Abstract
The role of [18F]fluorodeoxyglucose ([18F]FDG) PET in staging of sarcoma is well established. The aim of this preclinical study was to compare [18F]fluorothymidine ([18F]FLT) PET to [18F]FDG PET regarding early metabolic changes of sarcoma in the course of targeted cancer therapy. SCID mice bearing sarcoma A673 xenotransplants were used for investigation of tumor response after treatment with the multikinase inhibitor Sorafenib. [18F]FLT and/or [18F]FDG-PET were performed prior to and early after initiation of treatment. Tumoral uptake (% Injected Dose per gram (%ID/g) of [18F]FLT-PET was compared to [18F]FDG-PET. Results were correlated with histopathology and in vitro data including cellular uptake, cell cycle-related protein expression, cell cycle distribution and apoptosis. In vitro experiments showed that A673 cells were sensitive to Sorafenib. In vivo, tumor growth was inhibited in comparison to a 4-fold increase of the tumor volume in control mice. Using [18F]FDG as tracer, a moderate reduction in tracer uptake (n=15, mean relative %ID/g 74%, range 35%-121%, p=0.03) was observed. The decrease in %ID/g using [18F]FLT-PET was significantly higher (p=0.003). The mean relative %ID/g in [18F]FLT uptake on day + 5 was significantly reduced to 54% compared to baseline (n=15, range 24%-125%, SD=29%). The PET analysis 24 hr after therapy showed a significant reduction of the mean [18F]FLT-%ID/g (p=0.04). The reduction of %ID/g on day + 1 in [18F]FDG-PET was not statistically significant (p=0.99). In conclusion, both [18F]FDG- and [18F]FLT-PET were able to predict response to Sorafenib treatment. In contrast to [18F]FDG-PET, [18F]FLT-PET was more predictive for very early response to treatment.
Keywords: Molecular imaging, sarcoma, PET, proliferation, [18F]FLT, [18F]FDG
Introduction
Ewing Sarcoma is a malignant small round cell tumor of the musculoskeletal system and ranks as the 2nd most frequent bone related cancer in children and young adults. In the past few decades, long-term patient survival has considerably improved as a result of the success of multimodal therapeutic regimens, including su-rgery, chemotherapy, and radiotherapy. Today, the 10-year survival rate has reached approximately 50%. However, patients with metastatic disease have a poor prognosis. 30-40% of patients with local disease and approximately 80% of patients with metastatic disease will die because of disease progression [1-3]. In addition, long-term survival can be compromised by late side effects of chemotherapy and radiotherapy.
New insights into the molecular pathogenesis of human cancer have led to the identification of several proteins acting as signaling molecules and modulators of tumor growth. Targeting these proteins using novel pharmacologic agents can be effectively used to control tumor growth [4]. Sorafenib, a small-molecule multikinase inhibitor, is a potent inhibitor of RAF kinase in vitro. Sorafenib is also a strong inhibitor of vascular endothelial growth factor (VEGF) receptor 1, 2, and 3, platelet derived growth factor (PDGF) b-receptor, c-kit, and RET. Sorafenib demonstrates antitumor activity in a broad range of human xenograft animal models including colon, mammary, pancreatic, ovarian cancer, hepatocellular carcinoma [5,6] and sarcoma [7].
To assess therapy response, noninvasive imaging modalities like computed tomography (CT), magnetic resonance imaging (MRI) or bone scintigraphy are commonly used [8-10]. Due to the fact that a change in lesion size is mostly observed several weeks after the start of chemotherapy, other staging techniques such as PET with the radiopharmaceutical [18F]fluorodeoxyglucose ([18F]FDG), complemented with CT are widely used. [18F]FDG-PET/CT has the potential to provide an accurate assessment of early response to treatment, with the ultimate goal of identifying responding and non-responding tumors and of tailoring therapy according to the information obtained by non-invasive imaging [11-13].
Various forms of infection, inflammation, granulomatous disease and many other physiological or pathological conditions also utilize [18F]FDG and are the major cause of false-positive results [14]. While both [18F]FDG and [18F]fluorothymidine ([18F]FLT) are very sensitive markers, [18F]FLT-PET is expected to be more specific for tumor assessment [15,16]. Shields et al. [17] first reported the potential utility of [18F]FLT for the non-invasive detection of cell proliferation by PET. [18F]FLT undergoes monophosphorylation to [18F]FLT phosphate catalyzed by the cytosolic enzyme thymidine kinase 1 (TK1), which, being cell cycle regulated, provides a surrogate measure of cells in S phase of the cell cycle [18,19]. Thus, changes in [18F]FLT uptake can be correlated directly with cell proliferation and [18F]FLT-PET could be a potentially effective method to monitor early response to targeted therapy in the setting of clinical studies [20,21].
In this report, severe combined immunodeficient (SCID) mice bearing A673 sarcoma xenotransplants were treated with Sorafenib. Small animal PET-CT imaging using [18F]FDG and [18F]FLT as tracers was applied to predict response to targeted therapy very early in the course of treatment.
Materials and methods
Cell lines and animal model
The cell line A673 derived from a patient with Ewing sarcoma (ATCC CRL-1598, LGC Standards GmbH, Wesel, Germany) was cultured in DMEM medium (Biochrom, Germany) containing 10% fetal bovine serum. Six to eight week old female immunodeficient mice (CB-17 SCID) were obtained from Charles River Laboratories. For induction of tumors 5×106 A673 cells suspended in sterile PBS (100 μl) were injected subcutaneously into the right shoulder region. All animal experiments were authorized by the regional government agency.
Inhibitors and drugs
Sorafenib was solubilized in DMSO to 200 μM or 1 μM stock solution, respectively, for in vitro treatment, and stored at -20°C. The primary solution was diluted to 0-1000 μM with DMEM cell culture medium. Doxorubicin (Sigma-Aldrich Chemie GmbH, Munich, Germany) was diluted to 0-250 μM with DMEM cell culture medium.
MTT assay
MTT assays were performed according to the manufacturer’s instruction (Promega GmbH, Mannheim, Germany). Briefly, 104 cells per well (96-well plate) were incubated at 37°C with different concentrations of inhibitors for 48 h. 20 μl of MTT dilution (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide in PBS) was added to each well and cells were incubated for 90 min. Absorbance was measured at 570 nm using a Bio-Tek ELx800 series universal microplate reader (Progen Scientific, London, UK).
Flow cytometry
Sarcoma cells (5×105 per well, 12-well plates) were incubated with different inhibitor concentrations at 37°C. After 48 h, cells were centrifuged (1.700 spins/min, 7 min) and washed with cold PBS buffer (0°C). To assess cell cycle distribution, 1×106 cells were stained with FITC-BrdU (BD Pharmingen, Heidelberg, Germany) and counterstained with propidium iodide (Sig-ma-Aldrich Chemie GmbH, Munich, Germany) and 7-AAD (BD Pharmingen, Heidelberg, Germany). To assess apoptosis, 1×106 cells were stained with Annexin V (BD Pharmingen, Hei-delberg, Germany) and counterstained with propidium iodide (Sigma-Aldrich Chemie GmbH, Munich, Germany). Finally, cells were resuspended in 500 μl PBS, and analyzed by flow cytometry (Beckman Coulter GmbH, Krefeld, Germany).
[18F]FLT - and [18F]FDG-uptake in vitro
Sarcoma cells (5×105 per well) were plated in 12-well plates and were incubated with different concentrations of inhibitors at 37°C for 48 h. After cell counting, 100 μl of tracer solution was added and incubated for 45 min at 37°C. Tracer solution consisted of 0.9% sodium chloride and [18F]FLT or [18F]FDG with an activity of 370 kBq. Cells were then washed three times with PBS before measuring activity (counts per minute, cpm) using an automated gamma-counter (WALLAC 1480 WIZARD 3, PerkinElmer, Rodgau, Germany). Results were adjusted to 106 cells per well.
Tumor volume and therapeutic regimens
The tumor diameters were measured daily with a shifting calliper and tumor volume was calculated using the formula [length × (width)2]/2. Treatment was performed when the xenotransplants reached a size of approximately 100 mm³. Sarcoma bearing animals were treated daily by intraperitoneal injection of Sorafenib (30 mg/kg bodyweight). The control group received PBS only.
PET-imaging
[18F]FLT and [18F]FDG were synthesized as previously described [22] and were obtained from the radiopharmacy unit of the TU Munich. Imaging was performed using a dedicated micro PET/CT system (Inveon, SIEMENS Preclinical Solutions), [18F]FLT or [18F]FDG was administered via tail vein injection (100 μl) at an activity dose of 5-10 MBq per mouse. The accumulation of radiotracer in the tumor was allowed for 45 min. Mice were then imaged for a 15 min (static data acquisition).
PET data analysis
% Injected Dose per gram (%ID/g) were calculated to semi-quantitatively assess the tracer accumulation in the tumor. Circular 3D regions of interest (ROIs) were placed manually in the area with the highest tumor activity. The diameter was not covering the entire tumor volume to avoid partial volume effects. For determination of background activity, two 3D ROIs were placed in the spinal muscle at the level of the kidneys.
Histological and immunohistochemical analysis
Formalin-fixed, paraffin-embedded sections (5 μm) of resected tumor tissue were dewaxed, rehydrated and microwaved for 30 min in a 0.01 M citrate buffer, pH 6.0 containing 0.1% Tween 20. Sections were washed in Tris-buffered saline (pH 7.6) containing 5% fetal calf serum (Life Technologies GmbH, Darmstadt, Germany) for 20 min. The primary antibodies used were: anti-Ki67 antigen antibody solution (MIB-1, M7240, Dako; 1:75 diluted with Antib-ody Diluent (Dako ChemMate, Dako Deutsch-land GmbH, Hamburg, Germany), anti-cleaved caspase-3 rabbit monoclonal antibody solution (Asp175, Cell Signaling Technology, New Engla-nd Biolabs GmbH, Frankfurt am Main, Germa-ny). The remainder of the procedure was performed on the automated immunostainer (DISCOVERY XT, Ventana Medical System, Roche, Mannheim, Germany). Diaminobenzidine (DAB, Ventana Medical Systems, Roche, Mann-heim, Germany) served as chromogen.
Statistical analysis
Statistical analyses were performed using the statistical function of Excel 2007 (Microsoft) or GraphPad Prism 5 (GraphPad Software). A p-value<0.05 was considered statistically significant, as assessed by t-test.
Results
A673 sarcoma is sensitive to Sorafenib and doxorubicin treatment in vitro
Using MTT assays, A673 sarcoma cells showed sensitivity to Sorafenib with 50% inhibition of cell viability of less than 20 μM (Figure 1A left panel). Doxorubicin inhibited the viability of A673 cells with a concentration between 100 and 500 μM (Figure 1A right panel). BrdU staining revealed that both drugs induced cell cycle arrest and cell death as demonstrated by a significant decrease of the S phase fraction as well as a marked increase of the G0/G1 (Figure 1B). Furthermore, treatment resulted in apoptotic cell death (Figure 1C).
Figure 1.
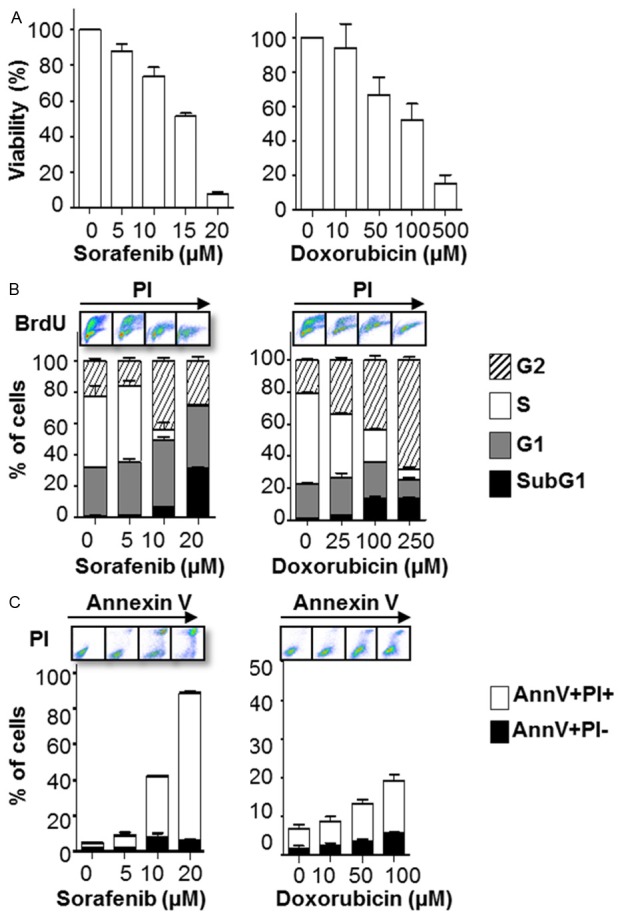
Sorafenib and doxorubicin treatment inhibits the cellular viability and induces apoptosis of A673 cells in vitro. A673 cells were treated with the indicated concentrations of Sorafenib (left panel) or doxorubicin (right panel) for 48 h. A: Cell viability was assessed by MTT assay. B: Cell cycle distribution assessed by BrdU flow cytometry. C: Apoptotic cell death upon Sorafenib treatment assessed by flow cytometry for Annexin V (AnnV) positivity. The bars represent the mean ± SD of the mean from n=3 individual experiments.
[18F]FDG and [18F]FLT uptake in vitro is a measure for viability and proliferation upon Sorafenib and doxorubicin treatment
To estimate whether multikinase targeted treatment with Sorafenib was assessable by measuring [18F]FDG and [18F]FLT uptake, we determined the cellular tracer uptake of [18F]FLT and [18F]FDG in Sorafenib treated cells. A673 cells were incubated with [18F]FLT or [18F]FDG for 45 min after a 48 hr Sorafenib treatment period and the activity was measured us-ing a gamma-counter. As shown in Figure 2A, Sorafenib treatment led to a significant reduction of both [18F]FLT and [18F]FDG uptake in A673 cells. However, sensitivity of [18F]FLT was significantly higher showing a strong reduction of [18F]FLT uptake in the presence of 10 μM Sorafenib (23%, SD=0.13). In contrast, [18F]FDG uptake remained at 52% of untreated control at the same dose level. As a control we included the an-tracyclin doxorubicin, an es-tablished cornerstone drug in Ewing sarcoma protocols [23,24]. The response as assessed by [18F]FLT and [18F]FDG uptake in A673 cells was also detected un-der doxorubicin treatment. As depicted in Figure 2B, response monitoring reve-aled superiority of [18F]FLT (10 μM: 93%, SD=6.6; 50 μM: 84.9%, SD=10.7) over [18F]FDG (10 μM: 111.6%, SD=3; 50 μM: 102.8%, SD=9.4) as early as 24 hr after Doxorubicin treatment. These data suggest that [18F]FLT uptake is more sensitive to determining very early treatment effectiveness in vitro.
Figure 2.
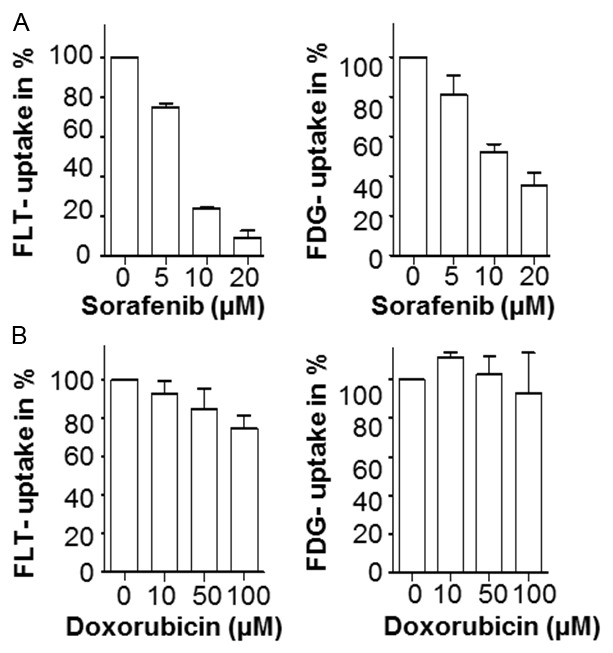
[18F]FLT uptake is a sensitive measure for Sorafenib treatment of A673 cells in vitro. A673 cells were treated with the indicated concentrations of Sorafenib for 48 h or with doxorubicin for 24 h. A: Cellular [18F]FLT (left panel) and [18F]FDG (right panel) -uptake measurements (gamma-counter in counts per minute, cpm) performed as described in the material and methods section 48 hours after treatment with the indicated concentration of Sorafenib. B: Cellular [18F]FLT (left panel) and [18F]FDG (right panel) -uptake measurements 24 hours after treatment with the indicated concentration of doxorubicin. Shown are the mean values ± SD of n=3 individual experiments.
Functional in vivo imaging reveals superiority of [18F]FLT over [18F]FDG for very early response assessment
To evaluate [18F]FDG- and [18F]FLT-PET imaging for response monitoring in vivo, we generated xenograft A673 tumors. To mimic the clinical situation treatment was initiated once the tumor volume reached approximately 100 mm³. The control group showed a 4-fold (n=7, SD=2-fold, range 2-9-fold) increase in tumor volume at day 7 (Figure 3A). In contrast, tumors of Sorafenib treated mice remained basically stable during the treatment period (range 0.2 cm3-0.3 cm3, SD=0.2 cm3) at d + 7. To determine the predictive value of very early functional imaging for response assessment we performed [18F]FDG- and [18F]FLT-PET scans using a micro PET/CT system before and six days after initiation of Sorafenib treatment. To quantify tracer uptake, the change of %ID/g on day 6 compared to pre-treatment %ID/g was calculated (relative %ID/g). The relative mean %ID/g of [18F]FLT uptake decreased significantly to 54% compared to baseline (n=15, mean=54%, SD=29%, range 24%-125%, p=0.003) 5 days after initiation of therapy. The mean %ID/g of pre- and post-treatment were 12.9 (range 4.5-21.5, SD=5.8) and 5.7 (range 3.9-8.5, SD=1.4), respectively (Figure 3B). The relative [18F]FLT %ID/g of untreated controls increased to 234% during the same time period (n=5, SD=70%, range 151%-294%). [18F]FDG-PET analysis of control animals revealed a significant increase in [18F]FDG uptake on day 6. In Sorafenib treated animals, relative %ID/g of [18F]FDG-PET showed a significant reduction compared to the pre-treatment [18F]FDG-PET (n=15, mean 74%, SD=32%, range 35%-121%, p=0.03). The mean %ID/g of pre- and post-treatment are 12.8 (range 7-20.4, SD=4.5) and 8.4 (range 5-12.9, SD=2.6), respectively (Figure 3C). To correlate the PET findings with the treatment effects of Sorafenib on A673 xenograft sarcoma in vivo, we stained fixed tumor sections for the S phase marker Ki67 and cleaved Caspase-3 to analyze proliferation and apoptosis. Sorafenib treatment led to a significant increase in apoptosis as quantified by cleaved Caspase-3 positive cells (Figure 3D, n=6, mean=12%, SD=5%) compared to untreated mice (n=6, mean=6%, SD=2%, p=0.04). Moreover, the percentage of proliferating cells was substantially decreased by Sorafenib as measured by Ki67 staining with a mean of 42% (n=9, range 12%-61%) for the control and 22% (7%-47%) for the treated tumors (p=0.029). These immunohistochemical analyses confirm that the observed changes in PET tracer uptake are associated with Sorafenib effects on sarcoma growth and survival. To assess very early therapy response mice bearing A673 xenograft tumors underwent PET imaging before a significant change of tumor volume at d + 1 after start of treatment. Again, treatment was started when mice had measurable xenograft tumors. As depicted in Figure 4A, tumor growth did not differ significantly between treated and control animals (p=0.2). [18F]FLT-PET was able to significantly discriminate between post-treatment (%ID/g=7.6, SD=0.8) and pre-treatment uptake (%ID/g=14.2, SD=4.8) with a reduction to 57% (n=4, SD=14%, range 44%-71%, p=0.04) (Figure 4B, right panel). In contrast, using [18F]FDG-PET it was not possible to detect a significant change in tracer uptake (n=3, p=0.99). The mean %ID/g of pre- and post-treatment are 14.0 (range 10.1-16.8, SD=3.5) and 14.0 (range 12.6-16.4, SD=2.1), respectively (Figure 4C, right panel). In summary, both [18F]FDG and [18F]FLT are useful to detect treatment res-ponse, but only [18F]FLT-PET predicts response immediately after treatment initiation.
Figure 3.
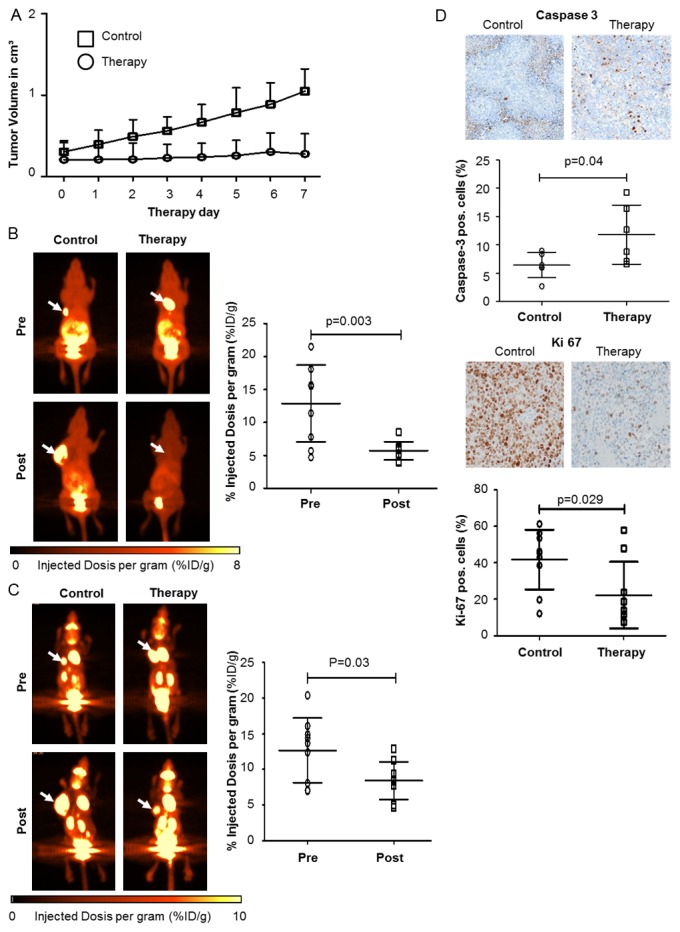
Functional in vivo monitoring of response to Sorafenib inhibition in A673 Sarcoma by [18F]FLT-PET and [18F]FDG-PET. Mice were treated with Sorafenib (n=15, 30 mg/kg i.p. daily) or DMSO/PBS solution (n=5, i.p. daily) once tumors had reached a volume of approximately 100 mm3 (day 0). (A) Tumor growth measurements for therapy- and control group; [18F]FLT- (B) or [18F]FDG-PET (C) scans were performed before (pre) and 5 or 6 days after initiation of therapy (post). Left panels, representative PET scans showing change of tumor tracer uptake (arrows). Right panels, %ID/g, before (pre) and after treatment (post), was calculated and served as an indicator of tracer uptake. Mean %ID/g of [18F]FLT-PET (p=0.003) and [18F]FDG-PET (p=0.03) are both significantly reduced after one week treatment with Sorafenib. (D) Immunohistochemistry of explanted tumors using the apoptosis marker cleaved caspase-3 and the proliferation marker Ki67. (A-D) Mean values ± SD are shown.
Figure 4.
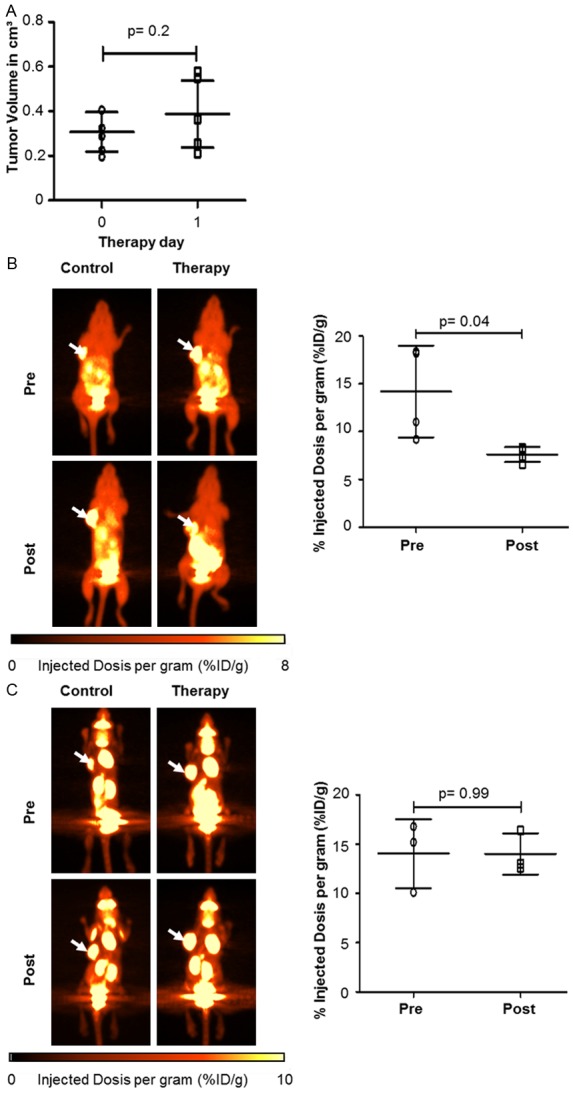
Functional in vivo imaging of A673 inhibition with [18F]FLT-PET is superior to [18F]FDG-PET. Mice were treated with Sorafenib (n=7, 30 mg/kg i.p. daily) or PBS solution (n=7, i.p. daily) when xenotransplants reached a volume of approximately 100 mm3 (day 0). (A) Tumor volume did not change after 24 h Sorafenib treatment in therapeutic group (p=0.2). [18F]FLT- (B) and [18F]FDG-PET (C) was performed before (day 0) and 24 h after initiation of therapy. Left panels: representative PET scans showing change of tumor tracer uptake (arrows). Right panels: tracer uptake, before and after treatment, was calculated. Mean %ID/g of [18F]FLT-PET (p=0.04) was significantly reduced in contrast to %ID/g of [18F]FDG-PET (p=0.99).
Discussion
In this preclinical study, we explored whether PET imaging of tumor proliferative activity using [18F]FDG and [18F]FLT can provide treatment response assessment in sarcoma cell lines and mice bearing sarcoma A673 xenotransplants. Both [18F]FDG as well as [18F]FLT uptake correlates with response to Sorafenib therapy in vivo. However, the reduction of %ID/g in [18F]FLT-PET was significantly more pronounced compared to that in [18F]FDG-PET. In particular, there was no significant difference in tracer uptake imaged by [18F]FDG at day + 1 after treatment initiation, while [18F]FLT-PET imaging showed a significant change in tracer uptake. Thus, our study provides strong evidence that [18F]FLT-PET is highly suitable to predict very early response to Sorafenib therapy in vivo. Considering the overall mo-derate efficacy that has been shown in sarcoma tre-ated with small molecule kinase inhibitors, the immediate identification of non-responders or the benefiting patients would be of immense clinical importance [25,26].
Non-invasive molecular or functional imaging using PET allows for biological processes to be visualized and quantified non-invasively over time. [18F]FDG is currently the most widely used radiotracer for imaging in oncology and proved useful for detecting and staging cancer [12,27,28]. Several studies have analyzed changes in [18F]FDG uptake following anti-cancer treatment, but with variable results [29,30]. [18F]FLT is used as a PET tracer for visualization of cell proliferation [17] and a correlation between [18F]FLT uptake and tumor cell proliferation in mouse xenografts and humans was previously shown [13,15,31-34]. The results from these studies vary; the earliest change in [18F]FLT uptake is found in the range of 24 hours to one week after initiation of treatment. Seemingly, the time frame for assessing changes in proliferation is very variable according to different treatment regimens [35].
The present study provides further evidence that [18F]FLT-PET represents a superior readout also for monitoring response in sarcoma of soft tissue or bone. Similarly, McKinley and coworkers showed in preclinical models of colorectal cancer cell lines that [18F]FLT-PET may be superior to [18F]FDG-PET for quantifying clinical response to targeted therapy [36]. [18F]FLT PET also predicts response to V600EBRAF-targeted therapy in preclinical models of colorectal cancer. Compared to [18F]FDG-PET, [18F]FLT-PET provides higher specificity and may be a more suitable option to evaluate response in clinical settings, in particular when used for response assessment very early in the course of therapy. Another approach is to directly radiolabel the therapeutic compound. Recently radiolabelling of Sorafenib has been desc-ribed, facilitating the estimation of the bioavailability of the drug and direct assessment of response to treatment [37]. Due to the limited availability of cyclotrons capable of providing 11C and the need for more sophisticated imaging protocols, PET using [11C]Sorafenib as the tracer will hardly be included in the therapeutic management of cancer patients in the near future.
Very early non-invasive and reliable response assessment is highly desirable for the management of cancer patients. This issue has become even more demanding with a plethora of novel small molecule therapeutics entering preclinical and clinical testing [25,26]. Currently, interim staging is mainly performed by CT, MRI or ultrasonography usually 6 to 8 weeks after initiation of therapy. Small molecule anti-cancer agents may be of immense clinical benefit by stabilizing the disease, and conventional assessment by morphology-based criteria could be misleading to assess effectiveness and clinical benefit [30,38]. It is therefore of foremost importance to further develop novel methods for assessing clinical response besides tumor volume shrinkage.
In vitro experiments with A673 sarcoma cells and Sorafenib or doxorubicin confirmed that sensitivity of [18F]FLT-PET for detecting early changes of tumor metabolism is significantly higher, as compared to [18F]FDG. These results are in line with a previous trial, where we showed that the classical cytotoxic drug doxorubicin led to dose-dependent reduction in [18F]FLT-uptake in lymphoma cells, in contrast to a variable and slower reduction in [18F]FDG-uptake [20,39-41]. These in vitro experiments thus substantiate our in vivo results of [18F]FLT as the superior early response marker.
The reasons for the observed superiority of [18F]FLT- over [18F]FDG-PET regarding very early assessment of targeted drug therapies remain a matter of debate. It has been suggested that imaging proliferation is a more tumor specific approach being less hampered by inflammatory changes caused by the therapeutic intervention, resulting in non-specific retention of the radiotracer [18F]FDG [17]. Our data suggest a close correlation of the tumoral proliferation fraction and retention of the thymidine analog [18F]FLT also in the early course of cytostatic or cytotoxic therapy. However, the underlying mechanisms may be even more complex, including also DNA repair mechanisms. The influence of DNA repair and other mechanisms related to thymidine metabolism need to be studied in detail in future preclinical studies.
Some limitations have to be considered when transfering our results to the clinic. The number of animals in the experimental arms (very early response after 24 hr and after d + 5/d + 6) significantly differ (n=7 and n=15, respectively). This limitation is related to the fact that the time interval between inoculation of tumor cells and development of local tumors is variable and radiotracers (except [18F]FDG) are not available on a daily basis. This led to the loss of a significant number of animals (n=8) scheduled for the very early assessment of response to treatment. Our results include exclusively in vitro and in vivo data and the findings observed in established sarcoma cell lines and xenotransplant models may not be transferred to the human disease. Despite the preliminary nature of our experiments, further analysis of [18F]FLT-PET also in sarcoma patients seems justified.
In conclusion our data indicate a strong concordance between very early response parameters assessable by [18F]FLT uptake in vivo and in vitro, as confirmed by histological and functional assays. Further studies of [18F]FLT as a very early response marker in clinical trials including patients with sarcoma undergoing targeted cancer drug therapy are therefore highly warranted.
Acknowledgements
We appreciate the excellent support of all members of the technical staff, in particular Sybille Reeder, Elisabeth Aiwanger and Brigitte Dze-was. This work was supported by the Wilhelm Sander-Stiftung (A. Buck), the Deutsche Fors-chungsgemeinschaft (U. Keller, A. Beer, M. Schwaiger), and the German Cancer Consortium (DKTK).
Disclosure of conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Paulussen M, Frohlich B, Jurgens H. Ewing tumour: incidence, prognosis and treatment options. Paediatr Drugs. 2001;3:899–913. doi: 10.2165/00128072-200103120-00003. [DOI] [PubMed] [Google Scholar]
- 2.Thacker MM, Temple HT, Scully SP. Current treatment for Ewing’s sarcoma. Expert Rev Anticancer Ther. 2005;5:319–331. doi: 10.1586/14737140.5.2.319. [DOI] [PubMed] [Google Scholar]
- 3.Ludwig JA. Ewing sarcoma: historical perspectives, current state-of-the-art, and opportunities for targeted therapy in the future. Curr Opin Oncol. 2008;20:412–418. doi: 10.1097/CCO.0b013e328303ba1d. [DOI] [PubMed] [Google Scholar]
- 4.Seddon BM, Whelan JS. Emerging chemotherapeutic strategies and the role of treatment stratification in Ewing sarcoma. Paediatr Drugs. 2008;10:93–105. doi: 10.2165/00148581-200810020-00004. [DOI] [PubMed] [Google Scholar]
- 5.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–11858. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 7.Maki RG, D’Adamo DR, Keohan ML, Saulle M, Schuetze SM, Undevia SD, Livingston MB, Cooney MM, Hensley ML, Mita MM. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J. Clin. Oncol. 2009;27:3133–3140. doi: 10.1200/JCO.2008.20.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aboagye EO, Bhujwalla ZM, Shungu DC, Glickson JD. Detection of tumor response to chemotherapy by 1H nuclear magnetic resonance spectroscopy: effect of 5-fluorouracil on lactate levels in radiation-induced fibrosarcoma 1 tumors. Cancer Res. 1998;58:1063–1067. [PubMed] [Google Scholar]
- 9.Zinreich SJ. Imaging in laryngeal cancer: computed tomography, magnetic resonance imaging, positron emission tomography. Otolaryngol Clin North Am. 2002;35:971. doi: 10.1016/s0030-6665(02)00037-3. [DOI] [PubMed] [Google Scholar]
- 10.Buck AC, Schirrmeister HH, Guhlmann CA, Diederichs CG, Shen C, Buchmann I, Kotzerke J, Birk D, Mattfeldt T, Reske SN. Ki-67 immunostaining in pancreatic cancer and chronic active pancreatitis: does in vivo FDG uptake correlate with proliferative activity? J Nucl Med. 2001;42:721. [PubMed] [Google Scholar]
- 11.Kalff V, Hicks RJ, Ware RE, Hogg A, Binns D, McKenzie AF. The clinical impact of 18F-FDG PET in patients with suspected or confirmed recurrence of colorectal cancer: a prospective study. J Nucl Med. 2002;43:492–499. [PubMed] [Google Scholar]
- 12.Herrmann K, Benz MR, Krause BJ, Pomykala KL, Buck AK, Czernin J. (18)F-FDG-PET/CT in evaluating response to therapy in solid tumors: where we are and where we can go. Q J Nucl Med Mol Imaging. 2011;55:620–32. [PubMed] [Google Scholar]
- 13.Herrmann K, Benz MR, Czernin J, Allen-Auerbach MS, Tap WD, Dry SM, Schuster T, Eckardt JJ, Phelps ME, Weber WA, Eilber FC. 18F-FDG-PET/CT Imaging as an early survival predictor in patients with primary high-grade soft tissue sarcomas undergoing neoadjuvant therapy. Clin Cancer Res. 2012;18:2024–2031. doi: 10.1158/1078-0432.CCR-11-2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strauss LG. Fluorine-18 deoxyglucose and false-positive results: a major problem in the diagnostics of oncological patients. Eur J Nucl Med. 1996;23:1409–1415. doi: 10.1007/BF01367602. [DOI] [PubMed] [Google Scholar]
- 15.Herrmann K, Erkan M, Dobritz M, Schuster T, Siveke JT, Beer AJ, Wester HJ, Schmid RM, Friess H, Schwaiger M, Kleeff J, Buck AK. Comparison of 3’-deoxy-3’-[18F] fluorothymidine positron emission tomography (FLT PET) and FDG PET/CT for the detection and characterization of pancreatic tumours. Eur J Nucl Med Mol Imaging. 2012;39:846–851. doi: 10.1007/s00259-012-2061-8. [DOI] [PubMed] [Google Scholar]
- 16.Halter G, Buck AK, Schirrmeister H, Aksoy E, Liewald F, Glatting G, Neumaier B, Muhling B, Nussle-Kugele K, Hetzel M, Sunder-Plassmann L, Reske SN. Lymph node staging in lung cancer using [18F] FDG-PET. Thorac Cardiovasc Surg. 2004;52:96–101. doi: 10.1055/s-2004-817844. [DOI] [PubMed] [Google Scholar]
- 17.Shields AF, Grierson JR, Dohmen BM, Machulla HJ, Stayanoff JC, Lawhorn-Crews JM, Obradovich JE, Muzik O, Mangner TJ. Imaging proliferation in vivo with [F-18] FLT and positron emission tomography. Nat Med. 1998;4:1334–1336. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- 18.Munch-Petersen B, Cloos L, Jensen H, Tyrsted G. Human thymidine kinase 1. Regulation in normal and malignant cells. Adv Enzyme Regul. 1995;35:69–89. doi: 10.1016/0065-2571(94)00014-t. [DOI] [PubMed] [Google Scholar]
- 19.Sherley J, Kelly TJ. Regulation of human thymidine kinase during the cell cycle. J Biol Chem. 1988;263:8350–8358. [PubMed] [Google Scholar]
- 20.Li Z, Graf N, Herrmann K, Jünger A, Aichler M, Feuchtinger A, Baumgart A, Walch A, Peschel C, Schwaiger M, Buck A, Keller U, Dechow T. FLT-PET Is Superior to FDG-PET for Very Early Response Prediction in NPM-ALK-Positive Lymphoma Treated with Targeted Therapy. Cancer Res. 2012;72:5014–24. doi: 10.1158/0008-5472.CAN-12-0635. [DOI] [PubMed] [Google Scholar]
- 21.Herrmann K, Buck AK, Schuster T, Rudelius M, Wester HJ, Graf N, Scheuerer C, Peschel C, Schwaiger M, Dechow T, Keller U. A Pilot Study to Evaluate 3’-Deoxy-3’-18F-Fluorothymidine PET for Initial and Early Response Imaging in Mantle Cell Lymphoma. J Nucl Med. 2011;52:1898–1902. doi: 10.2967/jnumed.111.094698. [DOI] [PubMed] [Google Scholar]
- 22.Machulla HJ, Blocher A, Kuntzsch M, Piert M, Wei R, Grierson J. Simplified labeling approach for synthesizing 3-deoxy-3-[18F] fluorothymidine ([18F] FLT) J Radioanal Nucl Chem. 2000;243:843–846. [Google Scholar]
- 23.DeGregorio MW, Lui GM, Macher BA, Wilbur JR. Uptake, metabolism, and cytotoxicity of doxorubicin in human Ewing’s sarcoma and rhabdomyosarcoma cells. Cancer Chemother Pharmacol. 1984;12:59–63. doi: 10.1007/BF00255912. [DOI] [PubMed] [Google Scholar]
- 24.Whelan J, Khan A, Sharma A, Rothermundt C, Dileo P, Michelagnoli M, Seddon B, Strausss S. Interval compressed vincristine, doxorubicin, cyclophosphamide alternating with ifosfamide, etoposide in patients with advanced Ewing’s and other Small Round Cell Sarcomas. Clin Sarcoma Res. 2012;2:12. doi: 10.1186/2045-3329-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryan CW, Desai J. The past, present, and future of cytotoxic chemotherapy and pathway-directed targeted agents for soft tissue sarcoma. Am Soc Clin Oncol Educ Book. 2013;2013:386–393. doi: 10.14694/EdBook_AM.2013.33.e386. [DOI] [PubMed] [Google Scholar]
- 26.Somaiah N, von Mehren M. New drugs and combinations for the treatment of soft-tissue sarcoma: a review. Cancer Manag Res. 2012;4:397–411. doi: 10.2147/CMAR.S23257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kruger S, Mottaghy FM, Buck AK, Maschke S, Kley H, Frechen D, Wibmer T, Reske SN, Pauls S. Brain metastasis in lung cancer. Comparison of cerebral MRI and 18F-FDG-PET/CT for diagnosis in the initial staging. Nuklearmedizin. 2011;50:101–106. doi: 10.3413/Nukmed-0338-10-07. [DOI] [PubMed] [Google Scholar]
- 28.Sessa C, Shapiro GI, Bhalla KN, Britten C, Jacks KS, Mita M, Papadimitrakopoulou V, Pluard T, Samuel TA, Akimov M, Quadt C, Fernandez-Ibarra C, Lu H, Bailey S, Chica S, Banerji U. First-in-Human Phase I Dose-Escalation Study of the HSP90 Inhibitor AUY922 in Patients with Advanced Solid Tumors. Clin Cancer Res. 2013;19:3671–80. doi: 10.1158/1078-0432.CCR-12-3404. [DOI] [PubMed] [Google Scholar]
- 29.Weber WA, Wieder H. Monitoring chemotherapy and radiotherapy of solid tumors. Eur J Nucl Med Mol Imaging. 2006;33(Suppl 1):27–37. doi: 10.1007/s00259-006-0133-3. [DOI] [PubMed] [Google Scholar]
- 30.Benz MR, Czernin J, Allen-Auerbach MS, Tap WD, Dry SM, Elashoff D, Chow K, Evilevitch V, Eckardt JJ, Phelps ME, Weber WA, Eilber FC. FDG-PET/CT imaging predicts histopathologic treatment responses after the initial cycle of neoadjuvant chemotherapy in high-grade soft-tissue sarcomas. Clin Cancer Res. 2009;15:2856–2863. doi: 10.1158/1078-0432.CCR-08-2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waldherr C, Mellinghoff IK, Tran C, Halpern BS, Rozengurt N, Safaei A, Weber WA, Stout D, Satyamurthy N, Barrio J, Phelps ME, Silverman DH, Sawyers CL, Czernin J. Monitoring antiproliferative responses to kinase inhibitor therapy in mice with 3’-deoxy-3’-18F-fluorothymidine PET. J Nucl Med. 2005;46:114–120. [PubMed] [Google Scholar]
- 32.Leyton J, Latigo JR, Perumal M, Dhaliwal H, He Q, Aboagye EO. Early detection of tumor response to chemotherapy by 3’-deoxy-3’-[18F] fluorothymidine positron emission tomography: the effect of cisplatin on a fibrosarcoma tumor model in vivo. Cancer Res. 2005;65:4202–4210. doi: 10.1158/0008-5472.CAN-04-4008. [DOI] [PubMed] [Google Scholar]
- 33.Buck AK, Halter G, Schirrmeister H, Kotzerke J, Wurziger I, Glatting G, Mattfeldt T, Neumaier B, Reske SN, Hetzel M. Imaging proliferation in lung tumors with PET: 18F-FLT versus 18F-FDG. J Nucl Med. 2003;44:1426–1431. [PubMed] [Google Scholar]
- 34.Vesselle H, Grierson J, Muzi M, Pugsley JM, Schmidt RA, Rabinowitz P, Peterson LM, Vallieres E, Wood DE. In vivo validation of 3’deoxy-3’-[(18)F] fluorothymidine ([(18)F] FLT) as a proliferation imaging tracer in humans: correlation of [(18)F] FLT uptake by positron emission tomography with Ki-67 immunohistochemistry and flow cytometry in human lung tumors. Clin Cancer Res. 2002;8:3315–3323. [PubMed] [Google Scholar]
- 35.Soloviev D, Lewis D, Honess D, Aboagye E. [(18)F] FLT: an imaging biomarker of tumour proliferation for assessment of tumour response to treatment. Eur J Cancer. 2012;48:416–424. doi: 10.1016/j.ejca.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 36.McKinley ET, Smith RA, Zhao P, Fu A, Saleh SA, Uddin MI, Washington MK, Coffey RJ, Manning HC. 3’-Deoxy-3’-18F-fluorothymidine PET predicts response to (V600E)BRAF-targeted therapy in preclinical models of colorectal cancer. J Nucl Med. 2013;54:424–430. doi: 10.2967/jnumed.112.108456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asakawa C, Ogawa M, Kumata K, Fujinaga M, Kato K, Yamasaki T, Yui J, Kawamura K, Hatori A, Fukumura T, Zhang MR. [11C] sorafenib: radiosynthesis and preliminary PET study of brain uptake in P-gp/Bcrp knockout mice. Bioorg Med Chem Lett. 2011;21:2220–2223. doi: 10.1016/j.bmcl.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Evilevitch V, Weber WA, Tap WD, Allen-Auerbach M, Chow K, Nelson SD, Eilber FR, Eckardt JJ, Elashoff RM, Phelps ME, Czernin J, Eilber FC. Reduction of glucose metabolic activity is more accurate than change in size at predicting histopathologic response to neoadjuvant therapy in high-grade soft-tissue sarcomas. Clin Cancer Res. 2008;14:715–720. doi: 10.1158/1078-0432.CCR-07-1762. [DOI] [PubMed] [Google Scholar]
- 39.Herrmann K, Buck AK, Schuster T, Junger A, Wieder HA, Graf N, Ringshausen I, Rudelius M, Wester HJ, Schwaiger M. Predictive value of initial 18F-FLT uptake in patients with aggressive non-Hodgkin lymphoma receiving R-CHOP treatment. J Nucl Med. 2011;52:690–696. doi: 10.2967/jnumed.110.084566. [DOI] [PubMed] [Google Scholar]
- 40.Herrmann K, Wieder HA, Buck AK, Schöffel M, Krause BJ, Fend F, Schuster T, Meyer zum Büschenfelde C, Wester HJ, Duyster J, Schwaiger M, Dechow T. Early response assessment using 3′-deoxy-3′-[18F] fluorothymidine-positron emission tomography in high-grade non-Hodgkin’s lymphoma. Clin Cancer Res. 2007;13:3552–3558. doi: 10.1158/1078-0432.CCR-06-3025. [DOI] [PubMed] [Google Scholar]
- 41.Graf N, Herrmann K, Numberger B, Zwisler D, Aichler M, Feuchtinger A, Schuster T, Wester HJ, Senekowitsch-Schmidtke R, Peschel C, Schwaiger M, Keller U, Dechow T, Buck AK. [18F] FLT is superior to [18F] FDG for predicting early response to antiproliferative treatment in high-grade lymphoma in a dose-dependent manner. Eur J Nucl Med Mol Imaging. 2013;40:34–43. doi: 10.1007/s00259-012-2255-0. [DOI] [PubMed] [Google Scholar]


