Abstract
Bone metastases (BM) of gastroenteropancreatic neuroendocrine tumours (GEP-NET) can be effectively controlled by peptide receptor radionuclide therapy (PRRT). Eventually, however, BM may become refractory and determine survival. We aimed to assess the clinical benefit of bone-targeted radionuclide therapy (BTRT) in this subgroup of patients failing PRRT. A small cohort of n=6 patients with progressive BM failing PRRT with 177Lu-octreotate (mean cumulative activity, 46.7 GBq) were treated with a total of 11 cycles BTRT using 2.6-3.3 GBq 188Re-HEDP per cycle and a median cumulative activity of 5.9 GBq. Pain palliation was quantified applying the visual analogue scale (VAS). The mean VAS decreased from 6.6 (range 5-8) to 3.7 (range 2-7). Five patients experienced partial resolution of bone pain (≥ 2 steps reduction on the VAS for at least 2 weeks) and one patient had no significant improvement. Flare phenomena occurred in 2 patients and lasted for 2-3 days. Tumor response consisted of stable disease in 2 and progressive disease in 4 patients. No regression of bone metastases has been observed. The median overall survival was 5 months (range 2-9). Relevant myelosuppression (grade 3-4; self-limited with no interventions or hospitalization), occurred 4-6 weeks post-treatment, and after 2 (18.1%) administrations or in 1 (16.7%) patient. No other relevant toxicities or treatment-related death was observed. 188Re-HEDP may be safely applied in patients with bone metastatic GEP-NET previously treated with 177Lu-octreotate. While acceptable pain relief may be expected, no tumor-regression or long-term disease stabilization with apparent survival benefit has been observed. This disputes the use of BTRT as salvage anti-tumor therapy in PRRT-refractory neuroendocrine bone metastases.
Keywords: Bone metastases, neuroendocrine tumors, peptide receptor radionuclide therapy, targeted radionuclide therapy, 188Re-HEDP
Introduction
Bone metastases (BM) are present in 8-15% of metastatic gastroenteropancreatic neuroendocrine tumours (GEP-NET), are frequently multiple and may be associated with a poorer prognosis [1-6]. They usually cause pain with a significant impact on quality of life [7]. PRRT is known to be a very effective systemic treatment for metastatic GEP-NET [8]. Bone metastases can be effectively controlled by PRRT with a 50% overall remission tendency after treatment with 177Lu-octreotate [9,10]. Nevertheless, a substantial number of patients with bone metastatic disease will experience disease progression after a period of remission or disease stabilization and standard pain palliation therapies are often inadequate [11-13].
It is well known, that bone-targeted radionuclide therapy (BTRT) with agents such as 89Sr, or radiolabelled bisphosphonates with 186Re, 153Sm, or 188Re may be effective in bone metastatic disease, predominantly in prostate and breast cancer patients [14-22]. However, there is no report - to the best of our knowledge - whether BTRT may be applied in refractory bone metastases in NET, especially in the salvage setting after PRRT. This study aims to assess the safety and efficacy of BTRT with 188Re-HEDP in a small cohort of GEP NET patients with bone metastases refractory to PRRT with 177Lu-octreotate.
Material and methods
Patients
Six patients (5 men, 1 women; age range, 43-70 y) with well-differentiated GEP-NET (2 pancreatic NET, 4 non pancreatic NET) and advanced bone metastases who underwent BTRT with 188Re-HEDP after failing previous PRRT with 177Lu-octreotate were retrospectively investigated. Before treatment with 188Re-HEDP, all patients had osseous tumor progression and uncontrolled bone pain despite other palliative treatments. Other prerequisites for the treatment with 188Re-HEDP were sufficient tumor uptake on conventional bone scintigraphy, preserved kidney function (i.e. a glomerular filtration rate of > 30 ml/min/1.73 m2) and bone marrow reserve (WBC count ≥ 2000/mm3, haemoglobin ≥ 8 g/dl, platelets ≥ 75000/mm3). Patients provided written informed consent for the scientific analysis of their data and the local ethics committee approved the study. Quantifying the bone pain, the mean Visual Analogue Scale (VAS) at baseline was 6.6 (range 5-8). Apart from the bone metastases, metastatic sites included the liver in 6, the lymph nodes in 4, and other organs in 4 patients (Table 1). The mean cumulative activity of 177Lu-octreotate was 48.7 GBq (range, 29.6-96.7 GBq). PRRT was well tolerated in all of these patients with no significant toxicity. Other previous treatments were comprised of surgery (n=2), biotherapy (n=4), chemotherapy (n=5), locoregional treatment (n=3), and radiation (n=1). The mean interval between the previous systemic treatment and initiation of 188Re-HEDP therapy was 21 months (range 13-47).
Table 1.
Patients characteristics, administered therapeutic doses, response and survival
| Patient (no) | Age/Sex | Primary site | Metastatic sites | Tx prior to BTRT | Cumulative activity (GBq) | VAS at baseline | Best response | OS (mo) | ||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| 177Lu-octreotate | 188ReHEDP | symptomatc* | morphologic | |||||||
| 1 | 70/m | GI | bone, liver, LN, lung | PRRT, CTx | 32.4 | 2.8 | 5 | partial | PD | 2 |
| 2 | 43/m | P | bone, liver, LN | PRRT, CTx, RE, SSA, Surgery | 51.8 | 3.2 | 6 | partial | PD | 5 |
| 3 | 69/m | GI | bone, liver, LN, peritoenal | PRRT, CTx, SSA, Radiation | 40.4 | 6.0 | 7 | no change | PD | 5 |
| 4 | 66/m | P | bone, liver, LN | PRRT, CTx, SSA | 96.7 | 6.4 | 7 | partial | SD | 5 |
| 5 | 56/m | GI | bone, liver, lung | PRRT, Surgery, RE | 41.2 | 5.8 | 8 | partial | PD | 4 |
| 6 | 49/w | GI | bone, liver, mesenterial | PRRT, CTx, RE, SSA | 29.6 | 7.8 | 7 | partial | SD | 9 |
GI, gastrointestinal (non-pancreatic); P, pancreatic; LN, lymph nodes; Tx, therapies; BTRT, bone-targeted radionuclide therapy; PRRT, peptide receptor radionuclide therapy; CTx, chemotherapy; SSA, biotherapy with somatostatin analogues; SD, stable disease; PD, progressive disease; OS, overall survival;
observed symptomatic response consisted of partial resolution (≥ 2 steps reduction on the VAS for at least 2 weeks) and no significant change.
BTRT with 188Re-HEDP
The main intention of BTRT is mostly bone pain palliation; however, in our patients with progressive refractory bone metastatic disease, tumor control and a survival benefit was also intended. 188Re was preferred over other radioisotopes used in BTRT (e.g. 153Sm, 89Sr, 186Re, 177Lu) due to the higher energy and thus longer penetration range of the emitted beta particles and based on the promising results of previous studies reporting survival improvement in patients who received repeated 188Re-HEDP injections [15,23]. 188Re was obtained from an alumina-based 188W/188Re generator. 188Re-HEDP was prepared according to the previously described method [14,24,25]. Treatments were performed with a mean of 2.6-3.3 GBq (70-90 mCi) 188Re-HEDP per cycle. Repeat cycles were performed based on the clinical response and patient’s request. The intervals between successive administrations of 188Re-HEDP were approximately 8 weeks. Table 1 shows the patients characteristics and administered thrapeutic doses.
Assessment of outcome and toxicity
To evaluate the response of bone metastases, patients underwent a diagnostic whole-body 99mTc-MDP bone scan 4 weeks after the treatment. Somatostatin receptor imaging (111In-DTPA-octreotide or 68Ga-DOTA-TOC) was also added for a more accurate evaluation (Figure 1). Response of BMs was determined in this study according to functional M.D. Anderson criteria [26] and modified for the purpose of assessment in NET. Symptomatic response was assessed according to the change in the osseous pain intensity quantified by the VAS. It was categorized into complete resolution, partial resolution (≥ 2 steps reduction on the VAS for at least 2 weeks), no significant change, and progression.
Figure 1.
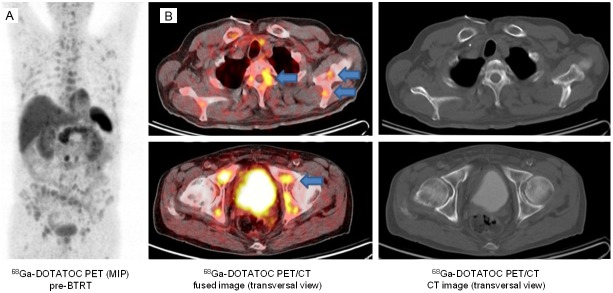
Images of a patient with disseminated PRRT-refractory bone metastases. The absence of corresponding morphologic correlates for several osseous findings of 68Ga-DOTATOC PET (arrows) supports the implementation of functional somatostatin receptor imaging for a more accurate evaluation of bone metastases of NET. A: Maximum-intensity-projection 68Ga-DOTATOC PET images (coronal view); B: Fused PET-CT (left) and corresponding CT images (right) of the same examination.
Hematological parameters were determined prior to each treatment course, in 2-4 weeks intervals between the courses, and 8-12 weeks after the last course of the treatment. Glomerular filtration rate was measured using a standardized 99mTc-DTPA blood clearance examination prior to each treatment course and in 3 monthly intervals after the last administration. Toxicity was recorded using the Common Terminology Criteria for Adverse Events v3.0 (CTCAE).
Results
11 courses with 188Re-HEDP were performed in 6 patients. Two patients received 1, three patients 2 cycles and one patient 3 cycles. The median activity was 5.9 GBq (range 2.8-7.8) and the median overall survival was 5 months (range 2-9). Figure 2 shows the Kaplan-Meier curve for overall survival in the study cohort.
Figure 2.
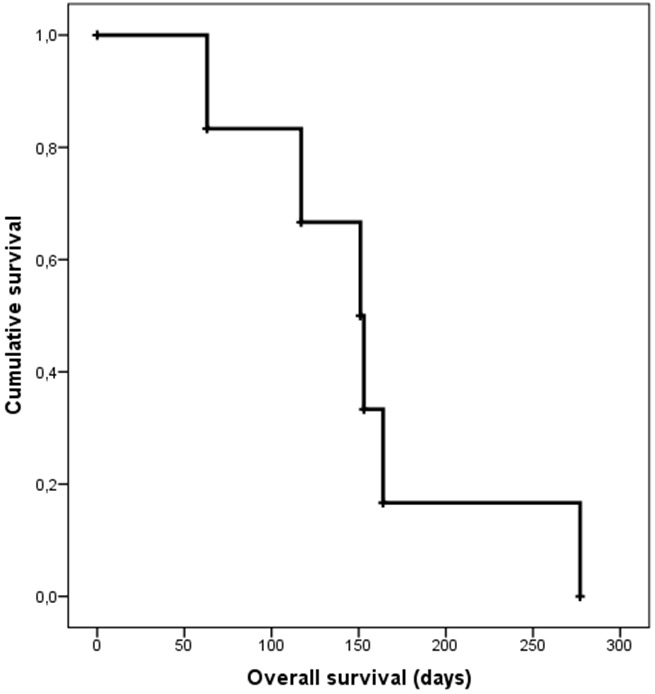
Overall survival of the patient cohort (n=6) depicted by a Kaplan-Meier curve with a median OS of 5 months (range 2-9).
Symptomatic and morphologic response
Rebound pain (flare phenomena) occurred in 2 patients and lasted for 2-3 days. Clinically evident pain relief occurred within 1 week in 5 patients (Table 1). No patient experienced complete pain relief and 5 patients partial resolution of metastatic bone pain. One patient had no significant symptomatic improvement (Figure 3). The mean VAS decreased from 6.6 (range 5-8) to 3.7 (range 2-7). Figure 4 illustrates the change of pain intensity following BTRT in each patient. This pain alleviation effect resulted in significant reduction of the required analgesic doses in 3 patients (≥ 50% reduction in patients) lasting for a mean of 3 months (range, 1-4 mo) from completion of 188Re-HEDP therapy. 2 patients showed ≥ 2 steps decrease of pain levels in the VAS but no decrease in analgesic consumption. Morphologic response consisted of stable disease in 2 patients and progressive disease in 4 patients. Figures 5 and 6 show examples of patients with progressive and stable disease after BTRT. No morphologic regression of bone metastases has been observed (Table 1). Median overall survival of the entire cohort was 5 months (range, 2-9 mo, Table 1).
Figure 3.
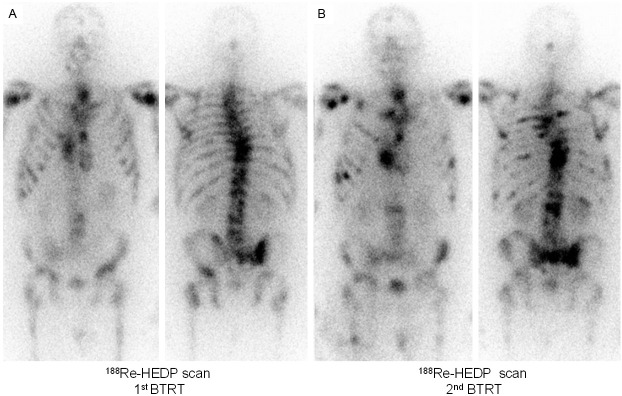
Intra-therapeutic 188Re-HEDP images of a patient with bone metastases of a rectal NET after the first (A) and second (B) BTRT cycle. The patient showed no morphologic or symptomatic response and died 5 months later.
Figure 4.
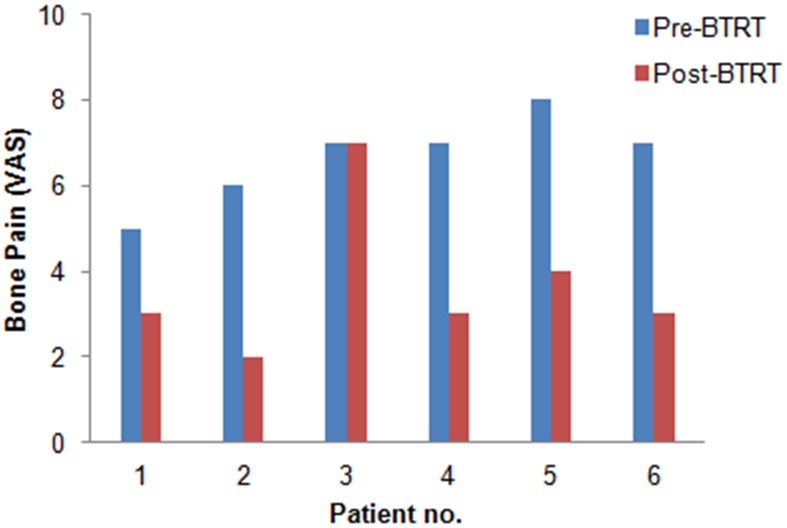
Intensity of bone pain before and after BTRT in each patient (n=6).
Figure 5.
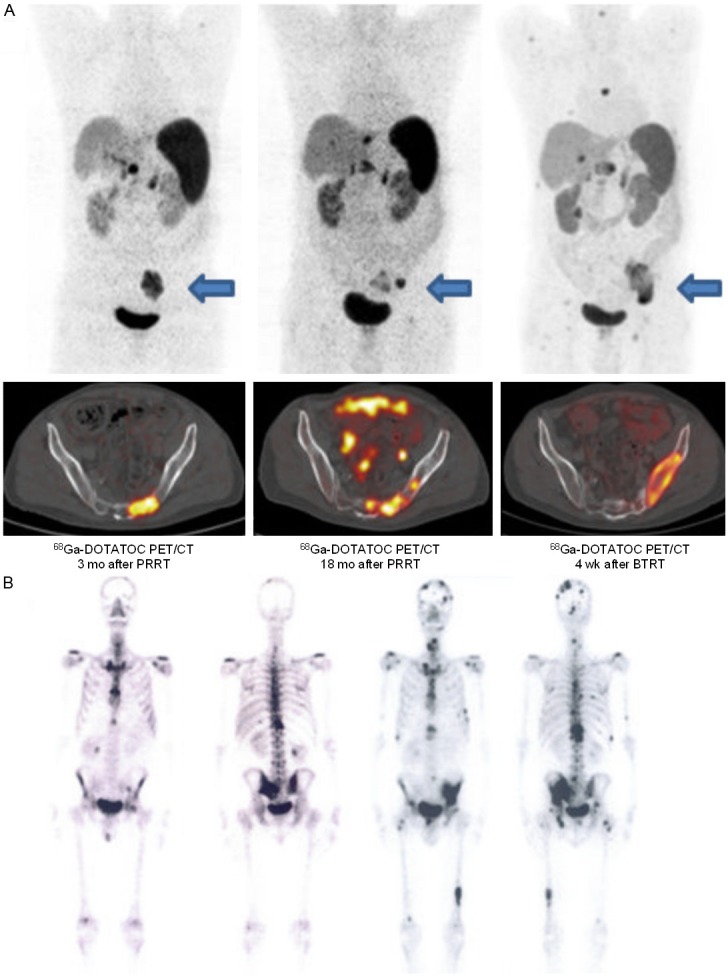
A: 68Ga-DOTATOC PET/CT images of a patient with initial response after PRRT (left) undergoing BTRT because of progressive and painful bone metastases (middle). The patient showed no morphologic response after 2 BTRT cycles (right) and died 5 months later. Above: Maximum-intensity-projection PET images (coronal view), below: fused PET-CT images (selected lesion indicated by arrow). B: 99mTc-MDP whole body bone scan images of the same patient before (left) and after (right) BTRT showing new osseous lesions (progressive disease).
Figure 6.
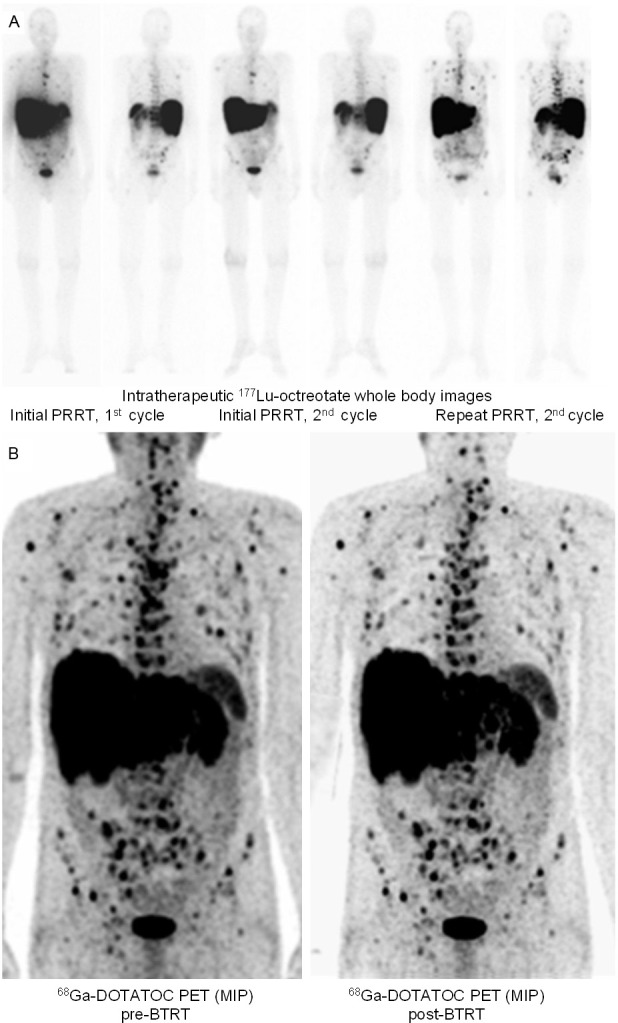
Patient with metastatic P-NET and initial response to PRRT undergoing BTRT after showing progression of recurrent bone metastases under repeat PRRT. A: 177Lu-octreotate therapy scans of the first (left) and last cycle (middle) of the initial PRRT, as well as the final cycle of the repeat PRRT (right). B: Maximum-intensity-projection 68Ga-DOTATOC PET images before (left) and after (right) BTRT, showing a stable disease lasting for 5 months.
Toxicity
Before the treatment, all patients had baseline reductions of at least one blood cell line: Anemia in 6 patients (5 grade I and 1 grade II), thrombocytopenia in 2 patients (1 grade I and 1 grade II), and leukopenia in 1 patient (1 grade I and 0 grade II) according to CTCAE criteria. Relevant hematotoxicity (grade III-IV) occurred 4-6 weeks post-treatment, observed after 2 (18.1%) administrations and in 1 (16.7%) patient. This patient developed isolated thrombocytopenia (grade III) after the first and combined thrombocytopenia and anemia (both grade III) after the second treatment. Overall, there were 10 cases of anaemia, 8 cases of thrombocytopenia, and 5 cases of leukopenia (Table 2). In none of the patients, the observed myelosuppression necessitated any interventions or hospitalization. No other relevant toxicities or treatment-related death was observed.
Table 2.
Post-BTRT toxicities according to CTCAE v.3
| Hematotoxicity | Incidence | |
|---|---|---|
|
| ||
| per patient n (%) | per cycle n (%) | |
| Leukopenia | ||
| Grade 1 | 2 (33.3) | 4 (36.4) |
| Grade 2 | 1 (16.7) | 1 (9.1) |
| Grade 3 | 0 (0) | 0 (0) |
| Grade 4 | 0 (0) | 0 (0) |
| Thrombocytopenia | ||
| Grade 1 | 3 (50.0) | 4 (36.4) |
| Grade 2 | 1 (16.7) | 2 (18.2) |
| Grade 3 | 1 (16.7) | 2 (18.2) |
| Grade 4 | 0 (0) | 0 (0) |
| Anemia | ||
| Grade 1 | 5 (88.3) | 7 (63.6) |
| Grade 2 | 2 (33.3) | 3 (27.3) |
| Grade 3 | 1 (16.7) | 1 (9.1) |
| Grade 4 | 0 (0) | 0 (0) |
Discussion
Our retrospective study indicates that radionuclide therapy with 188Re-HEDP may provide safe pain palliation for patients with bone metastatic GEP-NET who previously received PRRT with 177Lu-octreotate. However, we observed 1) no regression of bone metastases and 2) no obvious survival benefit in our small cohort, which disputes the use of BTRT as a salvage treatment for PRRT-refractory bone metastases in NET patients.
Skeletal metastases may cause pain and decrease the quality of life. Standard pain palliation therapies such as bisphosphonate are of limited benefit in the late stages of the disease, and extended-field radiation is often accompanied by serious side effects [27-29]. Bone-targeted radionuclide therapy with 188Re-HEDP has proved to be an effective therapeutic option in patients with bone metastatic pain from different malignancies with palliative response rate of 70-85% [14,21,22,30-32]. Consistent with previous studies on patients with other tumor origins we achieved a significant pain relief (≥ 2 steps reduction in VAS at least in two consecutive weeks without increase of analgesics intake) in 5 patients, lasting for a mean of 3 months. The incidence of flare syndrome (2 patients) was also in agreement to previous reports.
Myelosuppression may be the dose-limiting factor for 188Re-HEDP therapy [14,15,21]. Also, it is known that PRRT may lead to relevant cumulative bone marrow doses and reduced bone marrow reserve [8,33]. In our small study cohort on patients with previous history of PRRT with 177Lu-octreotate (mean cumulative activity: 46.7), undergoing dose-intensified 188Re-HEDP therapy, significant but reversible hematotoxicity was the only serious adverse effect and observed in 1 patient. This acceptable toxicity profile despite pretreatment with high applied total activities in our cohort disputes a major impact of previous PRRT on the incidence and intensity of bone marrow suppression in patients undergoing 188Re-HEDP therapy.
Repeated 188Re-HEDP therapy may improve survival in patients with prostate cancer and bone metastases [15]. Unfortunately, this form of BTRT with up to 3 cycles seemed to have no relevant impact on survival in our study. Two patients experienced disease stabilization with a short overall survival of 5 and 9 months. This outcome disputes the consideration of 188Re-HEDP as a salvage therapy for controlling the progressive neuroendocrine bone metastases after failure of PRRT (Figure 7).
Figure 7.

Initial response to PRRT of the same patient as in Figure 6: A: 99mTc-MDP whole body bone scan images before PRRT. B: 111In-DTPA-octreotide images before (left) and after PRRT (right), above: planar images, below: fused SPECT-CT images. C: 68Ga-DOTATOC PET/CT images before (left) and after PRRT (right), above: maximum-intensity-projection PET images (coronal view), below: fused PET-CT images.
The main limitations of this study are the very small population size and the retrospective setting, which restricts the conclusions to a preliminary context. The observations made in this small series may thus only indicate a potential goal (bone pain palliation) of BTRT in neuroendocrine bone metastases and at the same time portray a limitation for patient management in case this modality is considered as a salvage anti-proliferative treatment for refractory bone metastatic disease.
Conclusion
This report on a small population indicates that bone-targeted radionuclide therapy with 188Re-HEDP may be safely applied in patients with bone metastatic GEP-NET previously treated with 177Lu-octreotate and may produce acceptable pain relief. However, neither tumor-regression or long-term disease stabilization nor an apparent survival benefit has been observed, disputing the use of this bone-targeted modality as a salvage therapy form in PRRT-refractory neuroendocrine bone metastases.
Disclosure of conflict of interest
None.
References
- 1.Sabet A, Ezziddin S, Heinemann F, Guhlke S, Muckle M, Willinek W, Biersack HJ, Ahmadzadehfar H. Osseous metastases of gastro-enteropancreatic neuroendocrine tumours. Diagnostic value of intra-therapeutic 177Lu-octreotate imaging in comparison with bone scintigraphy. Nuklearmedizin. 2012;51:95–100. doi: 10.3413/Nukmed-0428-11-08. [DOI] [PubMed] [Google Scholar]
- 2.Lebtahi R, Cadiot G, Delahaye N, Genin R, Daou D, Peker MC, Chosidow D, Faraggi M, Mignon M, Le Guludec D. Detection of bone metastases in patients with endocrine gastroenteropancreatic tumors: bone scintigraphy compared with somatostatin receptor scintigraphy. J Nucl Med. 1999;40:1602–1608. [PubMed] [Google Scholar]
- 3.Panzuto F, Nasoni S, Falconi M, Corleto VD, Capurso G, Cassetta S, Di Fonzo M, Tornatore V, Milione M, Angeletti S, Cattaruzza MS, Ziparo V, Bordi C, Pederzoli P, Delle Fave G. Prognostic factors and survival in endocrine tumor patients: comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer. 2005;12:1083–1092. doi: 10.1677/erc.1.01017. [DOI] [PubMed] [Google Scholar]
- 4.Durante C, Boukheris H, Dromain C, Duvillard P, Leboulleux S, Elias D, de Baere T, Malka D, Lumbroso J, Guigay J, Schlumberger M, Ducreux M, Baudin E. Prognostic factors influencing survival from metastatic (stage IV) gastroenteropancreatic well-differentiated endocrine carcinoma. Endocr Relat Cancer. 2009;16:585–597. doi: 10.1677/ERC-08-0301. [DOI] [PubMed] [Google Scholar]
- 5.Meijer WG, van der Veer E, Jager PL, van der Jagt EJ, Piers BA, Kema IP, de Vries EG, Willemse PH. Bone metastases in carcinoid tumors: clinical features, imaging characteristics, and markers of bone metabolism. J Nucl Med. 2003;44:184–191. [PubMed] [Google Scholar]
- 6.Pape UF, Berndt U, Muller-Nordhorn J, Bohmig M, Roll S, Koch M, Willich SN, Wiedenmann B. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer. 2008;15:1083–1097. doi: 10.1677/ERC-08-0017. [DOI] [PubMed] [Google Scholar]
- 7.Kos-Kudla B, O’Toole D, Falconi M, Gross D, Kloppel G, Sundin A, Ramage J, Oberg K, Wiedenmann B, Komminoth P, Van Custem E, Mallath M, Papotti M, Caplin M. ENETS consensus guidelines for the management of bone and lung metastases from neuroendocrine tumors. Neuroendocrinology. 2010;91:341–350. doi: 10.1159/000287255. [DOI] [PubMed] [Google Scholar]
- 8.Kwekkeboom DJ, de Herder WW, van Eijck CH, Kam BL, van Essen M, Teunissen JJ, Krenning EP. Peptide receptor radionuclide therapy in patients with gastroenteropancreatic neuroendocrine tumors. Semin Nucl Med. 2010;40:78–88. doi: 10.1053/j.semnuclmed.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Ezziddin S, Sabet A, Heinemann F, Yong-Hing CJ, Ahmadzadehfar H, Guhlke S, Holler T, Willinek W, Boy C, Biersack HJ. Response and long-term control of bone metastases after peptide receptor radionuclide therapy with (177)Lu-octreotate. J Nucl Med. 2011;52:1197–1203. doi: 10.2967/jnumed.111.090373. [DOI] [PubMed] [Google Scholar]
- 10.Sabet A, Khalaf F, Haslerud T, Al-Zreiqat A, Sabet A, Simon B, Pöppel TD, Biersack HJ, Ezziddin S. Bone metastases in GEP-NET: response and long-term outcome after PRRT from a follow-up analysis. Am J Nucl Med Mol Imaging. 2013;3:437–45. [PMC free article] [PubMed] [Google Scholar]
- 11.Krenning EP, Kwekkeboom DJ, Valkema R, Pauwels S, Kvols LK, De Jong M. Peptide receptor radionuclide therapy. Ann N Y Acad Sci. 2004;1014:234–245. doi: 10.1196/annals.1294.026. [DOI] [PubMed] [Google Scholar]
- 12.Moll S, Nickeleit V, Mueller-Brand J, Brunner FP, Maecke HR, Mihatsch MJ. A new cause of renal thrombotic microangiopathy: yttrium 90-DOTATOC internal radiotherapy. Am J Kidney Dis. 2001;37:847–851. doi: 10.1016/s0272-6386(01)80135-9. [DOI] [PubMed] [Google Scholar]
- 13.Cybulla M, Weiner SM, Otte A. End-stage renal disease after treatment with 90Y-DOTATOC. Eur J Nucl Med. 2001;28:1552–1554. doi: 10.1007/s002590100599. [DOI] [PubMed] [Google Scholar]
- 14.Palmedo H, Guhlke S, Bender H, Sartor J, Schoeneich G, Risse J, Grunwald F, Knapp FF Jr, Biersack HJ. Dose escalation study with rhenium-188 hydroxyethylidene diphosphonate in prostate cancer patients with osseous metastases. Eur J Nucl Med. 2000;27:123–130. doi: 10.1007/s002590050017. [DOI] [PubMed] [Google Scholar]
- 15.Palmedo H, Manka-Waluch A, Albers P, Schmidt-Wolf IG, Reinhardt M, Ezziddin S, Joe A, Roedel R, Fimmers R, Knapp FF Jr, Guhlke S, Biersack HJ. Repeated bone-targeted therapy for hormone-refractory prostate carcinoma: tandomized phase II trial with the new, high-energy radiopharmaceutical rhenium-188 hydroxyethylidenediphosphonate. J. Clin. Oncol. 2003;21:2869–2875. doi: 10.1200/JCO.2003.12.060. [DOI] [PubMed] [Google Scholar]
- 16.Sartor O, Reid RH, Bushnell DL, Quick DP, Ell PJ. Safety and efficacy of repeat administration of samarium Sm-153 lexidronam to patients with metastatic bone pain. Cancer. 2007;109:637–643. doi: 10.1002/cncr.22431. [DOI] [PubMed] [Google Scholar]
- 17.Lee CK, Aeppli DM, Unger J, Boudreau RJ, Levitt SH. Strontium-89 chloride (Metastron) for palliative treatment of bony metastases. The University of Minnesota experience. Am J Clin Oncol. 1996;19:102–107. doi: 10.1097/00000421-199604000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Finlay IG, Mason MD, Shelley M. Radioisotopes for the palliation of metastatic bone cancer: a systematic review. Lancet Oncol. 2005;6:392–400. doi: 10.1016/S1470-2045(05)70206-0. [DOI] [PubMed] [Google Scholar]
- 19.Han SH, de Klerk JM, Tan S, van het Schip AD, Derksen BH, van Dijk A, Kruitwagen CL, Blijham GH, van Rijk PP, Zonnenberg BA. The PLACORHEN study: a double-blind, placebo-controlled, randomized radionuclide study with (186)Re-etidronate in hormone-resistant prostate cancer patients with painful bone metastases. Placebo Controlled Rhenium Study. J Nucl Med. 2002;43:1150–1156. [PubMed] [Google Scholar]
- 20.Paes FM, Serafini AN. Systemic metabolic radiopharmaceutical therapy in the treatment of metastatic bone pain. Semin Nucl Med. 2010;40:89–104. doi: 10.1053/j.semnuclmed.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Biersack HJ, Palmedo H, Andris A, Rogenhofer S, Knapp FF, Guhlke S, Ezziddin S, Bucerius J, von Mallek D. Palliation and survival after repeated (188)Re-HEDP therapy of hormone-refractory bone metastases of prostate cancer: a retrospective analysis. J Nucl Med. 2011;52:1721–1726. doi: 10.2967/jnumed.111.093674. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira S, Dormehl I, Botelho MF. Radiopharmaceuticals for bone metastasis therapy and beyond: a voyage from the past to the present and a look to the future. Cancer Biother Radiopharm. 2012;27:535–551. doi: 10.1089/cbr.2012.1258. [DOI] [PubMed] [Google Scholar]
- 23.Palmedo H, Bucerius J. Radionuclide therapy in oncology: repeated administrations of high dose rate radiopharmaceuticals. Eur J Nucl Med Mol Imaging. 2004;31:1556. doi: 10.1007/s00259-004-1665-z. [DOI] [PubMed] [Google Scholar]
- 24.Guhlke S, Beets AL, Oetjen K, Mirzadeh S, Biersack HJ, Knapp FF Jr. Simple new method for effective concentration of 188Re solutions from alumina-based 188W-188Re generator. J Nucl Med. 2000;41:1271–1278. [PubMed] [Google Scholar]
- 25.Knapp FF Jr. Rhenium-188--a generator-derived radioisotope for cancer therapy. Cancer Biother Radiopharm. 1998;13:337–349. doi: 10.1089/cbr.1998.13.337. [DOI] [PubMed] [Google Scholar]
- 26.Costelloe CM, Chuang HH, Madewell JE, Ueno NT. Cancer Response Criteria and Bone Metastases: RECIST 1.1, MDA and PERCIST. J Cancer. 2010;1:80–92. doi: 10.7150/jca.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Debes JD, Tindall DJ. The role of androgens and the androgen receptor in prostate cancer. Cancer Lett. 2002;187:1–7. doi: 10.1016/s0304-3835(02)00413-5. [DOI] [PubMed] [Google Scholar]
- 28.McEwan AJ. Palliative therapy with bone seeking radiopharmaceuticals. Cancer Biother Radiopharm. 1998;13:413–426. doi: 10.1089/cbr.1998.13.413. [DOI] [PubMed] [Google Scholar]
- 29.Lewington VJ. Targeted radionuclide therapy for bone metastases. Eur J Nucl Med. 1993;20:66–74. doi: 10.1007/BF02261248. [DOI] [PubMed] [Google Scholar]
- 30.Liepe K, Kropp J, Runge R, Kotzerke J. Therapeutic efficiency of rhenium-188-HEDP in human prostate cancer skeletal metastases. Br J Cancer. 2003;89:625–629. doi: 10.1038/sj.bjc.6601158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liepe K, Hliscs R, Kropp J, Gruning T, Runge R, Koch R, Knapp FF Jr, Franke WG. Rhenium-188-HEDP in the palliative treatment of bone metastases. Cancer Biother Radiopharm. 2000;15:261–5. doi: 10.1089/108497800414356. [DOI] [PubMed] [Google Scholar]
- 32.Maxon HR 3rd, Schroder LE, Washburn LC, Thomas SR, Samaratunga RC, Biniakiewicz D, Moulton JS, Cummings D, Ehrhardt GJ, Morris V. Rhenium-188(Sn)HEDP for treatment of osseous metastases. J Nucl Med. 1998;39:659–663. [PubMed] [Google Scholar]
- 33.Forrer F, Krenning EP, Kooij PP, Bernard BF, Konijnenberg M, Bakker WH, Teunissen JJ, de Jong M, van Lom K, de Herder WW, Kwekkeboom DJ. Bone marrow dosimetry in peptide receptor radionuclide therapy with [177Lu-DOTA(0),Tyr(3)] octreotate. Eur J Nucl Med Mol Imaging. 2009;36:1138–1146. doi: 10.1007/s00259-009-1072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


