Abstract
Unfavorable physiological, biological, and behavioral alterations during and following treatment for cancer may lead to chronic energy imbalance predisposing to a myriad of deleterious health conditions including obesity, dyslipidemia, and the metabolic syndrome. In addition to the cardiovascular and musculoskeletal effects of these conditions, energy imbalance and metabolic changes after cancer treatment can also affect cancer-related morbidity and mortality. To this end, lifestyle interventions such as diet and physical activity are especially relevant to mitigate the deleterious impact of chronic energy imbalance in cancer survivors.
INTRODUCTION
Energy balance is the homeostatic state achieved when energy expenditure equals energy intake.1 Energy balance requires the harmonization of caloric intake, physical activity, the thermic effect of food, and basal metabolic rate (reflecting resting energy expenditure and approximated by resting metabolic rate).1 In mammals, energy balance is complex. For example, major tissues and organs that regulate energy balance include, but are not limited to, the gut, the hypothalamus, adipose tissue, skeletal muscle, the liver and the pancreas.1,2 Positive energy balance (when intake exceeds expenditure) will result in energy storage and, under chronic conditions, weight gain, while negative energy balance (when intake is less than expenditure) will result in mobilization of energy stores and, under chronic conditions, weight loss.3
Among patients with a cancer diagnosis, herein referred to as cancer survivors,4,5 the problem of energy imbalance is more complicated, due in part to metabolic aberrations related to anticancer and supportive care therapy as well as to the tumor burden itself.6 Cancer is an umbrella term that covers a wide range of malignancies varying substantially in presentation, treatment, demography and prognosis – hence, the impact of energy balance also varies dramatically across cancer diagnoses.7,8
At one end of the energy balance continuum in the oncology setting is cachexia, a hypercatabolic state characterized by loss of skeletal muscle and marked negative energy balance. Cachexia has been a hallmark of cancer since the time of Hippocrates,9,10 and weight loss, either at diagnosis or during anticancer therapy, remains an important prognostic marker11 among patients with several cancers including breast, lung,12 and pancreas.13,14 Weight gain, at the other end of energy balance, is also becoming increasingly recognized as being associated with poor prognosis among patients diagnosed with breast cancer.15–17 Therefore, in totality, chronic energy imbalance, whether positive or negative, is associated with morbidity and mortality following a cancer diagnosis.18–20
In this paper, we will review the causes, consequences, and biologic mechanisms of positive energy imbalance in cancer survivors, with a focus on development of cardiovascular risk factors, overt cardiovascular disease, as well as cancer-specific outcomes. Examples from several cancer diagnoses will be provided, although evidence from breast cancer survivors is often strongest or most complete. Finally, we will discuss the evidence supporting the efficacy of dietary and exercise interventions to mitigate positive energy imbalance-related consequences among cancer survivors. For the impact of negative energy balance in cancer patients, the reader is referred to several recent excellent reviews.1, 2, 6,21–24
POSITIVE ENERGY BALANCE IN CANCER SURVIVORS
Under chronic conditions, positive energy balance will manifest as overweight and obesity. The general population has seen an explosion in the prevalence of overweight and obesity in the preceding four decades.25,26 In the contemporary setting of treatment for breast cancer, lymphoma, craniopharyngioma, or following stem cell transplant, weight gain and positive energy balance have been described.27–34 As a common cancer and a frequently studied group, discoveries among breast cancer survivors may provide guideposts for investigators in other cancers. Weight gain during breast cancer therapy typically ranges from 2.5 to 11 kilograms.35–38 Premenopausal women and those receiving chemotherapy are at higher risk.35,37–39 In the Health, Eating, Activity, and Lifestyle (HEAL) Study, 514 women with stage 0-IIIA breast cancer were followed from the first to the third year of diagnosis for weight and body fat changes. Weight and body fat (via DXA scan) were measured at baseline and at two-year follow-up. A large majority of women gained weight (68%) and body fat (74%).40
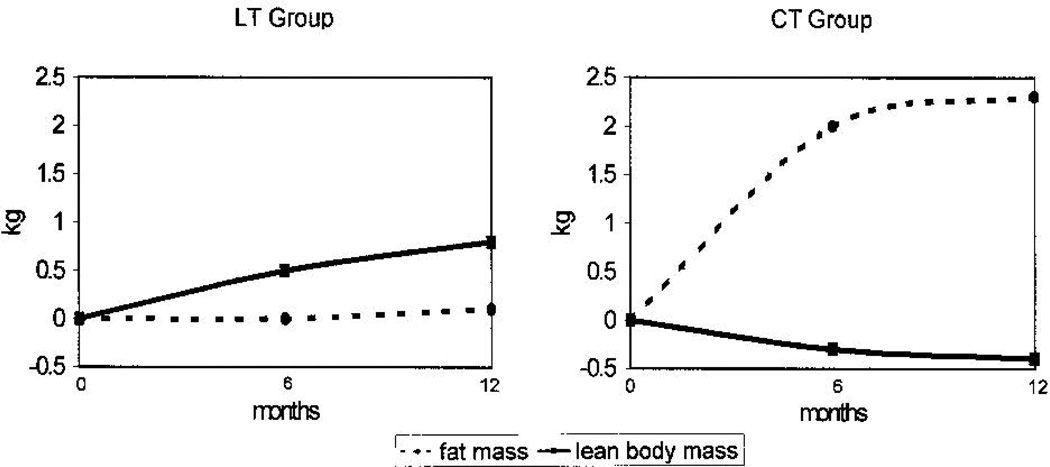
Weight gain in this setting appears to reflect loss of lean muscle mass and gain of adiposity, especially abdominal adiposity.40–42 A seminal study by Demark-Wahnefried and colleagues examined changes in weight and body composition among premenopausal breast cancer patients receiving chemotherapy. Patients were assessed at the time of diagnosis and throughout the first year of treatment with DXA for body composition, resting energy expenditure (via indirect calorimetry), dietary intake, and physical activity. Patients receiving chemotherapy were noted to gain more weight (2.1 kg on average) than those who received localized therapy alone (1.0 kg on average), although they did not have different resting energy expenditure or energy intake. Instead, patients receiving chemotherapy were found to lose lean body mass and substantially gain fat mass (Figure 1), a situation the authors described as sarcopenic obesity.42 Since that study, other work has confirmed these findings.41,43,44
Figure 1.
Change in fat mass and lean body mass from baseline among locally-treated (LT) compared to chemotherapy-treated (CT) premenopausal women with breast cancer.
Copyright 2001, American Society of Clinical Oncology. Reprinted with permission from: Demark-Wahnefried, et al. J Clin Oncol 2001;19:2381–2389.
CAUSES OF POSITIVE ENERGY BALANCE
Fluctuations in dietary intake, physical activity, and resting energy expenditure likely contribute to weight gain and positive energy balance among cancer survivors,7,36,45,46 although the relative contribution of each component requires further investigation. Cancer therapy may impact diet and physical activity directly or via underlying mechanisms, such as endocrine disruption or alterations in the leptin-adiponectin system.
Direct changes in dietary intake
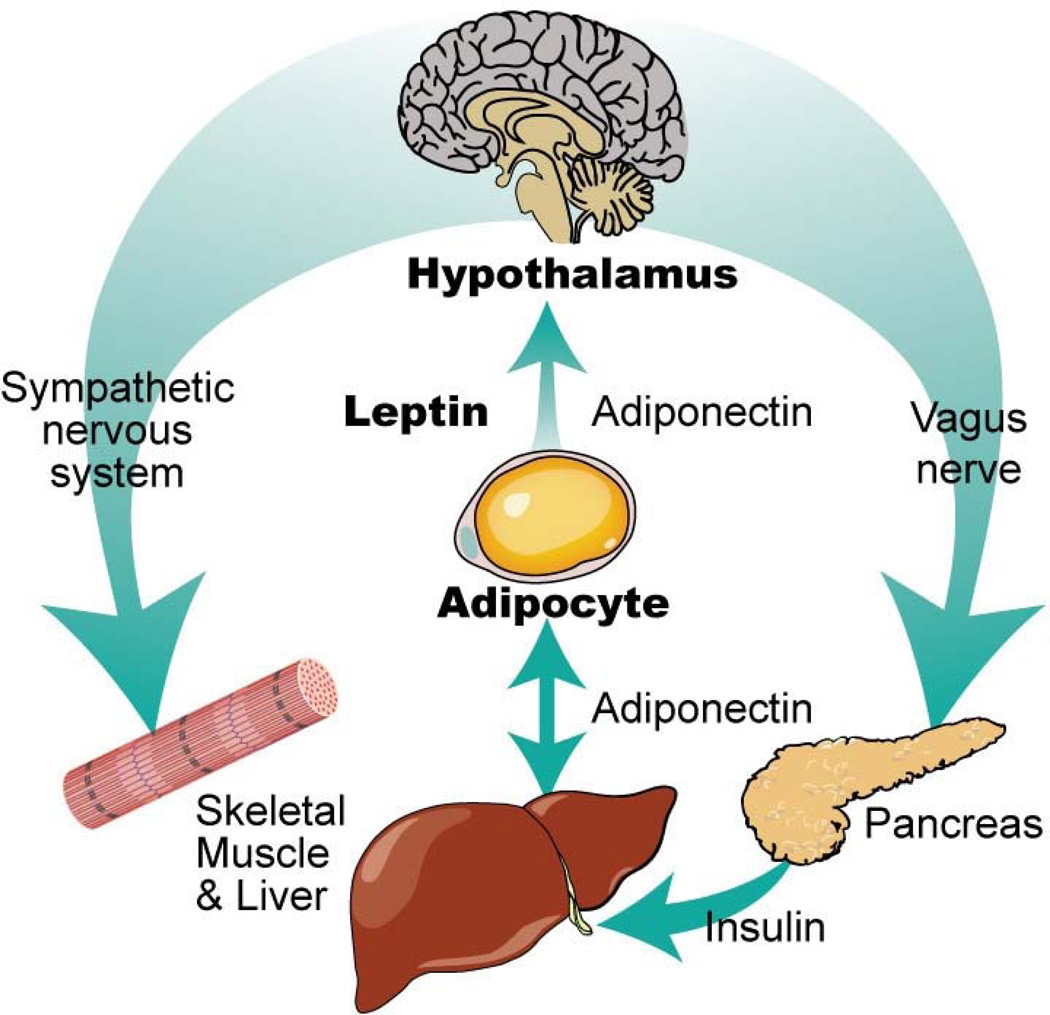
Brain tumor survivors, especially survivors of craniopharyngioma, are at risk for severe overeating. This caloric intake-related energy imbalance is termed hypothalamic obesity. Destruction of the ventromedial hypothalamus (VMH) during cancer treatment is thought to be responsible for hypothalamic obesity. In the healthy brain, the VMH regulates energy balance by interpreting hunger and satiety signals from circulating leptin, ghrelin, pancreatic polypeptide 3–36 and insulin (Figure 2).47,48 Damage to the VMH can result in excessive calorie intake and decreased caloric expenditure, either by inducing hyperphagia that results in obesity and insulin resistance, or via overactive vagal neural transmission resulting in excessive insulin secretion from the β-cells of the pancreas.49 Treatment decisions related to the primary tumor affect the likelihood and severity of subsequent obesity. Surgical excision of the tumor without radiation generally results in severe hypothalamic obesity and a typical adult body mass index (BMI) > 50. Less aggressive surgery in combination with radiation therapy typically results in less damage to the VMH and lower BMI, although survivors may not be spared completely. Unfortunately, hypothalamic obesity can be intractable and challenging to treat. Recent studies of octreotide and the combination of metformin plus diazoxide have been encouraging, although further study is needed.50–52
Figure 2.
Leptin and adiponectin system of communication.
Copyright 2011, Wiley. Reprinted with permission from: Tonorezos ES, et al. Ped Blood Cancer 2012; 58: 31–36.
Interestingly, survivors of breast cancer appear to change their diet, also. Among those patients, taste alteration may impact dietary changes and contribute to weight gain during chemotherapy for breast cancer. An investigation of breast cancer patients receiving docetaxel or paclitaxel described taste alteration resulting in behavioral changes including eating strongly flavored foods, honoring specific food cravings, eating candy before meals, and drinking sweetened beverages.53 Other studies have revealed poor intake of fruits and vegetables among various groups of cancer survivors (2.6 servings/day among uterine and colorectal cancer survivors; 2.2 servings/day among breast cancer survivors) that may reflect poor diet quality following treatment.54–57
Reduction in physical activity
As noted above, energy expenditure encompasses the resting metabolic rate, the thermic effect of food, and physical activity.1 Not surprisingly, physical activity, the other modifiable portion of the energy balance equation, also appears to be influenced by cancer treatment.61–63 Bed rest, fatigue, and direct toxic effects of therapy (such as neuropathy or cardiomyopathy) during the cancer experience modify fitness, endurance, and muscle strength.61 Reduced physical activity during cancer treatment has been documented by Demark-Wahnefried and colleagues among premenopausal breast cancer patients.36,42 Subsequent work in other populations has also described significant decreases, relative to pre-diagnosis baseline, in estimated leisure-time exercise.64 Interestingly, one investigation in breast cancer survivors36 and a separate study of children with acute lymphoblastic leukemia65 noted lower resting metabolic rates among survivors, possible due to loss of lean muscle mass, described further below, although other studies have not confirmed this observation.42
Among patients with ALL, glucocorticoid administration during therapy can directly alter appetite and physical activity, resulting in weight gain and height loss.58,59 Jansen and colleagues observed a group of ALL patients on a protocol that required intermittent dexamethasone. During dexamethasone administration, patients consumed more calories and were less active than healthy controls.60 In a separate study, Ness and colleagues tested 75 adult survivors of childhood ALL treated between 1970 and 1986. The survivors had decreased muscle strength and mobility compared to population normative values. Additionally, the survivors took longer to stand up out of a chair (the “Timed Up and Go” test) and walked shorter distances. Males had 4.5% more body fat and females had 2.3% more body fat (measured by dual energy x-ray absorptiometry) than population normative values. A history of treatment with cranial radiotherapy (CRT) and growth hormone deficiency were particularly relevant to lower body weakness among females.61
Hormonal and other endocrine-related dysregulation
Among breast cancer survivors, hormonal variation can alter energy balance, although data is conflicting. Chemotherapy-induced menopause results in rapid falls in estrogen and progesterone and increases in luteinizing hormone and follicle stimulating hormone. Some studies suggest that premenopausal breast cancer patients who receive chemotherapy that induces menopause appear to be at greater risk of weight gain,35,37–39,66 suggesting that estrogen may be relevant to energy balance in this setting. Other studies, however, have found greater weight gain among post menopausal women40,67 or no difference by menopausal status.29
Survivors of stem cell transplant, especially those who were treated with total body irradiation (TBI) are another high-risk group. Depending on the setting of the study, the prevalence of impaired glucose tolerance and overt type 2 diabetes mellitus (DM) among stem cell transplant survivors with a history of TBI ranges from 42% to 64%.68–70 Interestingly, the link between overweight or obesity and insulin resistance is not as clear in the TBI survivor population. Many hematopoietic stem cell transplant survivors are normal weight or even underweight.33,71 A recent laboratory investigation of 10 stem cell transplant survivors with a history of TBI found that all subjects were insulin resistant, while only 5 were overweight or obese (BMI ≥ 25).27 A study in Sweden showed similar results; many survivors were insulin resistant, but they did not have increased BMI compared to controls. Instead, survivors were shorter than controls and had lower BMI. When body composition was measured by DXA, survivors were found to have higher fat mass and lower lean body mass than non-cancer controls.30 In addition, surviovors had higher leptin and lower adiponectin than non-cancer controls, even after adjustment for fat mass.30
Indeed, these studies suggest that TBI followed by hematopoietic stem cell transplant may lead to a form of lipodystrophy. Impaired subcutaneous fat accumulation may lead to increased free fatty acids in the circulation and deposition of fat in visceral tissue.72,73 Impaired glucose tolerance results when subcutaneous adipocytes cannot expand because pre-adipocytes cannot proliferate in the post-radiation setting. In this setting, the body may respond by increasing leptin production in an attempt to recruit progenitor adipocytes from the bone marrow, although this hypothesis needs to be tested.73 Leptin is a hormone secreted by adipocytes (Figure 2). In the non-cancer setting, leptin is proportional to total body fat mass and communicates via the hypothalamus to signal that energy stores are sufficient; it can normally act as a mild appetite suppressant. Alternatively, increased leptin among stem cell transplant survivors may reflect leptin dysregulation in the setting of impaired glucose tolerance, as has been described among ALL survivors.74 After adjusting for fat mass (measured by DXA), leptin was higher among female ALL survivors (compared to male ALL survivors) and among those who had received cranial radiotherapy (CRT). Some evidence suggests that a biologic interaction between leptin receptor activity, CRT, and obesity may exist. A single nucleotide polymorphism of leptin receptor (Gln22Arg) interacts with CRT in its association with insulin resistance and is independently associated with obesity among female ALL survivors.75
CONSEQUENCES OF POSITIVE ENERGY BALANCE
Over time, positive energy balance will lead to obesity and can result in insulin resistance, DM, lipid abnormalities, and the metabolic syndrome, depending on the setting and the cancer population.70,71,81–83 Among cancer survivors, positive energy balance and weight gain can also adversely affect rates of relapse or recurrence and cancer-specific mortality. This relationship may be partially explained by the Warburg Effect. During the 1920’s, Otto Warburg discovered that cancer cells were using anaerobic metabolism via glycolysis even though oxygen was readily available. This persistent use of anaerobic metabolism results in a higher uptake of glucose into the cancer cell than into the non-cancer cell. Cancer cells, therefore, can survive despite fluctuations in oxygen levels but become “addicted” to glucose.46,84,85 Interestingly, not all tumors display the Warburg effect; those cancers which do not show Warburg features may not be amenable to caloric restriction.86 With the Warburg effect in mind, the importance of obesity and the readily available glucose supply for the modern-day cancer survivor becomes clear.
Relapse, recurrence and second malignant neoplasm
In 1990, Camoriano and colleagues published on the effect of weight gain among women treated with chemotherapy or observed following mastectomy for node-positive cancer. As in other studies, premenopausal women who received adjuvant chemotherapy were found to gain the most weight (median 5.9 kg), when compared to postmenopausal women who did not receive chemotherapy (median weight gain 1.8 kg) and to postmenopausal women who received adjuvant chemotherapy (median weight gain 3.6 kg; P < 0.01 for both comparisons). Furthermore, premenopausal women who gained more than the median amount of weight at 60 weeks had a risk of relapse 1.5-fold greater and a risk of death 1.6-fold greater than premenopausal women who gained less than the median amount of weight. Other studies have also found a relationship between weight gain during or following treatment and breast cancer recurrence.31, 87, 88,89,88 Furthermore, in a recent meta-analysis of thirteen prospective studies among women with breast cancer, obesity was associated with significantly increased risks of contralateral breast, endometrial, and colorectal second cancers. 90
Separate studies have described a similar relationship among women with colon cancer and among men with prostate cancer. In the setting of a longitudinal chemotherapy trial among 3759 patients with colon carcinoma, the effect of BMI on recurrence, survival, and treatment-related toxicity was examined. Obese women experienced significantly worse overall mortality and had a nonsignificant increase in the risk of recurrence.91 In another study of men with prostate cancer undergoing radical prostatectomy, obesity was associated with higher-grade tumors at baseline and higher risk of biochemical recurrence.92
Cancer-specific mortality
Many studies have documented a deleterious effect of obesity on cancer-specific mortality for a range of cancers, including breast, prostate, colon and rectum, non-Hodgkin lymphoma, cervix, uterus, hepatobiliary, esophagus, stomach, pancreas, kidney, and multiple myeloma.15–17 One early study by Petrelli, Calle, and colleagues, used data from the American Cancer Society’s Cancer Prevention Study II (CPS-II) to examine the impact of BMI and height on mortality among US adults who were cancer-free in 1982. Among 424,168 postmenopausal women, 2,852 breast cancer deaths were recorded. Breast cancer death rates were found to increase with BMI. For women in the highest BMI group (BMI ≥ 40 mg/kg2) compared to women with BMI of 18.5 to 20.9 mg/kg2, the risk ratio for death from breast cancer was 3.08 (95% CI: 2.09 to 4.51).28 Other studies have also found obesity to be an important negative predictor of breast cancer.32, 93 In a review of 26 studies, Chlebowski and colleagues described 17 studies that found increased weight to be a significant risk factor for recurrent disease and decreased survival.94
An evaluation of 5,204 Nurses’ Health Study participants diagnosed with breast cancer between 1976 and 2000 (with 860 total deaths and 533 breast cancer deaths) included BMI from prior to diagnosis as well as a year or more after diagnosis. Cox proportional hazard modeling revealed that weight before diagnosis was positively associated with breast cancer recurrence and death among non-smokers. Furthermore, non-smoking women who gained more than 0.5kg/m2, compared to women who maintained their weight, had an elevated risk of breast cancer death during follow-up. As in other studies, the findings were especially strong among premenopausal women.95
ROLE OF DIET AND PHYSICAL ACTIVITY
As noted, diet and physical activity are the modifiable portions of the energy balance equation. The quantity of calories consumed, the types of foods or dietary pattern, and the intensity and duration of physical activity are germane.
Caloric intake
The relative importance of dietary intake versus physical activity for energy balance has been debated. Early evidence from the non-cancer population via the Third National Health and Nutrition Examination Survey suggested that total daily energy intake in the United States had increased over the decades from the 1970s to the 1990s, principally due to increases in protein and carbohydrate consumption.96 An important subsequent examination used caloric intake data from the US food energy supply, assumed to be proportional to energy intake and adjusted for wastage. Measured weight gain was found to be equal to predicted weight gain based on increased food energy supply.97 In other words, according to these calculations, increased caloric intake since the 1970s explains weight gain in the US population.
Dietary composition
Dietary composition, including protein and sugar content, may be relevant to obesity and its consequences.98 Recent work has focused on the role of sugar-sweetened beverages on the positive energy balance among non-cancer populations.99 Intake of sugar-sweetened beverages has increased dramatically over the previous three decades,100 and studies suggest that liquid sugars do not lead to a sense of satiety. One study used a cross-over design to compare intake of liquid (soda) versus solid (jelly beans) sugars during two four-week periods separated by a four week washout. Subjects completed diet records and had body composition measurements. Interestingly, free-feeding intake during the solid (jelly bean) period was significantly lower than the prior intake, while free-feeding intake during the liquid (soda) period was unchanged. Body weight and BMI increased only during the liquid period, although physical activity and hunger were unchanged.101
Sugar-sweetened beverages, therefore, appear to be a candidate target for intervention. Although studies in cancer populations are required, a recent intervention among children in the Netherlands showed promising results. In that study, 641 primarily normal-weight children who were drinking sugar-sweetened beverages during snack time in school were randomized to receive a sugar-free artificially-sweetened beverage or an identical sugar-sweetened beverage that provided 104kcal. Although the caloric difference between groups was small, the sugar-free beverage group gained less weight over time. Skinfold-thickness, waist-to-height ratio, and fat mass also increased significantly less in the sugar-free beverage group.102
Studies of adult survivors of childhood leukemia suggest that the Mediterranean diet may be especially beneficial. A diet high in fruits and vegetables and low in meat, as was traditionally consumed on the island of Crete in the Mediterranean, has been shown to be beneficial in reducing all-cause mortality, DM, cardiovascular disease, and Alzheimer dementia.103–106 Other studies have found a reduction in cancer incidence among populations consuming a Mediterranean diet.107–109 Among ALL survivors in the ALLIFE Study, those who ate a more Mediterranean-style diet (measured with the Mediterranean Diet Score) had better anthropometric and metabolic measures, including lower levels of insulin resistance.45 In a separate study among women undergoing adjuvant chemotherapy for invasive breast cancer, those who adopted a Mediterranean-style diet intervention demonstrated an average weight loss of 2.9 kg, compared to controls.110 While cancer-related endpoints were not measured, further study of the Mediterranean diet among cancer survivors is warranted.
Unfortunately, other studies of dietary interventions among cancer survivors have had conflicting results. Results of the Women’s Intervention Nutrition Study (WINS) were encouraging. In WINS, 2437 women from 39 different clinical centers with a history of resected, early-stage breast cancer were randomized to a dietary intervention (focused on reducing dietary fat intake) or control groups. At five-year follow-up, dietary fat intake was lower in the intervention group, which had also lost an average of six pounds. Breast cancer relapse was less likely in the low-fat group (HR 0.76; 95% CI: 0.60 to 0.98). In contrast to prior studies, women with hormone receptor negative tumors appeared to achieve greater benefit than women with hormone receptor positive tumors.111 On the other hand, results from the Women’s Healthy Eating and Living (WHEL) trial, which focused on increasing fruit, vegetable, and fiber consumption and decreasing fat consumption, did not support a breast cancer-related benefit. In the WHEL trial, the intervention group did have a healthier diet, but the frequency of breast cancer events or mortality was not different.112
Clearly, further research in this area is needed. Specifically, the type and magnitude of dietary intervention required for cancer survivors to see a benefit should be clarified. Dietary and behavioral interventions are complex, as are motivators and barriers to action. The hormonal milieu following weight loss which may benefit tumor-related outcomes, also (unfortunately) works to replace lost fat stores.113 Nonetheless, results from work by Demark-Wahnefried among breast and colorectal survivors114,115 and by Appel in a non-cancer population116 suggests that dietary interventions can be effective.
Physical activity
Clinical and research interest in the application of physical activity and exercise following a cancer diagnosis has increased dramatically over the past decade.117 Several excellent systematic reviews and meta-analyses have extensively evaluated the available literature investigating the role of structured exercise therapy (physical activity) following a cancer diagnosis.118–122 In particular, studies have investigated whether exercise is an effective strategy to off-set the consequences of positive energy balance in patients with cancer both during and following cancer therapy. For example, Speck et al.123 identified a total of 66 ‘high quality’ studies that examined the effects of exercise on 60 different physiological, functional, biological, or psycho-social outcomes in adults with cancer. The same investigators later identified 22 studies (n=8, during therapy; n=14, after therapy) that examined the effects of exercise on body weight in cancer survivors; 2 (25%) and 7 (50%) of studies found that exercise was associated with favorable changes in body weight during and after therapy, respectively.53 The modest positive effects of exercise on weight loss in cancer survivors is consistent with that observed in other non-cancer clinical populations124 and healthy subjects.125
Nevertheless, exercise, in combination with a healthy diet, may have favorable effects on the adverse consequences of chronic positive energy balance independent of weight loss. Strong evidence suggests that interventions promoting increased physical activity can reduce the risk of DM via improvements in glycemic control. In the landmark US Diabetes Prevention Program, 3234 nondiabetic adults with elevated fasting glucose were randomized to metformin, 850 mg twice daily, lifestyle-modification (7% weight loss and >150 minutes of exercise/week) or placebo. After 2.8 years of follow-up, the incidence of DM was 11.0, 7.8, and 4.8 cases per 100 person-years in the placebo, metformin, and lifestyle intervention, respectively.126 These results were corroborated by Tuomilehto et al.127 In that study, lifestyle intervention reduced the risk of DM by 58% compared with a non-intervention group. Finally, Li and colleagues reported that 6 years of active intervention with diet alone, exercise alone, or diet plus exercise could prevent or delay incidence of DM up to 14 years after initial study recruitment. Specifically, patients with impaired glycemic control receiving lifestyle intervention (diet plus exercise) had a 43% lower risk of DM with new onset disease being delayed, on average, by 3.6 years.128
A growing number of randomized trials have investigated the effects of structured exercise training on glycemic control among adults with DM as well as those with impaired glucose control. In a recent meta-analysis of 14 randomized trials, Thomas et al.129 reported that standardized aerobic training (i.e., 3–5 sessions/week, 30–45 minutes/session at 60%–85% of baseline fitness, 12–24 weeks in duration) is associated with significant improvements in measures of glycemic control as well as reduction in visceral adipose tissue and subcutaneous adipose tissue. These results have been confirmed by several others systematic reviews and meta-analyses.130,131
At the present, no studies to our knowledge have investigated whether exercise favorably modulates the overt clinical consequences of positive energy balance such as development of DM and cardiovascular disease in cancer survivors.117 Similarly, few studies have assessed whether exercise favorably modulates traditional cardiovascular risk factors in cancer survivors. A recent review by Betof and colleagues identified a total of nine studies assessing the effects of exercise on changes in metabolic factors.132 Overall, exercise was associated with favorable changes in insulin-like growth factor-1 and insulin-like growth factor binding protein-3, but not insulin or glucose levels.132 Nonetheless, relatively robust data indicate that exercise training improves measures of exercise capacity. In a recent meta-analysis of six randomized trials involving 571 (n=344, exercise; n=227, control) cancer patients, Jones et al. found that exercise training was associated with a significant increase in peak oxygen consumption (VO2peak; WMD=2.90 mL kg−1min−1, 95% CI: 1.16 to 4.64).133 This finding may be clinically important since exercise capacity is a strong, independent predictor of cardiovascular as well as all-cause mortality in a broad spectrum of adult populations.134–136
Based on the demonstrated effects of exercise in non-cancer populations and emerging data in cancer survivors, it could be speculated that such effects may, in turn, influence long-term prognosis. Data from randomized trials are not currently available; however, a growing number of epidemiological studies have examined the relationship between self-reported exercise behavior or measures of exercise capacity measured after the cancer diagnosis and prognosis. As recently summarized, approximately 20 observational studies have examined the association between self-reported exercise behavior (assessed via surveys) and prognosis following a diagnosis of cancer.132 To our knowledge, none of these studies have examined the relationship between exercise or cardiorespiratory fitness and cardiovascular disease-related events in cancer patients, although the majority have examined all-cause mortality. To this end, the current evidence indicates a relatively consistent inverse relationship between higher levels of exercise behavior and significant reductions in all-cause mortality compared with sedentary individuals.137 Importantly, the relationship between exercise and cancer prognosis is independent of BMI.132,137
Given the well-known limitations of exercise exposure assessment via self-report methods, investigators have also started to examine the prognostic value of measures that provide an objective measure of exercise exposure through the use of exercise capacity (e.g., maximal cardiopulmonary exercise testing) and functional capacity (e.g., 6 minute walk testing) evaluation. To this end, eight studies to date have utilized such tests to examine the prognostic value of either VO2peak from a maximal cardiopulmonary exercise test or 6-minute walk distance. Similar to self-reported exercise behavior, both VO2peak and 6-minute walk distance are strong independent predictors of death in patients with inoperable non-small cell lung cancer and metastatic breast cancer.132
Beyond all-cause mortality, there is also interest in whether exercise behavior modulates cancer prognosis. Fewer studies have examined this question. Nevertheless, emerging data indicates that, in general, higher levels of exercise behavior are associated with significant reductions in cancer-specific mortality after controlling for BMI.136 For example, in two recent large observational studies, investigators found that vigorous exercise (brisk walking ≥3 hrs.wk-1) was associated with a 50%–60% reduction in tumor progression and prostate cancer mortality in men with localized prostate cancer.138,139 Similarly, in women with early breast cancer, Holmes et al. reported that women reporting ≥9 MET-hrs.wk−1 (equivalent to walking briskly for 30 mins, 5 days/week), had a 6% reduction in unadjusted absolute cancer-specific mortality risk at 10 years compared with women who reported less than 3 MET-hours per week (equivalent to walking 2–2.9 mph for 1 hour).140
Intriguingly, the exercise – cancer-specific mortality relationship is not uniform. For example, the magnitude of risk reduction (in cancer prognosis) appears to vary according to exercise dose. In the study by Holmes et al. ≥9 MET-hrs.wk−1 was associated with significant reductions in cancer-specific mortality. 140 A separate study by Holick and colleagues, however, found that ≥21 MET-hrs.wk-1 (brisk walking for 75 minutes, 5 d.wk−1) was required for significant reductions in cancer specific and all-cause mortality in women with invasive breast cancer. 141 Among patients with colorectal cancer, Meyerhardt and colleagues found that between ≥18 MET-hrs.wk−1 (equivalent to walking briskly for 60 mins, 5 days/week) and ≥27 MET-hrs.wk−1 (equivalent to walking briskly for 90 mins, 5 days/week) was required for significant reductions in cancer-specific mortality.91, 142, 143
In addition to exercise dose, the impact of exercise behavior may also be dependent on expression of certain tumor molecular features. Two studies to date have explored this question. In the first study, Meyerhardt et al.144 found that in patients with tumors exhibiting loss of p27, a protein that regulates cell cycle, the hazard ratio (HR) for colon cancer-specific mortality was 1.40 (95% CI, 0.41–4.72) for those reporting ≥18 MET-hrs.wk−1 compared with <18 MET-hrs.wk−1. The corresponding HR for patients with tumors exhibiting expression of p27, was 0.33 (95% CI, 0.12–0.85) for those reporting ≥18 MET-hrs.wk−1 compared with <18 MET-hrs.wk−1. In a separate study, also in colorectal cancer, Morikawa et al. found that patients reporting ≥18 MET-hrs.wk−1 whose tumors did not express CTNNB1 (β-catenin), a key mediator of the WNT signaling pathway, had an adjusted HR for colorectal cancer-specific survival of 0.33 (95% CI: 0.13–0.81) compared with patients reporting <18 MET-hrs.wk−1. 145 Conversely, there was no significant relationship between exercise and prognosis in patients with tumors that were positive for nuclear CTNNB1 (adjusted HR: 1.07; 95% CI: 0.50–2.30).
In summary, promising evidence suggests that physical activity and structured exercise interventions can favorably influence the clinical consequences of chronic energy imbalance. However, several major questions remain to be addressed to continue progress in this field of research. From an epidemiological perspective, more large studies to examine the association between exercise exposure and clinical outcomes (e.g., recurrence, therapy-related morbidity, cancer-specific mortality, competing cause of mortality, and all cause mortality) in a diverse range of malignancies is necessary. Similarly, examining whether the exercise – cancer progression is modulated as a function of histological or molecular features of tumors will be critical to inform future, mechanistically-driven studies. From the perspective of translational and related clinical studies, there is an urgent need to elucidate the optimal exercise prescription (or dose-response relationship) to modulate the consequences of chronic energy balance and understand, at a systemic and molecular level, the mechanisms underlying the exercise -cancer prognosis relationship.
CONCLUSIONS
The evidence for a unique relationship between energy imbalance and cancer is building. Positive energy balance following cancer treatment is a complex phenomenon with wide-ranging effects. Prevention and treatment of weight gain in the cancer survivor should be a major clinical goal, perhaps even more so than in the non-cancer population. Nonetheless, clinicians and patients need guidance as to what specific interventions are likely to have the highest impact. Future research should focus on mechanistic studies, to better understand how metabolic and anthropometric changes contribute to post-cancer morbidity and mortality, and dissemination studies, so that patients receive the proper public health messages regarding energy balance and health. A focus on sugar-sweetened beverages and a Mediterranean-style diet (less meat, more fruits and vegetables) is a reasonable place to start. Finally, further study of insulin resistance in the setting of an apparently normal body mass index is needed.
Acknowledgments
ET is supported in part by the American Cancer Society (ACS CCCDA 11-192-01). LWJ is supported in part by research grants from the National Cancer Institute (CA143254, CA142566, CA138624, CA133895).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: none
REFERENCES
- 1.Pi-Sunyer FX. The obesity epidemic: pathophysiology and consequences of obesity. Obes Res. 2002;10(Suppl 2):97S–104S. doi: 10.1038/oby.2002.202. [DOI] [PubMed] [Google Scholar]
- 2.Galgani J, Ravussin E. Energy metabolism, fuel selection and body weight regulation. Int J Obes (Lond) 2008;32(Suppl 7):S109–S119. doi: 10.1038/ijo.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332(10):621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 4.Institute NC. About survivorship research: survivorship definitions. 2004 [Google Scholar]
- 5.Hewitt M, Greenfield S, Stovall E. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: The National Academies Press; 2006. [Google Scholar]
- 6.Davis MP, Dreicer R, Walsh D, Lagman R, LeGrand SB. Appetite and cancer-associated anorexia: a review. J Clin Oncol. 2004;22(8):1510–1517. doi: 10.1200/JCO.2004.03.103. [DOI] [PubMed] [Google Scholar]
- 7.Brouwer BG, Visseren FL, van der Graaf Y, Group SS. The effect of leisure-time physical activity on the presence of metabolic syndrome in patients with manifest arterial disease. The SMART study. American heart journal. 2007;154(6):1146–1152. doi: 10.1016/j.ahj.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 8.Miller WR. Controversies in breast cancer 2010. Breast cancer research : BCR. 2010;12(Suppl 4):S1. doi: 10.1186/bcr2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotler DP. Cachexia. Ann Intern Med. 2000;133(8):622–634. doi: 10.7326/0003-4819-133-8-200010170-00015. [DOI] [PubMed] [Google Scholar]
- 10.Morley JE, Thomas DR, Wilson MM. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr. 2006;83(4):735–743. doi: 10.1093/ajcn/83.4.735. [DOI] [PubMed] [Google Scholar]
- 11.Nichols HB, Trentham-Dietz A, Egan KM, et al. Body mass index before and after breast cancer diagnosis: associations with all-cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1403–1409. doi: 10.1158/1055-9965.EPI-08-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staal-van den Brekel AJ, Dentener MA, Schols AM, Buurman WA, Wouters EF. Increased resting energy expenditure and weight loss are related to a systemic inflammatory response in lung cancer patients. J Clin Oncol. 1995;13(10):2600–2605. doi: 10.1200/JCO.1995.13.10.2600. [DOI] [PubMed] [Google Scholar]
- 13.Falconer JS, Fearon KC, Plester CE, Ross JA, Carter DC. Cytokines, the acute-phase response, and resting energy expenditure in cachectic patients with pancreatic cancer. Annals of surgery. 1994;219(4):325–331. doi: 10.1097/00000658-199404000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaughan VC, Martin P, Lewandowski PA. Cancer cachexia: impact, mechanisms and emerging treatments. Journal of cachexia, sarcopenia and muscle. 2012 doi: 10.1007/s13539-012-0087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index mortality in a prospective cohort of U.S adults. N Engl J Med. 1999;341(15):1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 16.Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer prevention research. 2011;4(4):486–501. doi: 10.1158/1940-6207.CAPR-10-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li D, Morris JS, Liu J, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301(24):2553–2562. doi: 10.1001/jama.2009.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thivat E, Therondel S, Lapirot O, et al. Weight change during chemotherapy changes the prognosis in non metastatic breast cancer for the worse. BMC Cancer. 2010;10:648. doi: 10.1186/1471-2407-10-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. The American journal of medicine. 1980;69(4):491–497. doi: 10.1016/s0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- 20.Ambrus JL, Ambrus CM, Mink IB, Pickren JW. Causes of death in cancer patients. Journal of medicine. 1975;6(1):61–64. [PubMed] [Google Scholar]
- 21.Tsoli M, Robertson G. Cancer cachexia: malignant inflammation, tumorkines, and metabolic mayhem. Trends in endocrinology and metabolism: TEM. 2012 doi: 10.1016/j.tem.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Shum AM, Polly P. Cancer cachexia: molecular targets and pathways for diagnosis and drug intervention. Endocrine, metabolic & immune disorders drug targets. 2012;12(3):247–259. doi: 10.2174/187153012802002910. [DOI] [PubMed] [Google Scholar]
- 23.Seyfried TN, Shelton LM. Cancer as a metabolic disease. Nutrition & metabolism. 2010;7:7. doi: 10.1186/1743-7075-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leibel RL, Hirsch J. Metabolic characterization of obesity. Ann Intern Med. 1985;103(6 (Pt 2)):1000–1002. doi: 10.7326/0003-4819-103-6-1000. [DOI] [PubMed] [Google Scholar]
- 25.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 26.Blanck HM, McCullough ML, Patel AV, et al. Sedentary behavior, recreational physical activity, 7-year weight gain among postmenopausal U.S women. Obesity (Silver Spring) 2007;15(6):1578–1588. doi: 10.1038/oby.2007.187. [DOI] [PubMed] [Google Scholar]
- 27.Chemaitilly W, Boulad F, Oeffinger KC, Sklar CA. Disorders of glucose homeostasis in young adults treated with total body irradiation during childhood: a pilot study. Bone Marrow Transplant. 2009;44(6):339–343. doi: 10.1038/bmt.2009.40. [DOI] [PubMed] [Google Scholar]
- 28.Petrelli JM, Calle EE, Rodriguez C, Thun MJ. Body mass index, height, and postmenopausal breast cancer mortality in a prospective cohort of US women. Cancer Causes Control. 2002;13(4):325–332. doi: 10.1023/a:1015288615472. [DOI] [PubMed] [Google Scholar]
- 29.Han HS, Lee KW, Kim JH, et al. Weight changes after adjuvant treatment in Korean women with early breast cancer. Breast cancer research and treatment. 2009;114(1):147–153. doi: 10.1007/s10549-008-9984-6. [DOI] [PubMed] [Google Scholar]
- 30.Frisk P, Rossner SM, Norgren S, Arvidson J, Gustafsson J. Glucose metabolism and body composition in young adults treated with TBI during childhood. Bone Marrow Transplant. 2011;46(10):1303–1308. doi: 10.1038/bmt.2010.307. [DOI] [PubMed] [Google Scholar]
- 31.Donegan WL, Hartz AJ, Rimm AA. The association of body weight with recurrent cancer of the breast. Cancer. 1978;41(4):1590–1594. doi: 10.1002/1097-0142(197804)41:4<1590::aid-cncr2820410449>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 32.Daling JR, Malone KE, Doody DR, et al. Relation of body mass index to tumor markers and survival among young women with invasive ductal breast carcinoma. Cancer. 2001;92(4):720–729. doi: 10.1002/1097-0142(20010815)92:4<720::aid-cncr1375>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 33.Meacham LR, Gurney JG, Mertens AC, et al. Body mass index in long-term adult survivors of childhood cancer: a report of the Childhood Cancer Survivor Study. Cancer. 2005;103(8):1730–1739. doi: 10.1002/cncr.20960. [DOI] [PubMed] [Google Scholar]
- 34.Lynce F, Pehlivanova M, Catlett J, Malkovska V. Obesity in adult lymphoma survivors. Leukemia & lymphoma. 2012;53(4):569–574. doi: 10.3109/10428194.2011.619606. [DOI] [PubMed] [Google Scholar]
- 35.Demark-Wahnefried W, Rimer BK, Winer EP. Weight gain in women diagnosed with breast cancer. Journal of the American Dietetic Association. 1997;97(5):519–526. 529. doi: 10.1016/s0002-8223(97)00133-8. quiz 527–518. [DOI] [PubMed] [Google Scholar]
- 36.Demark-Wahnefried W, Hars V, Conaway MR, et al. Reduced rates of metabolism and decreased physical activity in breast cancer patients receiving adjuvant chemotherapy. Am J Clin Nutr. 1997;65(5):1495–1501. doi: 10.1093/ajcn/65.5.1495. [DOI] [PubMed] [Google Scholar]
- 37.Demark-Wahnefried W, Winer EP, Rimer BK. Why women gain weight with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1993;11(7):1418–1429. doi: 10.1200/JCO.1993.11.7.1418. [DOI] [PubMed] [Google Scholar]
- 38.Dixon JK, Moritz DA, Baker FL. Breast cancer and weight gain: an unexpected finding. Oncol Nurs Forum. 1978;5(3):5–7. [PubMed] [Google Scholar]
- 39.Boyd NF. Nutrition and breast cancer. J Natl Cancer Inst. 1993;85(1):6–7. doi: 10.1093/jnci/85.1.6. [DOI] [PubMed] [Google Scholar]
- 40.Irwin ML, McTiernan A, Baumgartner RN, et al. Changes in body fat and weight after a breast cancer diagnosis: influence of demographic, prognostic, and lifestyle factors. J Clin Oncol. 2005;23(4):774–782. doi: 10.1200/JCO.2005.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vance V, Mourtzakis M, McCargar L, Hanning R. Weight gain in breast cancer survivors: prevalence, pattern and health consequences. Obes Rev. 2011;12(4):282–294. doi: 10.1111/j.1467-789X.2010.00805.x. [DOI] [PubMed] [Google Scholar]
- 42.Demark-Wahnefried W, Peterson BL, Winer EP, et al. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2001;19(9):2381–2389. doi: 10.1200/JCO.2001.19.9.2381. [DOI] [PubMed] [Google Scholar]
- 43.Winters-Stone KM, Schwartz A, Nail LM. A review of exercise interventions to improve bone health in adult cancer survivors. J Cancer Surviv. 2010;4(3):187–201. doi: 10.1007/s11764-010-0122-1. [DOI] [PubMed] [Google Scholar]
- 44.Irwin ML, Alvarez-Reeves M, Cadmus L, et al. Exercise improves body fat, lean mass, and bone mass in breast cancer survivors. Obesity (Silver Spring) 2009;17(8):1534–1541. doi: 10.1038/oby.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tonorezos ES, Robien K, Eshelman-Kent D, et al. Contribution of diet and physical activity to metabolic parameters among survivors of childhood leukemia. Cancer Causes Control. 2013;24(2):313–321. doi: 10.1007/s10552-012-0116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu Z, Haegele AD, Thompson HJ. Effect of caloric restriction on pre-malignant and malignant stages of mammary carcinogenesis. Carcinogenesis. 1997;18(5):1007–1012. doi: 10.1093/carcin/18.5.1007. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz GJ. The role of gastrointestinal vagal afferents in the control of food intake: current prospects. Nutrition. 2000;16(10):866–873. doi: 10.1016/s0899-9007(00)00464-0. [DOI] [PubMed] [Google Scholar]
- 48.Lustig RH, Post SR, Srivannaboon K, et al. Risk factors for the development of obesity in children surviving brain tumors. J Clin Endocrinol Metab. 2003;88(2):611–616. doi: 10.1210/jc.2002-021180. [DOI] [PubMed] [Google Scholar]
- 49.Lustig RH. Autonomic dysfunction of the beta-cell and the pathogenesis of obesity. Rev Endocr Metab Disord. 2003;4(1):23–32. doi: 10.1023/a:1021819318484. [DOI] [PubMed] [Google Scholar]
- 50.Lustig RH, Hinds PS, Ringwald-Smith K, et al. Octreotide therapy of pediatric hypothalamic obesity: a double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2003;88(6):2586–2592. doi: 10.1210/jc.2002-030003. [DOI] [PubMed] [Google Scholar]
- 51.Lustig RH, Greenway F, Velasquez-Mieyer P, et al. A multicenter, randomized, double-blind, placebo-controlled, dose-finding trial of a long-acting formulation of octreotide in promoting weight loss in obese adults with insulin hypersecretion. Int J Obes (Lond) 2006;30(2):331–341. doi: 10.1038/sj.ijo.0803074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamilton JK, Conwell LS, Syme C, et al. Hypothalamic Obesity following Craniopharyngioma Surgery: Results of a Pilot Trial of Combined Diazoxide and Metformin Therapy. Int J Pediatr Endocrinol. 2011;2011:417949. doi: 10.1155/2011/417949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Speck RM, Demichele A, Farrar JT, et al. Taste alteration in breast cancer patients treated with taxane chemotherapy: experience, effect, and coping strategies. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2013;21(2):549–555. doi: 10.1007/s00520-012-1551-3. [DOI] [PubMed] [Google Scholar]
- 54.von Gruenigen V, Frasure H, Kavanagh MB, et al. Survivors of uterine cancer empowered by exercise and healthy diet (SUCCEED): a randomized controlled trial. Gynecol Oncol. 2012;125(3):699–704. doi: 10.1016/j.ygyno.2012.03.042. [DOI] [PubMed] [Google Scholar]
- 55.Grimmett C, Bridgewater J, Steptoe A, Wardle J. Lifestyle and quality of life in colorectal cancer survivors. Qual Life Res. 2011;20(8):1237–1245. doi: 10.1007/s11136-011-9855-1. [DOI] [PubMed] [Google Scholar]
- 56.Paxton RJ, Garcia-Prieto C, Berglund M, et al. A randomized parallel-group dietary study for stages II-IV ovarian cancer survivors. Gynecol Oncol. 2012;124(3):410–416. doi: 10.1016/j.ygyno.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robien K, Ness KK, Klesges LM, Baker KS, Gurney JG. Poor adherence to dietary guidelines among adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2008;30(11):815–822. doi: 10.1097/MPH.0b013e31817e4ad9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arguelles B, Barrios V, Buno M, Madero L, Argente J. Anthropometric parameters and their relationship to serum growth hormone-binding protein and leptin levels in children with acute lymphoblastic leukemia: a prospective study. Eur J Endocrinol. 2000;143(2):243–250. doi: 10.1530/eje.0.1430243. [DOI] [PubMed] [Google Scholar]
- 59.Dalton VK, Rue M, Silverman LB, et al. Height and weight in children treated for acute lymphoblastic leukemia: relationship to CNS treatment. J Clin Oncol. 2003;21(15):2953–2960. doi: 10.1200/JCO.2003.03.068. [DOI] [PubMed] [Google Scholar]
- 60.Jansen H, Postma A, Stolk RP. Kamps WA. Acute lymphoblastic leukemia and obesity: increased energy intake or decreased physical activity? Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2009;17(1):103–106. doi: 10.1007/s00520-008-0531-0. [DOI] [PubMed] [Google Scholar]
- 61.Ness KK, Baker KS, Dengel DR, et al. Body composition, muscle strength deficits and mobility limitations in adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;49(7):975–981. doi: 10.1002/pbc.21091. [DOI] [PubMed] [Google Scholar]
- 62.Ness KK, Gurney JG, Baker KS, et al. Functional limitations, physical disability and social competence among HCT survivors transplanted during childhood or adolescence: A report from the bone marrow transplant survivor. Blood. 2004;104(11):20a–21a. [Google Scholar]
- 63.Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112(11 Suppl):2577–2592. doi: 10.1002/cncr.23448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andrykowski MA, Beacham AO, Jacobsen PB. Prospective, longitudinal study of leisure-time exercise in women with early-stage breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(3):430–438. doi: 10.1158/1055-9965.EPI-06-0735. [DOI] [PubMed] [Google Scholar]
- 65.Reilly JJ, Ventham JC, Ralston JM, Donaldson M, Gibson B. Reduced energy expenditure in preobese children treated for acute lymphoblastic leukemia. Pediatr Res. 1998;44(4):557–562. doi: 10.1203/00006450-199810000-00015. [DOI] [PubMed] [Google Scholar]
- 66.Goodwin PJ, Ennis M, Pritchard KI, et al. Adjuvant treatment and onset of menopause predict weight gain after breast cancer diagnosis. J Clin Oncol. 1999;17(1):120–129. doi: 10.1200/JCO.1999.17.1.120. [DOI] [PubMed] [Google Scholar]
- 67.Rock CL, Flatt SW, Newman V, et al. Factors associated with weight gain in women after diagnosis of breast cancer. Women's Healthy Eating and Living Study Group. Journal of the American Dietetic Association. 1999;99(10):1212–1221. doi: 10.1016/s0002-8223(99)00298-9. [DOI] [PubMed] [Google Scholar]
- 68.Chow EJ, Mueller BA, Baker KS, et al. Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Ann Intern Med. 2011;155(1):21–32. doi: 10.7326/0003-4819-155-1-201107050-00004. [DOI] [PubMed] [Google Scholar]
- 69.Lorini R, Cortona L, Scaramuzza A, et al. Hyperinsulinemia in children and adolescents after bone marrow transplantation. Bone Marrow Transplant. 1995;15(6):873–877. [PubMed] [Google Scholar]
- 70.Hoffmeister PA, Storer BE, Sanders JE. Diabetes mellitus in long-term survivors of pediatric hematopoietic cell transplantation. J Pediatr Hematol Oncol. 2004;26(2):81–90. doi: 10.1097/00043426-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 71.Meacham LR, Sklar CA, Li S, et al. Diabetes mellitus in long-term survivors of childhood cancer. Increased risk associated with radiation therapy: a report for the childhood cancer survivor study. Arch Intern Med. 2009;169(15):1381–1388. doi: 10.1001/archinternmed.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Danforth E., Jr. Failure of adipocyte differentiation causes type II diabetes mellitus? Nat Genet. 2000;26(1):13. doi: 10.1038/79111. [DOI] [PubMed] [Google Scholar]
- 73.Ablamunits V, Weisberg SP, Lemieux JE, Combs TP, Klebanov S. Reduced adiposity in ob/ob mice following total body irradiation and bone marrow transplantation. Obesity (Silver Spring) 2007;15(6):1419–1429. doi: 10.1038/oby.2007.170. [DOI] [PubMed] [Google Scholar]
- 74.Tonorezos ES, Vega GL, Sklar CA, et al. Adipokines, body fatness, and insulin resistance among survivors of childhood leukemia. Pediatr Blood Cancer. 2011 doi: 10.1002/pbc.22964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ross JA, Oeffinger KC, Davies SM, et al. Genetic variation in the leptin receptor gene and obesity in survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2004;22(17):3558–3562. doi: 10.1200/JCO.2004.11.152. [DOI] [PubMed] [Google Scholar]
- 76.Garofalo C, Koda M, Cascio S, et al. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res. 2006;12(5):1447–1453. doi: 10.1158/1078-0432.CCR-05-1913. [DOI] [PubMed] [Google Scholar]
- 77.Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24(4):683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 78.Moller DE, Flier JS. Insulin resistance-mechanisms, syndromes, and implications. N Engl J Med. 1991;325(13):938–948. doi: 10.1056/NEJM199109263251307. [DOI] [PubMed] [Google Scholar]
- 79.Pollak M. Insulin, insulin-like growth factors and neoplasia. Best practice & research Clinical endocrinology & metabolism. 2008;22(4):625–638. doi: 10.1016/j.beem.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 80.Hursting SD, Perkins SN, Phang JM, Barrett JC. Diet and cancer prevention studies in p53-deficient mice. The Journal of nutrition. 2001;131(11 Suppl):3092S–3094S. doi: 10.1093/jn/131.11.3092S. [DOI] [PubMed] [Google Scholar]
- 81.de Haas EC, Oosting SF, Lefrandt JD, et al. The metabolic syndrome in cancer survivors. The lancet oncology. 2010;11(2):193–203. doi: 10.1016/S1470-2045(09)70287-6. [DOI] [PubMed] [Google Scholar]
- 82.Haugnes HS, Aass N, Fossa SD, et al. Components of the metabolic syndrome in long-term survivors of testicular cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2007;18(2):241–248. doi: 10.1093/annonc/mdl372. [DOI] [PubMed] [Google Scholar]
- 83.Nuver J, Smit AJ, Wolffenbuttel BH, et al. The metabolic syndrome and disturbances in hormone levels in long-term survivors of disseminated testicular cancer. J Clin Oncol. 2005;23(16):3718–3725. doi: 10.1200/JCO.2005.02.176. [DOI] [PubMed] [Google Scholar]
- 84.Moreschi C. Beziehungen zwischen ernahrung und tumorwachstum. Seitschrift f Immunitatsforshung. 1909;2:651–675. [Google Scholar]
- 85.Rous P. The influence of diet on transplanted and spontaneous mouse tumors. The Journal of experimental medicine. 1914;20:433–451. doi: 10.1084/jem.20.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458(7239):725–731. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boyd NF, Campbell JE, Germanson T, et al. Body weight and prognosis in breast cancer. J Natl Cancer Inst. 1981;67(4):785–789. [PubMed] [Google Scholar]
- 88.Demark-Wahnefried W, Platz EA, Ligibel JA, et al. The role of obesity in cancer survival and recurrence. Cancer Epidemiol Biomarkers Prev. 2012;21(8):1244–1259. doi: 10.1158/1055-9965.EPI-12-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Champ CE, Volek JS, Siglin J, Jin L, Simone NL. Weight gain, metabolic syndrome, and breast cancer recurrence: are dietary recommendations supported by the data? International journal of breast cancer. 2012;2012:506868. doi: 10.1155/2012/506868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Druesne-Pecollo N, Touvier M, Barrandon E, et al. Excess body weight and second primary cancer risk after breast cancer: a systematic review and meta-analysis of prospective studies. Breast cancer research and treatment. 2012;135(3):647–654. doi: 10.1007/s10549-012-2187-1. [DOI] [PubMed] [Google Scholar]
- 91.Meyerhardt JA, Catalano PJ, Haller DG, et al. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer. 2003;98(3):484–495. doi: 10.1002/cncr.11544. [DOI] [PubMed] [Google Scholar]
- 92.Freedland SJ, Aronson WJ, Kane CJ, et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004;22(3):446–453. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]
- 93.Newman SC, Lees AW, Jenkins HJ. The effect of body mass index and oestrogen receptor level on survival of breast cancer patients. Int J Epidemiol. 1997;26(3):484–490. doi: 10.1093/ije/26.3.484. [DOI] [PubMed] [Google Scholar]
- 94.Chlebowski RT, Aiello E, McTiernan A. Weight loss in breast cancer patient management. J Clin Oncol. 2002;20(4):1128–1143. doi: 10.1200/JCO.2002.20.4.1128. [DOI] [PubMed] [Google Scholar]
- 95.Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23(7):1370–1378. doi: 10.1200/JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 96.McDowell MA, Briefel RR, Alaimo K, et al. Energy and macronutrient intakes of persons ages 2 months and over in the United States: Third National Health and Nutrition Examination Survey, Phase 1, 1988–91. Advance data. 1994;255:1–24. [PubMed] [Google Scholar]
- 97.Swinburn B, Sacks G, Ravussin E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am J Clin Nutr. 2009;90(6):1453–1456. doi: 10.3945/ajcn.2009.28595. [DOI] [PubMed] [Google Scholar]
- 98.Li Z, Heber D. Overeating and overweight: extra calories increase fat mass while protein increases lean mass. JAMA. 2012;307(1):86–87. doi: 10.1001/jama.2011.1959. [DOI] [PubMed] [Google Scholar]
- 99.Fowler SP, Williams K, Resendez RG, et al. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity (Silver Spring) 2008;16(8):1894–1900. doi: 10.1038/oby.2008.284. [DOI] [PubMed] [Google Scholar]
- 100.Company BD. Fact book. Bedford Hill, NY: Beverage Digest Compant; 1998. [Google Scholar]
- 101.DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2000;24(6):794–800. doi: 10.1038/sj.ijo.0801229. [DOI] [PubMed] [Google Scholar]
- 102.de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med. 2012;367(15):1397–1406. doi: 10.1056/NEJMoa1203034. [DOI] [PubMed] [Google Scholar]
- 103.Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr. 2010;92(5):1189–1196. doi: 10.3945/ajcn.2010.29673. [DOI] [PubMed] [Google Scholar]
- 104.Renaud S, de Lorgeril M, Delaye J, et al. Cretan Mediterranean diet for prevention of coronary heart disease. Am J Clin Nutr. 1995;61(6 Suppl):1360S–1367S. doi: 10.1093/ajcn/61.6.1360S. [DOI] [PubMed] [Google Scholar]
- 105.Scarmeas N, Stern Y, Mayeux R, Luchsinger JA. Mediterranean diet, Alzheimer disease, and vascular mediation. Archives of neurology. 2006;63(12):1709–1717. doi: 10.1001/archneur.63.12.noc60109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Estruch R, Ros E, Salas-Salvado J, et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N Engl J Med. 2013 doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 107.Knoops KT, de Groot LC, Kromhout D, et al. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA. 2004;292(12):1433–1439. doi: 10.1001/jama.292.12.1433. [DOI] [PubMed] [Google Scholar]
- 108.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348(26):2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 109.Trichopoulou A, Vasilopoulou E. Mediterranean diet and longevity. The British journal of nutrition. 2000;84(Suppl 2):S205–S209. doi: 10.1079/096582197388554. [DOI] [PubMed] [Google Scholar]
- 110.Villarini A, Pasanisi P, Raimondi M, et al. Preventing weight gain during adjuvant chemotherapy for breast cancer: a dietary intervention study. Breast cancer research and treatment. 2012;135(2):581–589. doi: 10.1007/s10549-012-2184-4. [DOI] [PubMed] [Google Scholar]
- 111.Chlebowski RT, Blackburn GL, Thomson CA, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women's Intervention Nutrition Study. J Natl Cancer Inst. 2006;98(24):1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 112.Pierce JP, Natarajan L, Caan BJ, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women's Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298(3):289–298. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597–1604. doi: 10.1056/NEJMoa1105816. [DOI] [PubMed] [Google Scholar]
- 114.Demark-Wahnefried W, Clipp EC, Lipkus IM, et al. Main outcomes of the FRESH START trial: a sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. J Clin Oncol. 2007;25(19):2709–2718. doi: 10.1200/JCO.2007.10.7094. [DOI] [PubMed] [Google Scholar]
- 115.Christy SM, Mosher CE, Sloane R, et al. Long-term dietary outcomes of the FRESH START intervention for breast and prostate cancer survivors. Journal of the American Dietetic Association. 2011;111(12):1844–1851. doi: 10.1016/j.jada.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Appel LJ, Clark JM, Yeh HC, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 2011;365(21):1959–1968. doi: 10.1056/NEJMoa1108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jones LW, Alfano CM. Exercise-oncology research: past, present, and future. Acta Oncol. 2013;52(2):195–215. doi: 10.3109/0284186X.2012.742564. [DOI] [PubMed] [Google Scholar]
- 118.Fong DY, Ho JW, Hui BP, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. Bmj. 2012;344:e70. doi: 10.1136/bmj.e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Craft LL, Vaniterson EH, Helenowski IB, Rademaker AW, Courneya KS. Exercise effects on depressive symptoms in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21(1):3–19. doi: 10.1158/1055-9965.EPI-11-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McNeely ML, Campbell KL, Rowe BH, et al. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. Cmaj. 2006;175(1):34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: a systematic review of randomized and controlled clinical trials. J Clin Oncol. 2005;23(16):3830–3842. doi: 10.1200/JCO.2005.02.148. [DOI] [PubMed] [Google Scholar]
- 122.Jones LW, Demark-Wahnefried W. Diet, exercise, and complementary therapies after primary treatment for cancer. Lancet Oncol. 2006;7(12):1017–1026. doi: 10.1016/S1470-2045(06)70976-7. [DOI] [PubMed] [Google Scholar]
- 123.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 124.Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286(10):1218–1227. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- 125.Garrow JS, Summerbell CD. Meta-analysis: effect of exercise, with or without dieting, on the body composition of overweight subjects. European journal of clinical nutrition. 1995;49(1):1–10. [PubMed] [Google Scholar]
- 126.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 128.Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet. 2008;371(9626):1783–1789. doi: 10.1016/S0140-6736(08)60766-7. [DOI] [PubMed] [Google Scholar]
- 129.Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;3:CD002968. doi: 10.1002/14651858.CD002968.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Conn VS, Hafdahl AR, Mehr DR, et al. Metabolic effects of interventions to increase exercise in adults with type 2 diabetes. Diabetologia. 2007;50(5):913–921. doi: 10.1007/s00125-007-0625-0. [DOI] [PubMed] [Google Scholar]
- 131.Balducci S, Alessi E, Cardelli P, et al. Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: a meta-analysis: response to Snowling and Hopkins. Diabetes Care. 2007;30(4):e25. doi: 10.2337/dc06-2495. author reply e26. [DOI] [PubMed] [Google Scholar]
- 132.Betof AS, Dewhirst MW, Jones LW. Effects and potential mechanisms of exercise training on cancer progression: A translational perspective. Brain Behav Immun. 2013;(30 Suppl):S75–S87. doi: 10.1016/j.bbi.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jones LW, Liang Y, Pituskin EN, et al. Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. The oncologist. 2011;16(1):112–120. doi: 10.1634/theoncologist.2010-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: the St James Women Take Heart Project. Circulation. 2003;108(13):1554–1559. doi: 10.1161/01.CIR.0000091080.57509.E9. [DOI] [PubMed] [Google Scholar]
- 135.Mora S, Redberg RF, Cui Y, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the lipid research clinics prevalence study. JAMA. 2003;290(12):1600–1607. doi: 10.1001/jama.290.12.1600. [DOI] [PubMed] [Google Scholar]
- 136.Myers J, Prakash M, Froelicher V, et al. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 137.Ballard-Barbash R, Friedenreich CM, Courneya KS, et al. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104(11):815–840. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. 2011;29(6):726–732. doi: 10.1200/JCO.2010.31.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Richman EL, Kenfield SA, Stampfer MJ, et al. Physical activity after diagnosis and risk of prostate cancer progression: data from the cancer of the prostate strategic urologic research endeavor. Cancer Res. 2011;71(11):3889–3895. doi: 10.1158/0008-5472.CAN-10-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293(20):2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 141.Holick CN, Newcomb PA, Trentham-Dietz A, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(2):379–386. doi: 10.1158/1055-9965.EPI-07-0771. [DOI] [PubMed] [Google Scholar]
- 142.Meyerhardt JA, Giovannucci EL, Holmes MD, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24(22):3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 143.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24(22):3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 144.Meyerhardt JA, Ogino S, Kirkner GJ, et al. Interaction of molecular markers and physical activity on mortality in patients with colon cancer. Clin Cancer Res. 2009;15(18):5931–5936. doi: 10.1158/1078-0432.CCR-09-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Morikawa T, Kuchiba A, Yamauchi M, et al. Association of CTNNB1 (beta-catenin) alterations, body mass index, and physical activity with survival in patients with colorectal cancer. JAMA. 2011;305(16):1685–1694. doi: 10.1001/jama.2011.513. [DOI] [PMC free article] [PubMed] [Google Scholar]