Abstract
Background
Patients with systemic lupus erythematosus (SLE) are at increased risk of herpes zoster (HZ). Although a vaccine for HZ has been FDA approved, its use in immunocompromised individuals remains controversial because it is a live-attenuated virus vaccine. We performed a pilot study of the immunogenicity of Zostavax® in SLE patients.
Methods
Ten SLE patients and 10 controls ≥50 years old participated in this open label vaccination study. All were seropositive for varicella zoster virus (VZV). SLE patients were excluded for SLEDAI>4, use of mycophenolatemofetil, cyclophosphamide, biologics, or >10 mg prednisone daily. Follow-up visits occurred at 2, 6, and 12 weeks. Clinical outcomes included the development of adverse events, particularly HZ or vesicular lesions, and SLE flare. Immunogenicity was assessed with VZV-specific IFN-γ producing ELISPOT assays and with antibody concentrations.
Results
All subjects were women. SLE patients were slightly older than controls (60.5 vs. 55.3 years, p<0.05) Median baseline SLEDAI was 0 (range 0–2) for SLE patients. No episodes of HZ, vesicular rash, serious adverse events, or SLE flares occurred. Three injection site reactions occurred in each group: mild erythema or tenderness. The proportion of subjects with a >50% increase in ELISPOT results following vaccination was comparable between both groups, although absolute SLE responses were lower than controls. Antibody titers increased only among controls following vaccination (p<0.05).
Conclusions
Zostavax vaccination yielded a measurable immuneresponse in this cohort of mild SLE patients on mild-moderate immunosuppressive medications. No herpetiform lesions or lupus flares were seen in this small cohort of patients.
Keywords: Systemic lupus erythematosus, herpes zoster, vaccine, Zostavax, infection, clinical trial
Introduction
Herpes Zoster (HZ) is caused by reactivation of latent varicella zoster virus (VZV) that usually occurs decades following initial exposure. The rash usually lasts 7–10 days, during which the virus may be transmissible through airborne particles. Complications include post herpetic neuralgia, bacterial superinfection, and disseminated disease with meningoencephalitis. HZ incidence increases with age, presumably as cell mediated immunity (CMI) naturally wanes. Studies of VZV-CMI in unvaccinated individuals estimate a 2.7%–3.9% decrease with each year of age after 60, whereas VZV-specific antibody levels remain essentially unchanged [1].
Several studies have shown that HZ is more common and can present with more severe manifestations among patients with systemic lupus erythematosus (SLE) [2–12]. Some studies suggest that the incidence may be increased in SLE even in the absence of immunosuppressive medications [3,9] and during periods of relative disease quiescence [13], possibly because of an inherent deficiency in cell mediated immunity associated with the disease process itself.
The HZ vaccine (Zostavax®, Merck) is a live, attenuated version of the Oka/Merck strain of VZV. It has at least 14-times the potency of Varivax® (primary varicella infection). Zostavax was licensed in the United States and Europe in 2006 for adults aged ≥ 60 years based on a large Phase III clinical trial that showed a reduction in the incidence of HZ by 51.3% [14]. It is now licensed for individuals ≥ 50 years old [15]. An immunological sub-study of the Shingles Prevention Study demonstrated a clear association between increased cell mediated immunity and protection from the development of HZ, although a threshold level could not be identified [16]. In contrast, VZV-specific antibody titers were not associated with protection.
The CDC Advisory Committee on Immunization Practices (ACIP) has published guidelines regarding use of the Zostavax vaccine [17] stating that vaccination should be safe for persons taking moderate doses of prednisone, methotrexate, or azathioprine for autoimmune diseases; however, this is not based upon any evidence of safety in these populations.
Because Zostavax is a live-attenuated vaccine, theoretical concerns remain about the safety of vaccination in immunocompromised patients, including SLE. To date, there are no published data regarding the tolerability and immunogenicity of Zostavax in subjects with autoimmune diseases; therefore, its use in this population remains the subject of controversy despite documented increased risk of HZ reactivation. Indeed, current guidelines from EULAR and the ACR recommend against Zostavax in many individuals with autoimmune diseases [18,19].
We performed a pilot study to ascertain preliminary estimates of immunogenicity to Zostavax vaccination in SLE patients compared to healthy control subjects.
Materials and Methods
Study Design
We performed a pilot, open -label, prospective 12-week study of commercially available Zostavax in 10 subjects with SLE and a comparison cohort of 10 healthy subjects [ClinicalTrials.gov ID:NCT01474720]. SLE subjects were recruited from the Oklahoma Rheumatic Disease Research Center (NIH AR053483) and clinics. Healthy controls were recruited from participants in the Oklahoma Immune Cohort (NIH GM103510). The study received local IRB approval prior to initiation, and all subjects provided written informed consent.
Inclusion criteria included: age ≥ 50 years; serologic evidence of primary varicella infection; diagnosis of SLE according to 1992 Modified ACR criteria [20] or healthy subject. SLE patients were required to have stable, mild disease activity defined by a clinical Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) [21] score ≤ 4 (clinical SLEDAI excluded complement levels or double stranded DNA antibodies); acceptable immunosuppressive medications must be stable for 60 days prior to screening and are limited to prednisone ≤ 10 mg daily; hydroxychloroquine ≤ 6.5 mg/kg daily; methotrexate ≤ 20 mg weekly; or azathioprine ≤ 150 mg daily. Other immunosuppressive or biologic medications, including mycophenolatemofetil (MMF), were excluded from the study because they were not addressed in the CDC guidelines for Zostavax vaccination. Changes in medications were permitted only in situations where increased immunosuppression may be needed to treat disease flare.
Exclusion criteria included: any prior receipt of a VZV-containing vaccine (primary varicella or zoster); history of HZ reactivation within 5 years of screening; receipt of any live vaccine within 6 weeks or recombinant vaccine within 2 weeks of enrollment; known hepatitis B or C virus or HIV infection; diabetes mellitus; malignancy within 5 years of screening; contraindication to use of famciclovir; active lupus nephritis or cerebritis; proteinuria >1.5 g/day; serum creatinine >1.5 mg/dL,; mycophenolatemofetil within 3 months of screening; cyclophosphamide within 6 months or rituximab within 2 years of screening. Healthy subjects were excluded if they were taking corticosteroids or other immunosuppressive medications for any reason.
Study assessments
At baseline, subjects underwent physical examination, review of concomitant medications, and SLE disease activity was assessed utilizing the SLEDAI and the SLEDAI flare index [22]. Peripheral blood and urine was obtained for clinical assessments; plasma and PBMCs were collected for assays of VZV-specific immune response. Following baseline study assessments, all subjects received a single subcutaneous dose of commercially-purchased Zostavax (Merck) (≥19,400 plaque forming units) according to manufacturer’s directions. Subjects were provided phone numbers and instructed to call for any signs of rash or vesicles near the injection site. Follow-up assessments occurred at weeks 2, 6, and 12. At each visit, subjects were assessed for adverse events with particular focus on injection site reactions and development of vesicular or bulliformlesions around the injection site. All other adverse events and medication changes were recorded. SLEDAI and the SLEDAI flare index were assessed for all SLE participants. Peripheral blood and urine were collected for clinical assessments. Plasma and PBMCs were processed and frozen for batched analyses of VZV-specific immune response.
Preparation of frozen PBMCs
PBMCs from each study time point were isolated and stored in liquid nitrogen. Cell samples from all visits for a lupus case and a control subject were analyzed on the same day. Frozen cells were suspended in 37°C media supplemented with 25 U/ml benzonase (Sigma). Cells were centrifuged at 1000 rpm for 8 minutes, washed twice. The viable cell concentration (cells/ml) was determined and the concentration was adjusted to 2×105 cells/ml and 1×106 cells/ml.
IFN-γ ELISpot assay for VZV response (ELISPOT Human IFNγ Set, BDBiosciences)
Multi-screen IP membrane plates were prepared per manufactures instructions. On day of assay, fresh media was added to each well and the plates were incubated at room temperature for 2 hours. PHA-M (Phytohemagglutinin-M) and antigens (Vero uninfected cell extract and VZV-Vero inactivated cell extract (Advanced Biotechnologies Inc.)) used for stimulation were prepared in media. Pre-incubation media was removed from the wells, 100 μl of PHA-M (50 μg/ml) or antigen (30 μg/ml) was added to the appropriate wells. One hundred μl of the 2×105 cells/ml cell suspension was plated for the PHA-M positive control wells, and 1×106 cells/ml was plated for the antigen wells. The plate was then incubated at 37°C, in a 5% CO2 and humidified incubator for 18 hours, then washed and developed according to manufacturer’s instructions. Spots were allowed to develop for 15–20 minutes, the wells washed with water, then air-dried at room temperature overnight. Spots were enumerated manually using an ELISPOT plate reader. Each sample was run in duplicate. The results are a comparison of the median number of spot forming units (SFU) of duplicate wells between SLE patients to control subjects at the different time points.
VZV-specific IgG antibody assessments
Anti-VZV IgG reactivity was assessed using the commercial Varicella-Zoster Virus IgG ELISA II kit (Wampole) according to manufacturer’s instructions. Positive and negative controls and calibrators were included in the kits. Results were assessed by determining a “cut-off OD value” for the positive sample using the following formula (CF x mean OD(calibrator)= OD(cut-off), where CF is the correction factor provided by the manufacturer for each lot of the kit. An “index” value (or OD ratio) for each sample is then calculated by dividing the OD(sample) by the OD(cut-off). The sample is considered negative if the index value is ≤ 0.90, equivocal if the value varies between 0.91 and 1.09 and positive for IgG antibodies against VZV if >1.1. Higher index values semi-quantitatively reflect increased anti-VZV titers.
Study design and Endpoints
The study was designed to provide preliminary experience with Zostavax vaccination in a small cohort of SLE patients compared to healthy subjects in order to obtain estimates of tolerability and immunogenicity. Clinical outcomes included the development of lesions suspicious for VZV or clinical HZ following vaccination; development of vaccine-related adverse events, including injection site reactions; and flare or significant increase in SLEDAI among SLE patient s. Immunogenicity endpoints included increase in VZV-specific IFN-γ producing ELISPot spot forming units and change in anti-VZV IgG concentrations following vaccination.
Statistical analyses
Because there is no previous data on immunogenic response to Zostvax in SLE populations from which compute power estimates, sample size was determined by feasibility of recruitment at a single site within a reasonable time frame. One of the goals of the study is to derive initial estimates of immunogenic response to power future studies. Therefore, the study was designed without statistical comparisons between groups. Descriptive results were presented as mean (standard deviation) or median (range) as appropriate. Comparisons between cell-mediated or antibody response to vaccination were performed using the Mann-Whitney test with significance set at α=0.05.
In order to determine potential relationships between SLE-related immunological variables and cell mediated response, Spearman’s correlation coefficients were determined individually for total leukocyte count, absolute lymphocyte count, Complement 3 and Complement 4, and VZV-specific or PHA-mediated ELISPOT results. This analysis was performed for SLE patients only.
Results
Baseline Characteristics of Study Population
Ten SLE patients and 10 healthy subjects were recruited from January through March 2012. SLE patients were slightly older than healthy subjects (60.5 vs. 55.3 years, p=0.03) (Table 1). Four SLE patients and two controls had a clinical history of HZ prior to enrollment. Seven SLE patients were receiving hydroxychloroquine; two were taking low dose methotrexate; and four were taking low dose prednisone. The median SLEDAI score at baseline was 0 (range 0–2). By entry criteria, no control subjects were receiving corticosteroids or other immunosuppressant medications. A side from age, there were no statistically significant differences between baseline demographics or history of HZ between the groups.
Table 1.
Baseline Demographic and Clinical Characteristics of Participants
| SLE | Healthy | |
|---|---|---|
| n | 10 | 10 |
| % female | 100 | 100 |
| Age, mean (SD) | 60.5 (5.4)* | 55.3 (4.2) |
| Caucasian, n | 7 | 7 |
| African American, n | 3 | 3 |
| h/o shingles, n | 4 | 2 |
| Taking prednisone, n | 4 | 0 |
| mean daily dose | 6.9 mg | - |
| Taking HCQ, n | 7 | 0 |
| Taking MTX, n | 2 | 0 |
| Baseline SLEDAI, median (range) | 0 (0–2) | - |
p<0.05 compared to healthy
Clinical Outcomes
All subjects completed the 12-week study. No serious adverse events, hospitalizations, episodes of HZ, or SLE flares occurred (Table 2). No immunosuppressive or other medication changes occurred during the 12-week study. Three patients in each group experienced injection site reactions: all were mild and consisted of self-limited erythema and/or tenderness. No vesicular or herpetiformlesions occurred, nor were any systemic complaints (fever, myalgias) reported. The median SLEDAI ranged from 0–1 (range 0–3) over the 12 week study (p=ns). In five subjects, SLEDAI did not change over time. In all other cases, SLEDAI changed by only 2 points during the study, all reflecting minor changes in complement levels that crossed thethreshold of the lower limit of normal. These modest changes were considered normal variations of stable disease and were not deemed clinically significant.
Table 2.
Adverse events and SLE disease activity following Zostavax® vaccination
| SLE | Healthy | |
|---|---|---|
| Herpes Zoster, n | 0 | 0 |
| Serious Adverse Event, n | 0 | 0 |
| ISR (any), n | 3 | 3 |
| Erythema, tenderness, n | 3 | 3 |
| Vesicular lesions, n | 0 | 0 |
| 2 week SLEDAI§ | 0 (0–3) | - |
| 6 week SLEDAI§ | 1 (0–3) | - |
| 12 week SLEDAI§ | 0 (0–3) | - |
| SLE flare, n | 0 | - |
median (range)
ISR= injection site reaction
SLEDAI=Systemic Lupus Erythematosus Disease Activity Index
Studies of immunogenicity
Cell mediated Response
At each time point, median VZV-specific ELISpot results were lower in SLE patients compared to controls (Figure 1A). The proportion of subjects with 50% increase in the frequency of spot forming units over baseline was similar in SLE patients and controls (63% SLE vs. 60% controls at 2 weeks; 44% SLE vs. 56% controls at 6 weeks; and 44% in both SLE and controls at 12-weeks post vaccination). In order to determine if the difference in VZV-specific cell mediated response was a function of a generally depressed cellular immunity in SLE patients, IFN-γ ELISPot results following stimulation with PHA were compared between groups at each time point (Figure 1B). Among control subjects, the interquartile range of spot forming units was similar at all time points, and did not change substantially following Zostavax vaccination. Except for the six-week time point where SLE patients had statistically lower median VZV-specific results (p=0.006), differences between SLE patients and controls were not clinically or statistically significant, and fewer than 20% of subjects in either group experienced a 50% increase in PHA-mediated response at any time following vaccination.
Figure 1.
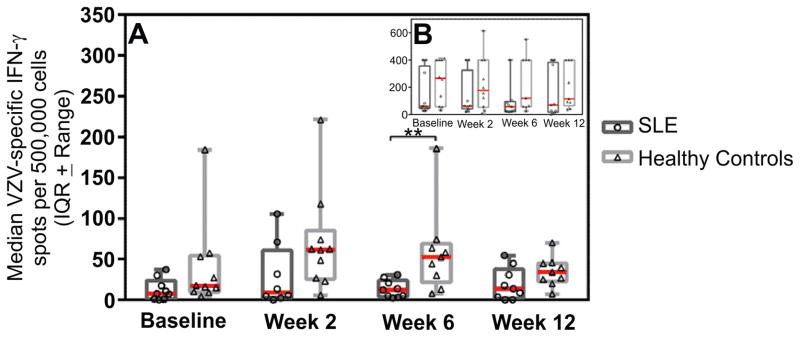
Panel A, number of IFN-γ ELISPot spot forming units with VZV stimulation by time point for SLE patients and control subjects. Inset panel B displays the number of IFN-γ ELISPot spot forming units with PHA stimulation by time point for SLE patients and control subjects. The median number of spots is displayed as a red bar with the interquartile range ± range graphed. Statistical significance was determined using a Mann-Whitney test with α=0.05 and is represented by **.
Humoral response
Although not protective, VZV-specific IgG concentrations were quantitated at the baseline and at each follow-up visit (Figure 2). Values were statistically increased from baseline at all time points among healthy adults, but no statistical significance was seen in change of IgG concentrations among SLE patients over time. By 12 weeks, fewer than 25% of subjects in either group had concentrations that were 50% higher than baseline levels.
Figure 2.
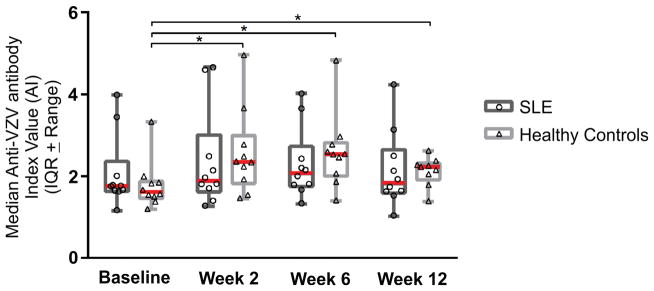
Median (Interquartile Range) anti-VZV IgG Index Value at each time point for SLE patients and control subjects. Statistical significance was determined using a Mann-Whitney test with α=0.05 and is represented by *.
Correlations between leukocyte count or complement and CMI
When all study visits were combined, a moderate positive correlation were seen between total leukocyte count, and VZV-specific ELISPOT results (r=0.59, p=0.02). At the baseline visit and 2 week visit, total leukocyte count, but not absolute lymphocyte count, had a stronger positive correlation with VZV-specific cell mediated immunity (r=0.78, p=0.02 and r=0.77, p=0.02 respectively), but this pattern was not seen at 6 or 12 weeks. At the baseline visit only, C3 level was strongly positively correlated with VZV-specific ELISPOT results (r=0.82, p=0.007). And at the 2 week visit only, C4 was positively correlated (r=0.71, p=0.047). No significant correlations were noted between any of the variables and PHA-mediated ELISPOT results.
Discussion
We conducted a prospective open-label pilot study of a commercially available live-attenuated zoster vaccine in stable SLE patients receiving no more than moderate immunosuppressive medications. The goal of this study was to obtain preliminary data on the short term safety, tolerability, and immunogenicity of Zostavax vaccine in a small cohort of SLE patients compared to healthy control subjects. Per licensure, all subjects were ≥ 50 years old. We did not identify any episodes of HZ or vesicular lesions at the injection site within 12 weeks following vaccination in any participant. Injection site reactions, consisting of mild erythema and tenderness, were seen at similar frequency in SLE patients and controls. Although VZV-specific cell mediated responses were diminished in SLE patients compared to controls, similar proportions of subjects increased responses by >50% following vaccination. Anti-VZV IgG concentrations were additionally similar between SLE patients and controls at each time point. We found only minimal changes in disease activity over the 12 weeks following vaccination (all increases in SLEDAI were from minor changes in complement levels that crossed the lower limit of normal), none of which were considered clinically significant or deemed to be disease flares. Immunosuppressive medications were not increased or added during the study.
When considering the risk-to-benefit analysis of vaccination in individuals with underlying autoimmune diseases including SLE, several concerns arise: the foremost being safety of vaccination. Particularly for live-attenuated virus vaccines, there is a concern about causing direct infection from the vaccine-strain of the virus. Additional safety considerations include the potential of causing a flare of underlying disease due to generalized immune stimulation in response to vaccination. These potential risks need to be balanced against the risk in the population of developing HZ if unvaccinated, as well as the efficacy of the vaccine to induce protection following vaccination.
Cumulative evidence has identified a nearly 10-fold increased risk of HZ among SLE patients compared to healthy individuals, and that elevated rates are seen in SLE patients at young ages [3–10,23]. Although rarely life threatening, HZ is associated with significant pain and associated morbidity despite early institution of antiviral therapy [24, 25], and may lead to disruption or discontinuation of otherwise necessary immunosuppressant medications. Given that the risk of HZ in SLE patients of all ages may be similar to or supersede the risk seen in elderly immunocompetent individuals for whom the vaccine is recommended, the study of the safety and efficacy of Zostavax vaccine is of high relevance. If found to be well tolerated, implementation of routine Zostavax administration to SLE patients may have an important impact on the disease experience by reducing the burden of comorbid HZ.
Specific guidelines about the use of Zostavax in SLE patients have been lacking, largely due to a theoretical concern of vaccine-induced infection as well as the lack of clinical or experimental data upon which to base recommendations [18,19].
We sought to minimize risk of vaccine-induced de novo HZ by confirming VZV seropositivity prior to vaccination in all subjects, and by restricting immunosuppressive medication use to those determined to be acceptable according to published guidelines. Within this restricted SLE population, no HZ-related reactions were identified.
Several limitations to this study need to be addressed. The small sample size is perhaps the greatest limitation. Others include the strict exclusion criteria and the short period of observation following vaccination. This pilot study was designed to assess Zostavax vaccination in lower-risk SLE patients for whom vaccination might be considered under current guidelines. The study design was not powered to perform meaningful statistical analyses. With the preliminary estimates of immunogenicity that have been established with this study, studies in SLE patients with expanded inclusion and restricted exclusion criteria can now be considered. Because the risk of HZ is increased even in adolescent and young adult SLE patients, the study of the safety and efficacy of vaccination in a wider age range are appropriate. Similarly, many patients are receiving long-term immunosuppressant therapy with MMF, which has been identified as an independent risk factor for HZ [10], and this group requires further study. The short time of follow-up allowed for close observation of participants during the time of highest risk for vaccine-induced HZ, and early estimates of the immune response.
Future, more definitive, studies will require a larger sample size, an unvaccinated control group, and longer term follow-up to assess the role of Zostavax in preventing episodes of HZ in this high risk population. Widespread vaccination among SLE patients should still proceed with caution, and preferably in the setting of controlled studies.
Acknowledgments
Funding source: This project was supported by GM103510, GM103456, AR053483, and the Autoimmunity Centers of Excellence, AI082714. Oklahoma Medical Research Foundation has received funding from the National Institutes of Health (NIH) to support an Institutional Development Award (IDeA) Center of Biomedical Research Excellence. The IDeA program builds research capacities in states that historically have had low levels of NIH funding by supporting basic, clinical and translational research; faculty development; and infrastructure improvements.
Footnotes
Conflicts of interest: none
ClinicalTrials.gov ID: NCT01474720
References
- 1.Levin MJ, Oxman N, Zhang JH, Johnson GR, Stanley H, Hayward AR, et al. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis. 2008;197:825–35. doi: 10.1086/528696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakravarty EF. Viral infection and reactivation in autoimmune diseases. Arthritis Rheum. 2008;58:2949–57. doi: 10.1002/art.23883. [DOI] [PubMed] [Google Scholar]
- 3.Park HB, Kim KC, Park JH, Kang TY, Lee HA, Kim TH, et al. Association of reduced CD4 T cell responses specific to varicella zoster virus with high incidence of herpes zoster in patients with systemic lupus erythematosus. J Rheumatol. 2004;31:2151–5. [PubMed] [Google Scholar]
- 4.Pope JE, Krizova A, Ouimet JM, Goodwin JL, Lankin M. Close association of herpes zoster reactivation and systemic lupus erythematosus (SLE) diagnosis: Case-control study of patients with SLE or noninflammatory musculoskeletal disorders. J Rheumatol. 2004;31:274–9. [PubMed] [Google Scholar]
- 5.Wolfe F, Michaud K, Chakravarty EF. Rates and predictors of herpes zoster in patients with rheumatoid arthritis and non-inflammatory musculoskeletal disorders. Rheumatology. 2006;45:1370–5. doi: 10.1093/rheumatology/kel328. [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa O, Abe M, Miyachi Y. Herpes zoster in Japanese patients with systemic lupus erythematosus. Clin Exp Dermatol. 1999;24:327–328. doi: 10.1046/j.1365-2230.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- 7.Manzi S, Kuller LH, Kutzer J, Pazin GJ, Sinacore J, Medsger TA, Jr, Ramsey-Goldman R. Herpes zoster in systemic lupus erythematosus. J Rheumatol. 1995;22:1254–1258. [PubMed] [Google Scholar]
- 8.Kahl LE. Herpes zoster infections in systemic lupus erythematosus: risk factors and outcome. J Rheumatol. 1994;21:84–86. [PubMed] [Google Scholar]
- 9.Moutsopoulos HM, Gallagher JD, Decker JL, Steinberg AD. Herpes zoster in patients with systemic lupus erythematosus. Arthritis Rheum. 1978;21:789–802. doi: 10.1002/art.1780210710. [DOI] [PubMed] [Google Scholar]
- 10.Chakravarty EF, Michaud K, Katz R, Wolfe F. Increased incidence of herpes zoster among patients with systemic lupus erythematosus. Lupus. 2013;22:238–44. doi: 10.1177/0961203312470186. [DOI] [PubMed] [Google Scholar]
- 11.Chen HH, Chen YM, Chen TJ, Lan JL, Lin CH, Chen DY. Risk of herpes zoster in pateints with systemic lupus erythematosus: a three-year follow-up study using a nationwide population-based cohort. Clinics (Sao Paulo) 2011;66:1177–82. doi: 10.1590/S1807-59322011000700009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hata A, Kuniyoshi M, Ohkusa Y. Risk of herpes zoster inpatients with underlying diseases: a retrospective hospital-based cohort study. Infection. 2011;39:537–44. doi: 10.1007/s15010-011-0162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borba EF, Ribeiro AC, Martin P, Costa LP, Guedes LK, Bonfa E. Incidence, risk factors, and outcomes of herpes zoster in systemic lupus erythematosus. J Clin Rheum. 2010;16:119–22. doi: 10.1097/RHU.0b013e3181d52ed7. [DOI] [PubMed] [Google Scholar]
- 14.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. NEJM. 2005;352:2271–72. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 15. [Accessed 1/3/13]; http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm248608.htm.
- 16.Weinberg A, Chang JH, Oxman MN, Johnson GR, Hayward AR, Caulfield MJ, et al. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis. 2009;200:1068–77. doi: 10.1086/605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. 2008;57:RR-5. [PubMed] [Google Scholar]
- 18.vanAssen S, Agmon-Levin N, Ilkayam O, Cervera R, Doran MF, Dougados M, et al. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2011;70:414–22. doi: 10.1136/ard.2010.137216. [DOI] [PubMed] [Google Scholar]
- 19.Curtis JR, Saag KG, Winthrop K. Update on Herpes Zoster (Shingles) vaccine for autoimmune disease patients. American College of Rheumatology Hotline. 2012 Sep 21; [Google Scholar]
- 20.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 21.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The committee on prognosis studies in SLE. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 22.Petri M, Buyon J, Kalunian KC, Urowitz MB, Strand V, Merrill J, et al. Revision of the SELENA flare index. Arthritis Rheum. 2009;60:S339. [Google Scholar]
- 23.Lee PP, Lee TL, Ho MH, Wong WH, Lau YL. Herpes zoster in juvenile-onset lupus erythematosus: incidence, clinical characteristics and risk factors. Pediatr Infect Dis J. 2006;25:728–732. doi: 10.1097/01.inf.0000226841.03751.1f. [DOI] [PubMed] [Google Scholar]
- 24.Drolet M, Brisson M, Levin MJ, Schmader KE, Oxman MN, Johnson RW, et al. A prospective study of the herpes zoster severity of illness. Clin J Pain. 2010;26:656–66. doi: 10.1097/AJP.0b013e3181eef686. [DOI] [PubMed] [Google Scholar]
- 25.Wang TJ, Hu CC, Lin HC. Increased risk of anterior uveitis following herpes zoster: a nationwide population-based study. Arch Ophthalmol. 2012;130:451–5. doi: 10.1001/archophthalmol.2011.357. [DOI] [PubMed] [Google Scholar]


