Abstract
Retinoblastoma is the most common primary cancer of the eye in children. The incidence of second tumors in survivors of bilateral retinoblastoma and in survivors of unilateral retinoblastoma who presumably carry a germline RB1 mutation is documented. This paper describes the previously unrecognized association of sinonasal adenocarcinoma as a second malignancy in retinoblastoma survivors. We present three cases who received radiation therapy as a part of their treatment and developed sinonasal adenocarcinoma as a second malignancy. Sinonasal adenocarcinoma should be considered as a second malignancy in retinoblastoma survivors who present with vague sinus symptoms.
Keywords: Retinoblastoma, Sinonasal adenocarcinoma, second malignancy
INTRODUCTION
Retinoblastoma is the most common primary cancer of the eye in children. Although it represents only approximately 4% of childhood cancer and less than 1% of all human cancers, it is of widespread interest because the gene responsible for retinoblastoma, RB1, was the first cancer gene identified and cloned (in 1986) [1]. Retinoblastoma may present in two ways: hereditary (40%) and non hereditary (60%). RB1 and is located on the long arm of chromosome 13 [2]. In hereditary retinoblastoma, the mutated gene is presented in all somatic and germ cells of the patient. A retinoblastoma will develop when a subsequent somatic mutation inactivates the second allele in a retina cell [3,4].
The incidence of second tumors in survivors of bilateral retinoblastoma and in survivors of unilateral retinoblastoma who carry the RB1 mutation is well documented and has been reviewed extensively [3,4,5]. Among these, several studies have demonstrated that the use of external beam radiation therapy for the treatment of retinoblastoma can increase the risk significantly of subsequent tumors developing if given in the first year of life or at high doses. Nearly 95% of all children in the United States with retinoblastoma survive the primary disease. Approximately 0.5%-1% of those with bilateral disease go on to have second non-ocular tumors each year and approximately 25% of these patients die of these tumors by 50 years of age [5].
This paper summarizes the previously unrecognized association of sinonasal adenocarcinoma as a second malignancy in retinoblastoma survivors.
METHODS
We saw a patient in clinic who is a long term survivor of retinoblastoma and subsequently developed sinonasal adenocarcinoma as a second malignancy. We carried out a search in Pub med and Medline by using the phrases “retinoblastoma and second malignancies” and “retinoblastoma and sinonasal adenocarcinoma”. Our search did not reveal any reported cases. Hence we decided to report this unusual entity. We did a retrospective review of our database and the National Cancer Institute (NCI) database and found 2 other cases of sinonasal adenocarcinoma among the 277 cases of second malignancies in retinoblastoma survivors.
Case 1
Thirty year old male with a history of retinoblastoma of the left eye at the age of two weeks and a positive family history, treated with radiation therapy, presented initially with symptoms of runny nose and congestion to his primary physician. He was advised to use Saline nasal drops. Symptoms worsened and he developed difficulty breathing, epistaxis and headache. CT and MRI of maxillofacial area showed mass in the right nasal cavity extending through the cribriform plate, displacing the dura posteriorly and involvement of left side of the posterior nasopharynx and fossa of Rosenmüller on the right. Preoperative MRI showed 5.3 cm mass entering the body of sphenoid sinus, ethmoid air cells, nasal cavity, posterior nasopharynx and the anterior cranial fossa superiorly. Resection of mass was done. Pathology report showed sinonasal papillary adenocarcinoma, intermediate grade with moderate to marked tumor necrosis and abundant mitoses, positive for CK7 and CEA and tumor involved bone. Morphologic features of the tumor were suggestive intestinal type phenotype but the immunophenotype was consistent with non-intestinal type of sinonasal adenocarcinoma (non-ITAC). Post-operative MRI was consistent with a gross total resection. He was treated with proton beam therapy to a maximum dose of 66.6 CGE to the affected area. He is now disease free for 15 months post diagnosis.
Case 2
Sixty-eight year old white male with a history of bilateral retinoblastoma for which he was treated with radiation therapy dating to childhood presented to his local ENT for ‘sinus problems’. CT scan of the sinus was done which showed a 1×1.3 cm left nasal cavity soft tissue mass. Biopsy showed intestinal type sinonasal papillary adenocarcinoma. His past history was significant for soft tissue sarcoma of the knee and bladder cancer. He underwent orbital exenteration with free flap reconstruction followed by radiation to the site. He is now disease free 25 months post diagnosis.
Case 3
Forty-six year old white male with a history of bilateral retinoblastoma as an infant , treated with left eye enucleation and 4000cGy radiation to the right eye, presented with symptoms of clear nasal discharge for few weeks prior to diagnosis. MRI revealed a 14mm ×15mm right nasal polyp. Biopsy showed adenocarcinoma, non-intestinal type, moderately to poorly differentiated with neuroendocrine features. He underwent endoscopic right medial maxillectomy, total ethmoidectomy and sphenoidectomy. There was no residual tumor after the procedure. No radiation was administered. He is disease free 9 months post diagnosis.
DISCUSSION
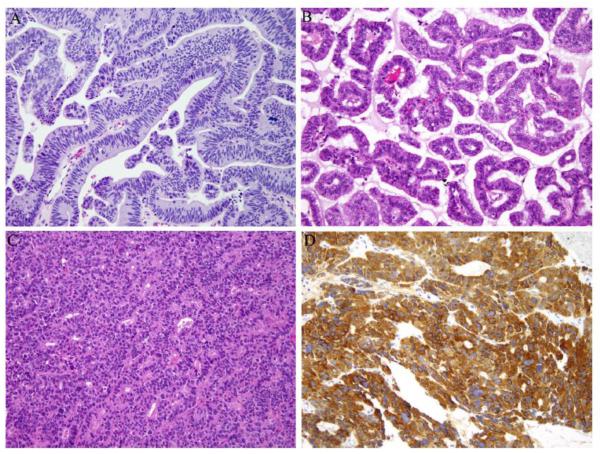
Sinonasal adenocarcinomas are rare tumors accounting for about 10-20% of all sinonasal malignancies. Broadly, these tumors are divided into salivary-type adenocarcinomas and non-salivary adenocarcinomas. Non-salivary adenocarcinomas are divided into intestinal and non-intestinal types. [6] In our series of 3 cases, two are intestinal type adenocarcinomas (ITAC) (Figure 1A-B) and one is a high-grade non-intestinal type adenocarcinoma (Figure 1C).
Fig. 1.
Sinonasal adenocarcinoma, intestinal-type, from patient 1 (A) and patient 2 (B) with colonic and papillary architecture, respectively. Sinonasal adenocarcinoma, high-grade nonintestinal-type. from patient 3 (C) with neuroendocrine features as evidenced by immunohistochemical staining with chromogranin (D). 200× magnification.
ITAC, as the name implies, morphologically resembles colonic or small intestinal adenocarcinoma or adenoma. Occupational exposure to wood or leather dust is an established risk factor [7]. Grossly, these tumors are often polypoid or fungating and may be hemorrhagic. Microscopically, several architectural patterns (papillary, colonic, solid, mucinous, mixed) with varying degrees of differentiation (well, moderate, poor) are recognized and have been used in subtyping and prognosticating ITAC. However, in general, ITAC has a poorer clinical outcome than non-ITAC and thus it is important to distinguish between the two [8]. In addition to morphology, immunohistochemistry is helpful in this aspect as ITAC is often positive for the intestinal markers CK20 and CDX-2 while non-ITAC is not. However, ITAC can also be positive for CK7 similar to non-ITAC.
Non-ITAC is an adenocarcinoma that neither shows minor salivary gland or intestinal-type features. There are no known etiologic factors. Non-ITAC is divided into low and high grade subtypes and it is crucial to do so as the former has a better prognosis [9]. Grossly, these tumors can be flat and invasive or exophytic. Microscopically, low-grade non-ITAC demonstrates a glandular or papillary architecture without much cytologic atypia. On the other hand high grade non-ITAC shows a more solid growth pattern with greater cellular pleomorphism, increased mitoses and necrosis. Additionally, our case of high-grade non-ITAC showed neuroendocrine features with positive immunohistochemical staining for chromogranin (Figure 1D) and synaptophysin.
Survivors of retinoblastoma with RB1 germline mutations are at increased risk for development of a variety of additional non-ocular neoplasms, including osteogenic sarcoma of the skull and long bones, soft tissue sarcomas, cutaneous melanomas and pineoblastomas [5,10]. Among patients with hereditary retinoblastoma patients surviving more than 40 years after retinoblastoma diagnosis, more than half of the absolute excess risk could be attributed to epithelial cancers [4]. External beam radiation therapy is a contributory factor in the development of additional tumors in retinoblastoma survivors [11,12, 13]. Our patients received radiation therapy as a part of their treatment and developed sinonasal adenocarcinoma as a second malignancy. Although an increased risk for sinonasal cancers has been reported following radiotherapy for retinoblastoma in a large series of Rb survivors (10), the histology of these tumors was not provided. Additionally, there have been individual case reports of leiomyosarcoma [14] and neuroendocrine sinonasal tumors following radiation for retinoblastoma [15]. However, adenocarcinoma of the sinonasal cavity has not been previously reported.
Among irradiated hereditary retinoblastoma survivors, the standardized mortality ratio for subsequent malignant neoplasms was 2.2 times higher for those who were irradiated at 12 months of age or younger that for those irradiated at older ages [16,17]. All our patients were irradiated as children. For radiation induced tumors a long latency period is characteristic. For solid tumors this period is presumed to be at least 10 years [4]. All our patients developed adenocarcinoma more than 10 years after being treated for retinoblastoma with radiation therapy. Occupational exposure to hardwood dust has been associated with intestinal type sinonasal adenocarcinoma[18]. However, none of our patients had such an occupational exposure. Survivors of childhood cancers other than retinoblastoma are also at an increased risk for second malignancies due to treatment [19]. Radiation induces 1.8 neoplasms per million person-years per rad in long term childhood cancer survivors treated with radiation for their primary tumors [20]. Excluding retinoblastoma, Hodgkin’s lymphoma and Wilm’s tumor are the next most common childhood tumors that are associated with second malignancies. For all of these diseases, survivors, especially those who receive radiation as a part of therapy, may develop solid tumors as second malignancy [19].
Sinonasal adenocarcinomas have not been reported as a second malignancy in retinoblastoma survivors. All three cases presented with vague sinus symptoms. Thus, in such cases, the patient, family and the primary physician must be aware that these otherwise innocent symptoms may be potentially dangerous and hence early detection and a better outcome may be possible. Hereditary retinoblastoma survivors are at a risk of developing multiple cancers through their lives. Therefore, lifelong close vigilance is needed. It is a challenge to manage retinoblastoma survivors whose second malignancy requires radiation therapy for disease control, knowing that that may increase the risk of a subsequent malignancy.
Acknowledgement
This research was supported in part by the Intramural Research Program of the National Institutes of Health, the National Cancer Institute, Division of Cancer Epidemiology and Genetics.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Abramson DH. Retinoblastoma in the 20th Century: Past Success and Future Challenges. The Weisenfeld Lecture Invest Ophthalmol Vis Sci. 2005;46:2684–2691. doi: 10.1167/iovs.04-1462. [DOI] [PubMed] [Google Scholar]
- 2.Friend SH, Horowitz JM, Gerber MR, et al. Deletions of a DNA sequence in retinoblastoma’s and mesenchymal tumors: Organization of the sequence and its encoded protein. Proc Natl Acad Sci USA. 1987;84:9059–63. doi: 10.1073/pnas.84.24.9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunkel IJ, Gerald WL, Rosenfield NS, et al. Outcome of patients with a history of bilateral retinoblastoma treated for a second malignancy. The Memorial Sloan Kettering experience. Med Pediatr Oncol. 1998;30:59–62. doi: 10.1002/(sici)1096-911x(199801)30:1<59::aid-mpo14>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Marees T, Moll AC, Imhof SM, et al. Risk of second malignancies in survivors of retinoblastoma: More than 40 years of follow-up. J Natl Cancer Inst. 2008;100:1771–1779. doi: 10.1093/jnci/djn394. [DOI] [PubMed] [Google Scholar]
- 5.Abramson DH, Melson MR, Dunkel IJ, et al. Third (fourth and fifth) nonocular tumors in survivors of retinoblastoma. Ophthalmology. 2001;108:1868–1876. doi: 10.1016/s0161-6420(01)00713-8. [DOI] [PubMed] [Google Scholar]
- 6.Leivo I. Update on Sinonasal Adenocarcinoma: Classification and Advances in Immunophenotype and Molecular Genetic Make-Up. Head Neck Pathol. 2007;1:38–43. doi: 10.1007/s12105-007-0025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes L, Eveson J, Reichart P, et al. Pathology and Genetics of Head and Neck Tumors. IARC; Lyon: 2005. pp. 20–23. [Google Scholar]
- 8.Wenig Bruce M. Atlas of Head and Neck Pathology. Saunders/Elsevier; Philadelphia, Pa.: 2008. pp. 121–127. [Google Scholar]
- 9.Stelow Edward B, Mills SE, et al. Adenocarcinoma of the Upper Aerodigestive Tract. Advances in Anatomic Pathology. 2010;17:262–69. doi: 10.1097/PAP.0b013e3181e3bf80. [DOI] [PubMed] [Google Scholar]
- 10.Kleinerman RA, Tucker MA, Tarone RE, et al. Risk of New Cancers After Radiotherapy in Long-Term Survivors of Retinoblastoma: An Extended Follow-Up. J Clin Oncol. 2005;23:2272–2279. doi: 10.1200/JCO.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 11.Mohney BG, Robertson DM, Schomberg PJ, et al. Second nonocular tumors in survivors of heritable retinoblastoma and prior radiation therapy. Am J Ophthalmol. 1998;126:269–277. doi: 10.1016/s0002-9394(98)00146-9. [DOI] [PubMed] [Google Scholar]
- 12.Schefler AC, Kleinerman RA, Abramson DH. Genes and environment: Effects on the development of second malignancies in retinoblastoma survivors. Expert Rev Ophthalmol. 2008;3:51–61. doi: 10.1586/17469899.3.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowe LD, Lane R, Snow JB, Jr., et al. Adenocarcinoma of the ethmoid following radiotherapy for bilateral retinoblastoma. Laryngoscope. 1980;90(1):61–69. doi: 10.1288/00005537-198001000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Fitzpatrick SG, Woodworth BA, Montiero C, Makary M. Nasal sinus leiomyosarcoma in a patient with history of non-hereditary unilateral treated retinoblastoma. Head and Neck Pathol. 2011;5:57–62. doi: 10.1007/s12105-010-0207-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franchi A, Sardi I, Cetica V, et al. pediatric sinonasal neuroendocrine carcinoma after treatment of retinoblastoma. Human Path. 2009;40:750–755. doi: 10.1016/j.humpath.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Chu-Ling Yu, Tucker MA, Abramson DH, et al. Cause-Specific Mortality in Long-Term Survivors of Retinoblastoma. J Natl Cancer Inst. 2009;101:581–591. doi: 10.1093/jnci/djp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong FL, Boice JD, Jr, Abramson DH, et al. Cancer incidence after retinoblastoma: radiation dose and sarcoma risk. JAMA. 1997;278:1262–7. doi: 10.1001/jama.278.15.1262. [DOI] [PubMed] [Google Scholar]
- 18.Leclerc A, Martinez Cortes M, Gérin M, et al. Sinonasal cancer and wood dust exposure: results from a case-control study. Am J Epidemiol. 1994;15(140):340–9. doi: 10.1093/oxfordjournals.aje.a117256. [DOI] [PubMed] [Google Scholar]
- 19.Meadows AT, Freidman DL, Neglia JP, et al. Second neoplasms in survivors of childhood cancer: Findings from the childhood cancer survivor study cohort. J Clin Oncol. 2009;27:2356–2362. doi: 10.1200/JCO.2008.21.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li FP, Cassady JR, Jaffe N, et al. Risk of second tumors in survivors of childhood cancer. Cancer. 1975;35:1230–5. doi: 10.1002/1097-0142(197504)35:4<1230::aid-cncr2820350430>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]