
FDG, fluorodeoxyglucose; NA, not available; PET, Positron Emission Tomography; SUV, Standardized uptake value.
Table 3.
Methodological quality assessment of diagnostic accuracy studies*
| Grade | Criteria |
|---|---|
| A | Studies with broad generalizability to a variety of patients and no significant flaws in research methods |
| ● ≥35 patients with disease and ≥35 without disease | |
| ● Patients drawn from a clinically relevant sample (not filtered to include only severe disease) whose clinical symptoms are completely described | |
| ● Diagnoses defined by an appropriate reference standard | |
| PET studies technically of high quality and evaluated independently of the reference | |
| B | Studies with a narrower spectrum of generalizability, and with only a few flaws that are well described (and impact on conclusion that be assessed) |
| ● ≥35 cases with and without disease | |
| ● More limited spectrum of patients, typically reflecting referral bias of University Centres (more severe illness) | |
| ● Free of other methods flaws that promote interaction between test result and disease determination | |
| Prospective study still required | |
| C | Studies with several methods flaws |
| ● Small sample size | |
| ● Incomplete reporting | |
| Retrospective studies of diagnostic accuracy | |
| D | Studies with multiple flaws in methods |
| ● No credible reference standard for diagnosis | |
| ● Test result and determination of final diagnosis not independent | |
| ● Source of patient cohort could not be determined or was obviously influenced by the test result (work up bias) | |
| Opinions without substantiating data |
Flynn K, Anderson D.
MDRC Technology Assessment Program - PET report, 1996. MDRC is as per June 2000 known as Veteran Affairs Technology Assessment Programme.
It was not possible to perform a formal assessment of publication bias due to the small number of studies (n=7) and the high level of heterogeneity.
Data analysis
The purpose of applying PET in a routine follow-up program is to be able to accurately distinguish between relapse and no relapse. To assess the ability of PET and PET/CT to discriminate between malignant and benign, or relapse and no-relapse, the nosographic and diagnostic probabilities (sensitivity, specificity, positive and negative predictive values) was calculated from the reconstructed 2x2 contingency tables of true positive (TP), true negative (TN), false positive (FP), and false negative (FN) results. The sensitivity, defined as the proportion of true positives correctly identified as such together with the specificity, defined as the proportion of true negatives correctly identified as such in combination with positive and negative predictive values, defined as, respectively, the proportion of test positives that are truly positive, and the proportion of test negatives that are truly negative reflects the ability of the diagnostic test to correctly detect malignant disease [12,17]. The values of TP, FP, TN and FN derived from each study together with the grading of Level of Evidence can be found in Table 4. These values were then used to calculate sensitivity, specificity and predictive values for each study. To calculate sensitivity, specificity, and predictive values on the pooled data, the values of each TP, FP, TN and FN from level B and C studies (n=6) were summed (Table 5) and entered into a 2x2 contingency table in Microsoft Excel spreadsheet (Confidence Interval Calculator (v4, November 2002)).
Table 4.
Level of evidence and diagnostic performance of PET and PET/CT in follow-up of CMM
| Study | Grade | TP | FP | TN | FN | Sens. (%) | Spec. (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|---|
| 2012, Beasley [17] | B | 59 | 13 | 19 | 5 | 92 | 59 | 82 | 79 |
| 2011, Raymond [18] | B | 43 | 0 | 29 | 0 | 100 | 100 | 100 | 100 |
| 2011, AbbottA [19] | C | 2 | 0 | 17 | 1 | 67 | 100 | 100 | 94 |
| 2011, AbbottB [19] | C | 4 | 1 | 9 | 0 | 100 | 90 | 80 | 100 |
| 2011, Peric [20] | C | 93 | 2 | 19 | 1 | 99 | 90 | 98 | 95 |
| 2007, Koskivuo [15] | C | 6 | 1 | 22 | 1 | 86 | 96 | 86 | 96 |
| 2006, Reinhardt [21] | C | 2 | 0 | 65 | 1 | 67 | 100 | 100 | 98 |
| 1999, Nguyen [16] | D | NA | NA | 12 | NA | NA | NA | NA | NA |
TP, true positives; FP, false positives; TN, true negatives; FN, false negatives; NA, not available. Sens, sensitivity; Spec, specificity; PPV, positive predictive value; NPV, negative predictive value. A + B: The study by Abbott et al. contained two patient groups, respectively patients with microscopic (A) and macroscopic (B) stage III disease. The study by Nguyen et al. only reported number of TN, and therefore sens., spec. PPV and NPV could not be calculated.
Table 5.
Pooled data on follow-up of CMM
| n | Diagnostic values | % | 95% CI | |
|---|---|---|---|---|
| TP | 209 | Sensitivity | 96 | 92-98 |
| FP | 17 | Specificity | 92 | 87-95 |
| TN | 180 | PPV | 92 | - |
| FN | 9 | NPV | 95 | - |
TP, true positives; FP, false positives; TN, true negatives; FN, false negatives. The values are summed from 6 studies [15,17-21], not including the study by Nguyen et al. due to incomplete reporting of diagnostic and nosographic values. TPtotal, FPtotal, TNtotal and FNtotal were entered into a 2x2 contingency table to calculate sensitivity, specificity, PPV and NPV on the pooled data. 95% CI interval was only calculated for sensitivity and specificity.
The confidence intervals for sensitivity and specificity were produced with the Wilson score method without continuity correction as described by Robert G. Newcombe [14]. All the score methods are designed to produce two-sided intervals whenever this is possible given the data, and the methods are constructed so as to try to align lower and upper tail probabilities symmetrically with α/2.
Results
Descriptive analysis
Of the seven articles eligible for further analysis, two addressed FDG-PET [15,16] and five FDG-PET/CT [17-21]. There were no randomised studies, and no studies met the methodological quality criteria of grade A. Table 4 contains the grading of Level of Evidence for the included studies together with the report of true positives, false positives, true negatives and false negatives, which were used to calculate sensitivity, specificity, positive and negative predictive values for each study.
The studies by Beasley et al. [17] and Raymond et al. [18] had PET/CT in follow-up of CMM patients as a secondary aim. The primary aim in these studies was to examine the use of PET/CT in therapy response, and it is important to note that the study population of these two studies may be overlapping with up to 68 patients. It was decided to include both studies in this systematic qualitative review, because they presented a thoroughly described purpose, method, and result section, and were graded level B in methodological quality. Beasley et al. evaluated the utility of [18F]FDG-PET/CT to detect responders to isolated limb infusion (ILI) in patients with Stage IIIB-IIIC extremity melanoma. The diagnostic performance of PET/CT was found to be quite sensitive (92%), less specific (59%), and with a positive predictive value of 82% and a negative predictive value of 79%. In the study by Raymond et al. PET/CT was used to compare the outcome of two different chemotherapy treatments: ILI and hyperthermic isolated limb perfusion (HILP). The study population contained a relatively large group of subjects with advanced extremity melanoma, Stage IIIB, IIIC and IV. The study stated the true-positive and true-negative findings causing the diagnostic values of PET/CT to be equal to 100%.
The study by Abbott et al. [19] contained two study populations where the aim was to evaluate PET/CT as a surveillance tool in asymptomatic CMM patients. One group contained 20 patients with microscopic Stage III and another group contained 14 patients with macroscopic Stage III melanoma. The patients were retrospectively identified from a PET database and the description of scan interval and follow-up program was unclear thereby grading the study as level C. The diagnostic value of PET/CT in the group of patients with microscopic disease showed a sensitivity of 67%, a specificity and positive predictive value of 100% and a negative predictive value of 94%. In the group of patients with macroscopic disease PET/CT was found to have a sensitivity of 100%, a specificity of 90%, a positive predictive value of 80%, and a negative predictive value of 100%.
Peric et al. [20] evaluated the role of serum S100B and PET/CT in follow-up of CMM. The study included 115 patients in all stages of disease. Due to the incomplete reporting of follow-up and inconsistency in the time interval between the scans the study was graded as level C. The sensitivity, specificity, positive and negative predictive values of PET/CT in all patients were found to be, respectively, 99%, 90%, 98% and 95%.
In a prospective Finnish study by Koskivuo et al. [15], 30 asymptomatic patients Stage IIB-IIIC were PET scanned 7-24 months after primary surgery. PET had a sensitivity of 86%, a specificity of 96%, positive and negative predictive values of respectively 86% and 96%. This study was graded as level C due to incomplete reporting of data and inconsistency in scan interval.
Reinhardt et al. [21] compared PET, CT and PET/CT in 250 symptomatic patients , and PET/CT in follow-up was found to have a sensitivity of 67%, a specificity and positive predictive value of 100% and a negative predictive value of 98%. The methodological quality of the study was graded as level C due to the retrospective design and incomplete description of follow-up program.
In an older study on the benefits and limitations of PET in CMM, Nguyen et al. [16] included 45 symptomatic patients of either high risk or with known recurrent melanoma. The patients were enrolled from a retrospective database and included a particular high degree of incomplete data reporting only stating number of false negative findings. The study was graded as level D and was not included in the final analysis. Table 4 summarizes the diagnostic and nosographic probabilities of PET in follow-up.
Quantitative analysis of the pooled data
In accordance with the grading system in Table 3, no level A studies, two level B studies, four level C studies and one level D study could be identified. It was decided to perform a systematic review on level B and C studies including data from a total of six studies and 714 patients to examine the diagnostic value of PET in follow-up.
Our results on the pooled data (Table 5) from studies on PET and PET/CT in follow-up indicate that PET is very sensitive, 96% (95% CI, 92-98), but less specific, 92% (95% CI 87-95), in discriminating relapse (malignant) from no relapse (benign) neoplasm. The positive predictive value was found to be 92% and the negative predictive value was 95%. These results show that 1) a negative PET scan can, with near certainty, exclude malignancy and 2) that a positive PET scan means high probability of malignancy and further examinations should be initiated.
Discussion
The desired goal of this study was to qualitatively evaluate the diagnostic value of PET and to develop a medical algorithm for PET in regular follow-up programs of CMM. A presentation of each study was performed, including a systematic methodological assessment of the study quality. Data on the diagnostic performance of PET and PET/CT from the eligible 6 studies were pooled, indicating a high sensitivity and negative predictive value of PET in the follow-up setting. But, from this systematic assessment of the present evidence on PET in follow-up of CMM, we also found that there were no randomised studies, and many of the existing studies had several methodological flaws.
Many types of biases can influence the validity of a study, and when defining a search strategy, bias will inevitably be introduced into the retrieved material. An example is referral bias, which applies to a large part of the studies included in this review [16-18,20,21]. The patients are referred to PET scans on the physicians’ suspicion of recurrence, thereby not representing the population of our primary interest: asymptomatic patients likely to enter a routine follow-up program. It would be most favourable to only include studies on asymptomatic patients because this group constitute the population of patients who will benefit most from entering a follow-up program. Patients with suspected recurrence enters into a different management plan. However, the number of studies and patient data eligible for this review would become too low, to draw any conclusions.
Another important bias influencing the validity of our study is verification bias, which occurs when the result of the diagnostic test evaluated influences the application of the gold standard for verification. i.e. a patient with a solitary focal uptake on FDG-PET/CT suspicious for malignancy is more likely than subjects without focal uptake to receive a biopsy for verification. Verification bias is difficult to avoid, especially in studies on whole body PET/CT, thereby accentuating the importance of performing larger prospective trials including data on survival.
A potential risk of referral and verification bias is an overestimation of the number of true positives respectively underestimation of false negatives, leading to an overestimation of both sensitivity and negative predictive value.
Lastly, when examining the effectiveness of a routine follow-up program of cancer patients, it is a prerequisite to maintain consistency and continuity throughout the work-up period to be able to compare outcome. CMM patients have a certain risk of relapse after resection of a primary melanoma, and 80% of the recurrences are seen within the first 3 years [22]. However, there is still a risk of relapse later in life an therefore, when the desired goal is to determine for how many years follow-up should be conducted in CMM patients, studies on follow-up monitored for 2 or 3 years are not adequate to address the question of relevance. None of the included studies presented with satisfying consistency or continuity in the time interval in which the PET scans were performed nor did the longest follow-up period extend beyond 3 years.
Naturally, the above-mentioned leads to the questions of whether the data in the literature are sufficient and, most importantly, whether the results of this study are valid. In the follow-up programs of cancer patients, it is desirable to use a diagnostic test with high sensitivity and high negative predictive value to identify patients with recurrence of malignant disease. A diagnostic test with high negative predictive value is important, because a negative test with almost certainty can exclude disease. The sensitivity and negative predictive value on the pooled data were found to be, respectively, 96% and 95%, which indicate that PET could be a useful tool in the routine follow-up program of CMM patients. When comparing the diagnostic performance of PET with the results of another meta-analysis, the values are found to vary. In a study by Xing et al. [23], the sensitivity of PET/CT to detect distant metastases was found to be 86% (95% CI, 76-93%). The difference to our study can be explained by a number of things, i.e. the dissimilarities in inclusion criteria: To raise the validity of our systematic review, our inclusion criteria were set within narrow limits, only allowing the best methodological studies to enter the analysis. The difference can also be explained by the fact that a closer look on the studies included in the surveillance meta-analysis by Xing mainly addresses staging and restaging [24-27], rather than follow-up and surveillance as is the focus of this review.
Three studies [15,16,21] were used in both analyses, and the last four studies [17-20] in our analysis were published after Xing’s final literature search in June, 30, 2009. With the continuous development of new and better technology, the PET scanners are now able to detect even smaller foci resulting in higher sensitivities, as was found on the pooled data in this study. In spite of the numerous differences between the two analyses, the conclusion of our study is consistent with that of Xing: “Future comparative effectiveness analyses should use decision-analytic modelling to simulate the effectiveness and cost-effectiveness of various surveillance strategies with respect to imaging modality and frequency on stage-specific patient outcomes” [23]. However, it should be stressed that it is indeed very possible that the results of our analysis as well as the analysis by Xing slightly overestimates the diagnostic value of PET and PET/CT due to a number of inevitable bias as described above.
The large heterogeneity of research questions, study designs, patient cohort, inclusion criteria, reference standards and follow-up periods, as well as the fact that no studies could be methodologically compared according to the rules of evidence-based medicine, indicate that the true evidence of PET’s role in routine follow-up programs of asymptomatic CMM patients has yet to be properly clarified.
When performing diagnostic imaging in patients, especially as follow-up examinations in asymptomatic patients, the physician always has to consider the radiation burden due to the injection of radioactive tracers to the subject and the amount of radiation exposure from x-ray examinations. If PET were to be established in the routine follow-up program of CMM patients, it would be strongly beneficial if the radiation could be restricted to an absolute minimum. One path to follow is to use single-modality PET or PET with low-dose CT. The total amount of radiation exposure received from a PET/CT (CT of diagnostic quality) is typically 12-20 mSv, of which the CT-scan alone accounts for app. 10 mSv. Reinhardt et al. [21] found that FDG-PET and FDG-PET/CT performed identically at follow-up, and this is an important finding, which can restrict the amount of radiation exposure to the patient since follow-up scans can be made with PET alone, thereby avoiding radiation from repetitive CT. However, this needs to be further investigated.
Introducing a blood sample for screening purpose and only applying PET/CT as a second step test would be ideal, both regarding radiation and cost-effectiveness, but such a test is unfortunately not available at present. The S-100B protein has been used as a routine immunohistochemical marker, but there is no actual agreement on its utility. Few studies have evaluated the advantages of combining PET and S-100B in the surveillance of CMM [20,30-34], and the protein by itself lacks sufficient sensitivity. A high value of S-100B can indicate metastases, and should lead to further examinations (i.e. PET scans). Despite this, normal values of S-100B cannot exclude metastases, and further investigations should be carried out.
Another serologic marker, melanoma-inhibitory activity (MIA) protein, is comparable to the findings of S-100B [35,36]; unfortunately this marker also lacks sensitivity.
Furthermore, the recent introduction of the combined PET/MR creates possibilities for dose reduction while maintaining, and perhaps even improving, soft tissue contrast. The combined PET/MR scanner is a new hybrid imaging modality combining PET and MRI in one scanner with simultaneous acquisitions [28]. The advantages of this combination include improved soft tissue contrast and decreased exposure of the patient to potential harmful radiation. In a recent study by Dellestable et al. [29] comparing PET/CT and MRI in the management of patients with melanoma, MRI was found to have a higher sensitivity (83%) than PET/CT (74%). MRI was found distinctly superior for both hepatic and pulmonary lesions. Combining the ability of PET to detect foci with a pathological uptake of glucose with the anatomical precision of MRI may therefore play an important role in future surveillance of CMM.
Finally, the systematic use of routine PET scans in cancer follow-up programs is a costly affair, and this extra expense needs to be proven worthy and beneficial. One way to evaluate whether a new method should be implemented into regular use is to apply the Health Technology Assessment (HTA) methodology, which is a multidisciplinary process that summarises information about the medical, social, economic, and ethical issues related to the use of a health technology in a systematic, unbiased, robust manner. The first step in an HTA-analysis is a thorough and complete rating of the evidence, as is this paper.
Conclusion
Introduction of PET as a routine diagnostic tool in the follow-up of CMM could make it possible - with one examination - to exclude or detect possible melanoma recurrence. With a sensitivity of 96% and a specificity of 92% PET is useful for decisions on the best future management of the patient.
The high rate of incidence together with better surgical treatment causes an increase in the number of melanoma survivors, and because of limited health-care resources, it is becoming increasingly critical to adjust current consensus-based guidelines toward a suitable evidence-based and cost-effective medical algorithm for an efficient follow-up program to monitor the patients.
Prior to testing the proficiency of PET in follow-up of CMM, a cost-effectiveness analysis has to be made, and whether there is a survival benefit from early detection of metastases must be clarified. Subsequently, the gold standard to examine PET in follow-up of CMM would be a randomized controlled study including asymptomatic patients stages IIB-IIIC.
Disclosure of conflict of interest
This study has nothing to declare.
References
- 1.Little EG, Eide MJ. Update on the current state of melanoma incidence. Dermatol Clin. 2012;30:355–361. doi: 10.1016/j.det.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC Jr, Morton DL, Ross MI, Sober AJ, Sondak VK. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Vries M, Speijers MJ, Bastiaannet E, Plukker JT, Brouwers AH, van Ginkel RJ, Suurmeijer AJ, Hoekstra HJ. Long-term follow-up reveals that ulceration and sentinel lymph node status are the strongest predictors for survival in patients with primary cutaneous melanoma. Eur J Surg Oncol. 2011;37:681–687. doi: 10.1016/j.ejso.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Mooney MM, Kulas M, McKinley B, Michalek AM, Kraybill WG. Impact on survival by method of recurrence detection in stage I and II cutaneous melanoma. Ann Surg Oncol. 1998;5:54–63. doi: 10.1007/BF02303765. [DOI] [PubMed] [Google Scholar]
- 5.Leiter U, Buettner PG, Eigentler TK, Forschner A, Meier F, Garbe C. Is detection of melanoma metastasis during surveillance in an early phase of development associated with a survival benefit? Melanoma Res. 2010;20:240–246. doi: 10.1097/CMR.0b013e32833716f9. [DOI] [PubMed] [Google Scholar]
- 6.Fischer BM, Olsen MW, Ley CD, Klausen TL, Mortensen J, Hojgaard L, Kristjansen PE. How few cancer cells can be detected by positron emission tomography? A frequent question addressed by an in vitro study. Eur J Nucl Med Mol Imaging. 2006;33:697–702. doi: 10.1007/s00259-005-0038-6. [DOI] [PubMed] [Google Scholar]
- 7.Horn J, Lock-Andersen J, Sjostrand H, Loft A. Routine use of FDG-PET scans in melanoma patients with positive sentinel node biopsy. Eur J Nucl Med Mol Imaging. 2006;33:887–892. doi: 10.1007/s00259-006-0077-7. [DOI] [PubMed] [Google Scholar]
- 8.Eigtved A, Andersson AP, Dahlstrom K, Rabol A, Jensen M, Holm S, Sorensen SS, Drzewiecki KT, Hojgaard L, Friberg L. Use of fluorine-18 fluorodeoxyglucose positron emission tomography in the detection of silent metastases from malignant melanoma. Eur J Nucl Med. 2000;27:70–75. doi: 10.1007/pl00006666. [DOI] [PubMed] [Google Scholar]
- 9.Altman DG, Bland JM. Diagnostic tests. 1: Sensitivity and specificity. BMJ. 1994;308:1552. doi: 10.1136/bmj.308.6943.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rinne D, Baum RP, Hor G, Kaufmann R. Primary staging and follow-up of high risk melanoma patients with whole-body 18F-fluorodeoxyglucose positron emission tomography: results of a prospective study of 100 patients. Cancer. 1998;82:1664–1671. doi: 10.1002/(sici)1097-0142(19980501)82:9<1664::aid-cncr11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Holder WD Jr, White RL Jr, Zuger JH, Easton EJ Jr, Greene FL. Effectiveness of positron emission tomography for the detection of melanoma metastases. Ann Surg. 1998;227:764–769. doi: 10.1097/00000658-199805000-00017. discussion 769-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dummer R, Hauschild A, Guggenheim M, Keilholz U, Pentheroudakis G ESMO Guidelines Working Group. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii86–91. doi: 10.1093/annonc/mds229. [DOI] [PubMed] [Google Scholar]
- 13.Xing Y, Cromwell KD, Cormier JN. Review of diagnostic imaging modalities for the surveillance of melanoma patients. Dermatol Res Pract. 2012;2012:941921. doi: 10.1155/2012/941921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 15.Koskivuo IO, Seppanen MP, Suominen EA, Minn HR. Whole body positron emission tomography in follow-up of high risk melanoma. Acta Oncol. 2007;46:685–690. doi: 10.1080/02841860600972885. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen AT, Akhurst T, Larson SM, Coit DG, Brady MS. PET Scanning with (18)F 2-Fluoro-2-Deoxy-D-Glucose (FDG) in Patients with Melanoma. Benefits and Limitations. Clin Positron Imaging. 1999;2:93–98. doi: 10.1016/s1095-0397(99)00006-0. [DOI] [PubMed] [Google Scholar]
- 17.Beasley GM, Parsons C, Broadwater G, Selim MA, Marzban S, Abernethy AP, Salama AK, Eikman EA, Wong T, Zager JS, Tyler DS. A multicenter prospective evaluation of the clinical utility of F-18 FDG-PET/CT in patients with AJCC stage IIIB or IIIC extremity melanoma. Ann Surg. 2012;256:350–356. doi: 10.1097/SLA.0b013e318256d1f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raymond AK, Beasley GM, Broadwater G, Augustine CK, Padussis JC, Turley R, Peterson B, Seigler H, Pruitt SK, Tyler DS. Current trends in regional therapy for melanoma: lessons learned from 225 regional chemotherapy treatments between 1995 and 2010 at a single institution. J Am Coll Surg. 2011;213:306–316. doi: 10.1016/j.jamcollsurg.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbott RA, Acland KM, Harries M, O’Doherty M. The role of positron emission tomography with computed tomography in the follow-up of asymptomatic cutaneous malignant melanoma patients with a high risk of disease recurrence. Melanoma Res. 2011;21:446–449. doi: 10.1097/CMR.0b013e3283480256. [DOI] [PubMed] [Google Scholar]
- 20.Peric B, Zagar I, Novakovic S, Zgajnar J, Hocevar M. Role of serum S100B and PET-CT in follow-up of patients with cutaneous melanoma. BMC Cancer. 2011;11:328. doi: 10.1186/1471-2407-11-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reinhardt MJ, Joe AY, Jaeger U, Huber A, Matthies A, Bucerius J, Roedel R, Strunk H, Bieber T, Biersack HJ, Tuting T. Diagnostic performance of whole body dual modality 18F-FDG PET/CT imaging for N- and M-staging of malignant melanoma: experience with 250 consecutive patients. J. Clin. Oncol. 2006;24:1178–1187. doi: 10.1200/JCO.2005.03.5634. [DOI] [PubMed] [Google Scholar]
- 22.Fields RC, Coit DG. Evidence-based follow-up for the patient with melanoma. Surg Oncol Clin N Am. 2011;20:181–200. doi: 10.1016/j.soc.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Xing Y, Bronstein Y, Ross MI, Askew RL, Lee JE, Gershenwald JE, Royal R, Cormier JN. Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: a meta-analysis. J Natl Cancer Inst. 2011;103:129–142. doi: 10.1093/jnci/djq455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfannenberg C, Aschoff P, Schanz S, Eschmann SM, Plathow C, Eigentler TK, Garbe C, Brechtel K, Vonthein R, Bares R, Claussen CD, Schlemmer HP. Prospective comparison of 18F-fluorodeoxyglucose positron emission tomography/ computed tomography and whole-body magnetic resonance imaging in staging of advanced malignant melanoma. Eur J Cancer. 2007;43:557–564. doi: 10.1016/j.ejca.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Veit-Haibach P, Vogt FM, Jablonka R, Kuehl H, Bockisch A, Beyer T, Dahmen G, Rosenbaum S, Antoch G. Diagnostic accuracy of contrast-enhanced FDG-PET/CT in primary staging of cutaneous malignant melanoma. Eur J Nucl Med Mol Imaging. 2009;36:910–918. doi: 10.1007/s00259-008-1049-x. [DOI] [PubMed] [Google Scholar]
- 26.Gritters LS, Francis IR, Zasadny KR, Wahl RL. Initial assessment of positron emission tomography using 2-fluorine-18-fluoro-2-deoxy-D-glucose in the imaging of malignant melanoma. J Nucl Med. 1993;34:1420–1427. [PubMed] [Google Scholar]
- 27.Boni R, Boni RA, Steinert H, Burg G, Buck A, Marincek B, Berthold T, Dummer R, Voellmy D, Ballmer B, et al. Staging of metastatic melanoma by whole-body positron emission tomography using 2-fluorine-18-fluoro-2-deoxy-D-glucose. Br J Dermatol. 1995;132:556–562. doi: 10.1111/j.1365-2133.1995.tb08711.x. [DOI] [PubMed] [Google Scholar]
- 28.Balyasnikova S, Lofgren J, de Nijs R, Zamogilnaya Y, Hojgaard L, Fischer BM. PET/MR in oncology: an introduction with focus on MR and future perspectives for hybrid imaging. Am J Nucl Med Mol Imaging. 2012;2:458–474. [PMC free article] [PubMed] [Google Scholar]
- 29.Dellestable P, Granel-Brocard F, Rat AC, Olivier P, Regent D, Schmutz JL. [Impact of whole body magnetic resonance imaging (MRI) in the management of melanoma patients, in comparison with positron emission tomography/computed tomography (TEP/CT) and CT] . Ann Dermatol Venereol. 2011;138:377–383. doi: 10.1016/j.annder.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 30.Beyeler M, Waldispuhl S, Strobel K, Joller-Jemelka HI, Burg G, Dummer R. Detection of melanoma relapse: first comparative analysis on imaging techniques versus S100 protein. Dermatology. 2006;213:187–191. doi: 10.1159/000095034. [DOI] [PubMed] [Google Scholar]
- 31.Aukema TS, Olmos RA, Korse CM, Kroon BB, Wouters MW, Vogel WV, Bonfrer JM, Nieweg OE. Utility of FDG PET/CT and brain MRI in melanoma patients with increased serum S-100B level during follow-up. Ann Surg Oncol. 2010;17:1657–1661. doi: 10.1245/s10434-010-0963-x. [DOI] [PubMed] [Google Scholar]
- 32.Mruck S, Baum RP, Rinne D, Hor G. Diagnostic accuracy and predictive value of the tumor-associated antigen S100 in malignant melanomas: validation by whole body FDG-PET and conventional diagnostics. Anticancer Res. 1999;19:2685–2690. [PubMed] [Google Scholar]
- 33.Strobel K, Skalsky J, Kalff V, Baumann K, Seifert B, Joller-Jemelka H, Dummer R, Steinert HC. Tumour assessment in advanced melanoma: value of FDG-PET/CT in patients with elevated serum S-100B. Eur J Nucl Med Mol Imaging. 2007;34:1366–1375. doi: 10.1007/s00259-007-0403-8. [DOI] [PubMed] [Google Scholar]
- 34.Strobel K, Dummer R, Steinert HC, Conzett KB, Schad K, Lago MP, Soyka JD, Veit-Haibach P, Seifert B, Kalff V. Chemotherapy response assessment in stage IV melanoma patients-comparison of 18F-FDG-PET/CT, CT, brain MRI, and tumormarker S-100B. Eur J Nucl Med Mol Imaging. 2008;35:1786–1795. doi: 10.1007/s00259-008-0806-1. [DOI] [PubMed] [Google Scholar]
- 35.Djukanovic D, Hofmann U, Sucker A, Rittgen W, Schadendorf D. Comparison of S100 protein and MIA protein as serum marker for malignant melanoma. Anticancer Res. 2000;20:2203–2207. [PubMed] [Google Scholar]
- 36.Juergensen A, Holzapfel U, Hein R, Stolz W, Buettner R, Bosserhoff A. Comparison of two prognostic markers for malignant melanoma: MIA and S100 beta. Tumour Biol. 2001;22:54–58. doi: 10.1159/000030147. [DOI] [PubMed] [Google Scholar]


