Abstract
Bone is the most common site of metastasis of breast cancer, affecting most women with advanced disease. Procollagen type I N-terminal propeptide (P1NP), osteocalcin, CTX, and IL-6 are markers of bone turnover. Our objective was to determine whether serum levels of these proteins have clinical utility as predictors of breast cancer metastasis to bone. Blood was collected before treatment from 164 patients with stage I–III breast cancer from September 2001 to December 2008. erum levels of P1NP, CTX, IL-6, and OC were measured using an automated immunoassay system. Correlations of the levels of these markers with time to bone metastasis development and with overall survival (OS) rate were assessed using Cox proportional hazards regression analysis and the Kaplan–Meier method. Fifty-five patients with stage I–III disease at the time of blood sample collection subsequently experienced metastasis to bone. A baseline P1NP level of at least 75 ng/mL predicted increased risk of bone metastasis (hazard ratio, 2.7 [95 % confidence interval, 1.2–6.0]; P = 0.031) and a poor OS rate (P = 0.031). Serum P1NP levels at or above 75 ng/mL correlate with a short time to development of bone metastasis and low overall survival in patients with stage I–III breast cancer.
Keywords: Breast neoplasm, Bone metastasis, Tumor marker, P1NP, Prognosis
Introduction
Breast cancer accounts for almost 25 % of all new cancer cases diagnosed in the United States each year [1]. Bone is the most common site of breast cancer metastases, affecting more than 50 % of women with advanced disease [2, 3]. Although therapies for bone metastases of breast cancer are available, patients still experience significant morbidity and mortality, with a median life expectancy of 4 years after diagnosis [4].
As with other bone-related diseases, development of bone metastasis of breast cancer is a multistep process-dependent on a balance between bone resorption and bone formation. In general, this process consists of detachment of tumors cells from the primary tumor, migration, and attachment of tumor cells to the bone marrow, cellular invasion of the bone matrix, and interactions between the tumor and bone matrix cells (e.g., seed and soil theory) with resultant development of osteolytic lesions [5]. Researchers have proposed that development of bone metastasis in breast cancer patients is driven by proteins released by tumor cells and the bone microenvironment that serve as “homing markers,” directing tumor cells to bone.
Over the past 20 years, researchers have identified more than a dozen proteins that may be considered biomarkers of bone metastasis in preclinical models of breast cancer and in human breast tumors (Table 1) [6-12]. Some of these biomarkers appear to be useful for monitoring bone metabolism in patients with various bone-related diseases, including osteoporosis, as well as monitoring patients receiving antiresorptive agents [13, 14]. However, biomarkers for the identification of patients at increased risk for bone metastasis of breast cancer have yet to be validated and implemented clinically. Furthermore, as understanding of the biological processes involved in metastasis of breast cancer to bone evolves and targeted treatments of bone metastases enter the clinic, identification of markers that assist in assessing therapeutic response to these agents will be needed [15-19].
Table 1.
Common biomarkers of bone homeostasis
| Markers of bone formation |
| Serum total alkaline phosphatase |
| Serum bone-specific alkaline phosphatase |
| Serum OC |
| Serum P1NP/procollagen type I C-terminal propeptide |
| Markers of bone resorption |
| Urinary hydroxyproline |
| Urinary total pyridinoline |
| Urinary free deoxypyridinoline |
| Urinary collagen type I cross-linked N-telopeptide |
| Urinary or serum CTX |
| Bone sialoprotein |
| Tartrate-resistant acid phosphatase 5b |
The identification of clinically useful predictive markers of bone metastasis in patients with early-stage breast cancer will allow us to design appropriate interventions to interfere with the metastatic process in this population. The aim of this study was to determine the association of serum levels of the biomarker procollagen type I N-terminal propeptide (P1NP) with the development of bone metastasis in patients with early stage breast cancer. Our hypothesis was that elevated levels of circulating P1NP would correlate with an increased risk of bone metastasis. Discovering circulating biomarkers associated with the development of metastatic breast cancer to bone is critical to identifying patients for whom early intervention could decrease the risk of this potentially fatal complication.
Patients and methods
Patients
Blood samples were collected from 164 patients with stage I–III breast cancer seen at The University of Texas MD Anderson Cancer Center from September 2001 to December 2008. All patients were staged with chest X-ray, bone scan, and CT of the abdomen to rule out distant metastasis at the time of diagnosis. Table 2 shows the characteristics of the patients included in this study. The samples were collected before definitive surgery or neoadjuvant chemotherapy and banked for future use. Sample collection, banking and analyses were conducted according to protocols approved by an Institutional Review Board.
Table 2.
Patients characteristics (N = 164)
| Age (median, range): 51 years (25, 85) |
| Stage I: 10; II: 86; III: 68 |
| Histology: invasive ductal carcinoma: 139; invasive lobular carcinoma: 10; |
| Invasive mixed carcinoma: 10; other* histology: 5 |
| Nuclear grade I: 4; II: 51; III: 109 |
| HR positive and HER2 negative: 70; HR positive and HER2 positive: 11; |
| HR negative and HER2 positive: 16; triple negative: 65; |
| Adj/neoadj anthracycline and taxane: 145 yes; 19 no |
| Endocrine therapy: 75 yes; 89 no |
Other histologies include 3 metaplastic/sarcomatoid, 1 medullary, and 1 invasive micropapillary
Blood samples
The blood samples were collected from the patients in evacuated Monovette plastic tubes (Sarstedt, Newton, NC) and centrifuged at 2,000×g for 15 min at room temperature within 2 h after venipuncture. Samples were taken in the morning, but fasting was not required. The samples were then aliquoted and stored at −80 °C.
Measurement of biomarker levels in serum
Serum levels of P1NP, CTX, IL-6, and OC were measured using an Elecsys 2010 automated immunoassay system (Roche, Indianapolis, IN). This electrochemiluminescent method uses streptavidin-labeled microparticles and monoclonal antibodies labeled with a ruthenium complex. Following validation of accuracy, precision, linearity, and limits of detection, the assay was used to simultaneously measure the levels of the selected analytes in serum samples. The samples were shuffled before testing, and the level of each biomarker was measured in duplicate (average of duplicates was used for analysis). Laboratory personnel were blinded to the patients’ identities and clinical data. Time to metastasis was measured from the date of blood sample to the date of discovery of metastasis or last follow-up visit. Overall survival (OS) was measured from the date of blood sample to date of death or last contact.
Statistical analysis
Correlations of the biomarker levels with time to bone metastasis development, disease-free survival (DFS) rate and OS rate were assessed using Cox proportional hazards regression analysis and the Kaplan–Meier method with the S-PLUS software program (version 8.0; Tibco, Palo Alto, CA). For patients who underwent serial measurement of P1NP levels, the association between time to distant metastasis and P1NP level was fit in a Cox proportional hazards regression model with P1NP fit as a time-varying covariate using the SAS software program (version 9.2; SAS Institute Inc., Cary, NC) [20].
Results
Correlation of P1NP with osteocalcin and CTX
Both osteocalcin and CTX were significantly (P<0.0001) correlated with P1NP based on Spearman’s rank correlation coefficients (osteocalcin: ρ = 0.72, CTX: ρ = 0.53).
Association of serum biomarkers levels with time to development of bone metastasis
Of the 164 study patients, 55 had bone metastases. Of note is that for 21 of the 107 patients without bone metastases, the follow-up duration was under 2 years. As a result, we were not able to treat bone metastasis development as a binary endpoint and thus could not directly compare patients with and without bone metastases.
Residual analysis suggested the existence of nonlinear relationships between each biomarker and risk of developing bone metastasis during follow-up. Thus, we fit Cox proportional hazards regression models for each biomarker with quadratic polynomials (on the log scale). Univariate analysis revealed no associations of risk of subsequent development of bone metastasis with serum levels of IL-6, OC, CTX, or P1NP (P = 0.18, 0.31, 0.37, and 0.20, respectively). However, adjusting the analysis for clinical factors (disease stage, age, race, post-menopause, estrogen receptor/progesterone receptor status, HER2 status, nuclear grade) yielded statistical significance for the quadratic polynomial for log P1NP (P = 0.043). The endpoint is bone recurrence at any time after baseline sample was obtained.
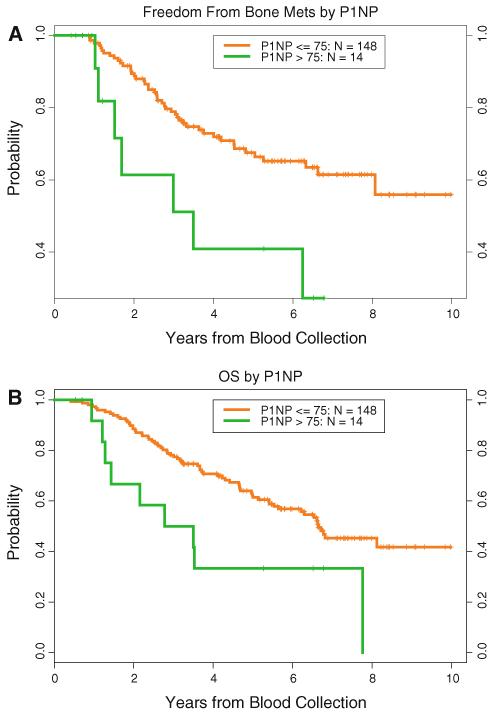
The serum levels of P1NP ranged from 12.8 to 212.0 ng/mL, with a median level of 44.3 ng/mL. Only four of the patients’ levels were greater than 100 ng/mL. A cut point of 75 ng/mL identified patients with short times to development of bone metastasis (hazard ratio (HR), 2.7 [95 % confidence interval (CI), 1.2–6.0]; P = 0.031) (Fig. 1a). The cut-off of 75 was selected as the best choice after investigation of several potential cut offs. Since it was selected from among a set of candidates, we cannot be sure of the true significance of this cut-off value. It would need to be validated in an independent dataset. The 1-, 2-, 3-, and 4-year freedom-from-bone-metastasis probabilities were 100, 89, 79, and 73 %, respectively, in patients with P1NP levels less than 75 ng/mL and 98, 61, 51, and 41 % in patients with P1NP levels at or greater than 75 ng/mL. After adjustment for clinical factors, the HR was 2.9 (95 % CI, 1.2–7.2; P = 0.019).
Fig. 1.
Correlation of serum P1NP level with development of bone metastasis (a) and overall survival (b) of breast cancer using a P1NP level of 75 ng/mL as the cut point
Correlation between the bone biomarkers and first relapse
There were 80 distant metastasis events (time to first distant metastasis), Kaplan–Meier freedom-from-distant mets: 2 years = 73 %, 4 years = 55 %, 6 years = 48 %. Cox PH models with biomarkers analyzed as continuous variables on the log scale showed none of the four markers were predictive. There were 82 local/regional/distant metastasis events (time to first recurrence), Kaplan–Meier freedom from recurrence: 2 years = 73 %, 4 years = 54 %, 6 years = 47 %. In only 2 out of 164 patients is the time to first distant met much different from the time to first recurrence of any type, suggesting that the results for time to first recurrence will not be much different from those for time to first distant metastasis.
Association of serum biomarker level with survival rate
Univariate analysis demonstrated that serum levels of P1NP, IL-6, OC, and CTX at diagnosis of breast cancer did not significantly correlate with OS. However, after adjustment for clinical factors, multivariate Cox proportional hazards regression analysis showed that the OC level was an independent predictor of OS (P = 0.04).
When modeled as a quadratic polynomial on the log scale, the serum P1NP level was associated with OS (P = 0.022) even after adjustment for clinical factors (P = 0.0056). When dichotomized at 75 ng/mL, high levels of P1NP were associated with poor survival (HR, 2.4 [95 % CI, 1.2–4.7]; P = 0.031) even after adjustment for clinical factors (HR, 3.3 [95 % CI, 1.5–7.3]; P = 0.0036) (Fig. 1b).
Discussion
We evaluated several proteins in serum as potential biomarkers for identifying women with early-stage breast cancer who are at increased risk for bone metastasis. This study is unique that in addition to evaluating some of the standard proteins that investigators have used to assess bone homeostasis (OC and CTX), we also evaluated two other proteins shown to have roles in signaling pathways involved in the development of bone metastasis (P1NP and IL-6) [21-23]. We found that a serum P1NP level of at least 75 ng/mL at the time of breast cancer diagnosis correlated with a short time to development of bone metastasis and short overall survival rate in patients with stage I–III breast cancer.
A few studies have demonstrated associations between elevated P1NP levels and development of certain cancers, particularly those with a high prevalence of metastasis to bone, such as breast and prostate cancer [22-24]. Furthermore, researchers have shown that P1NP is a potential predictor of bone metastasis and of disease progression and survival in patients with prostate cancer [23]. More recent studies have examined the use of the serum P1NP level as a tool for estimating the extent of bone involvement and detecting bone metastases in patients with breast cancer [25-27].
Studies have demonstrated elevated serum concentrations of P1NP in up to 70 % of patients with breast cancer having confirmed bone metastases [26-28]. In a randomized trial, patients with estrogen receptor-positive metastatic breast cancer treated with exemestane plus everolimus had higher P1NP levels than did patients who received exemestane alone, suggesting that P1NP is a predictive biomarker for bone metastasis in this population [29]. McCloskey et al. evaluated the predictive role of P1NP in patients treated with clodronate in the adjuvant setting. In this study, patients who received clodronate had a median 26 % reduction in levels of serum P1NP after 2 years of therapy, compared with a median 5 % increase in patients who received placebo (P<0.0001). Early changes in PINP were associated with changes in bone mineral density and the likelihood of developing bone metastases [30]. However, to our knowledge, this is the first study to demonstrate that baseline levels of P1NP can be used to identify women with early-stage breast cancer at increased risk for bone metastasis. This is highly significant in light of the fact that bone metastasis is a major contributor to the morbidity and mortality of breast cancer [31-34]. In addition, preliminary data demonstrating that use of bisphosphonates and the RANK ligand (RANKL) inhibitor denosumab is able to decrease the time to development of the first and subsequent skeletal-related events (e.g., pathological fractures, spinal compression, radiation or surgery to bone or tumor-induced hypocalcaemia) supports a potential role for these agents in adjuvant treatment of breast cancer [15-18, 35-39]. However, what patients are likely to benefit from this type of intervention is not known [40].
The correlation of elevated serum levels of OC at diagnosis of breast cancer and poor prognosis in the present study is in line with the results of previously published reports [41, 42] thus, we used it as a reference protein for bone homeostasis in the present study. However, the lack of prognostic value for serum levels of CTX are somewhat surprising. CTX is a marker of bone resorption, and elevated serum levels have been documented in patients with prostate or breast cancer having bone metastasis [43-45]. CTX levels are usually measured in urine samples, and measurement of this biomarker in blood samples obtained from breast cancer patients as performed in our study is fairly unique and may account for the discrepancy regarding prognosis.
Researchers have also evaluated the role of IL-6 in prognosis for bone metastasis, finding that elevated serum levels of IL-6 and soluble IL-6 receptor were associated with poor clinical outcome in patients with metastatic breast cancer [21, 46, 47]. IL-6, which is produced in the bone marrow microenvironment, also can enhance bone degradation in several ways, including stimulation of RANKL production by osteoblasts [48, 49]. Thus, the correlation we observed between serum IL-6 level and DFS rate (P = 0.056) is not surprising. Evaluation of IL-6 levels has offered the unique opportunity to identify effective targeted agents for treatment of breast cancer, as monoclonal antibodies and small molecule inhibitors of IL-6-mediated signaling molecules are now undergoing testing in phase 1 and 2 clinical trials [21].
New clinical trial designs are needed to prevent development of bone metastasis in patients at high risk [50]. Our study was limited by the small sample size, which was determined by the available samples in our serum bank at the time we performed the study. We had baseline samples for all patients but follow-up samples for only a subset of them. Serial measurement of the four markers tested in serum likely will be more informative than single biomarkers, although this hypothesis must be tested prospectively. This study is retrospective and therefore hypothesis-generating. If our observations are confirmed by larger studies, a randomized clinical trial would be justified for women with serum P1NP levels of at least 75 ng/mL at the time of breast cancer diagnosis to determine whether adjuvant bisphosphonates or denosumab improves DFS and OS rates.
Translational relevance.
Bone is the most common site of metastasis in patients with advanced breast cancer. Development of bone metastasis is associated with high mortality and morbidity. There are currently no biomarkers to identify patients with early-stage breast cancer who are likely to develop metastatic disease to the bone. We measured serum levels of bone turnover markers, including procollagen type I N-terminal propeptide (P1NP), osteocalcin, CTX, and IL-6 in patients with clinical stage I-III breast cancer, before initiation of definitive treatment. Serum procollagen type I N-terminal propeptide (P1NP) levels of at least 75 ng/mL at the time of breast cancer diagnosis correlated with an shorter time to development of bone metastasis and shorter overall survival rate in patients with stage I-III disease. Serum P1NP levels may be used for patient selection in future clinical trials of agents designed to prevent bone metastasis.
Acknowledgments
We thank our patients for providing the blood samples for our biomarker research. This study was supported by an investigator-initiated grant provided by Roche Diagnostics to Francisco J. Esteva. This research is supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672.
Footnotes
Present Address: W. Dean-Colomb, University of South Alabama Mitchell Cancer Institute, Mobile, AL, USA
Conflict of interest The authors made no disclosures.
Contributor Information
Windy Dean-Colomb, Department of Breast Medical Oncology, Unit 1354, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
Kenneth R. Hess, Department of Biostatistics, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA
Elliana Young, Department of Breast Medical Oncology, Unit 1354, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
Terrie G. Gornet, Department of Laboratory Medicine, The University of Texas, MD Anderson Cancer Center, Houston, TX, USA
Beverly C. Handy, Department of Laboratory Medicine, The University of Texas, MD Anderson Cancer Center, Houston, TX, USA
Stacy L. Moulder, Department of Breast Medical Oncology, Unit 1354, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA
Nuhad Ibrahim, Department of Breast Medical Oncology, Unit 1354, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
Lajos Pusztai, Department of Breast Medical Oncology, Unit 1354, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
Daniel Booser, Department of Breast Medical Oncology, Unit 1354, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
Vicente Valero, Department of Breast Medical Oncology, Unit 1354, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
Gabriel N. Hortobagyi, Department of Breast Medical Oncology, Unit 1354, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA
Francisco J. Esteva, Department of Breast Medical Oncology, Unit 1354, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Scheid V, Buzdar AU, Smith TL, Hortobagyi GN. Clinical course of breast cancer patients with osseous metastasis treated with combination chemotherapy. Cancer. 1986;58:2589–2593. doi: 10.1002/1097-0142(19861215)58:12<2589::aid-cncr2820581206>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 3.Alexandraki I. The United States-Mexico border: an area in need of cancer screening interventions. J Women’s Health. 2011;20:653–655. doi: 10.1089/jwh.2010.2700. [DOI] [PubMed] [Google Scholar]
- 4.Mehta RS, Barlow WE, Albain KS, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med. 2012;367:435–444. doi: 10.1056/NEJMoa1201622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipton A. Bone metastases in breast cancer. Curr Treat Options Oncol. 2003;4:151–158. doi: 10.1007/s11864-003-0016-9. [DOI] [PubMed] [Google Scholar]
- 6.Lipton A, Costa L, Ali S, Demers L. Use of markers of bone turnover for monitoring bone metastases and the response to therapy. Semin Oncol. 2001;28:54–59. doi: 10.1016/s0093-7754(01)90233-7. [DOI] [PubMed] [Google Scholar]
- 7.Bellahcene A, Kroll M, Liebens F, Castronovo V. Bone sialoprotein expression in primary human breast cancer is associated with bone metastases development. J Bone Miner Res. 1996;11:665–670. doi: 10.1002/jbmr.5650110514. [DOI] [PubMed] [Google Scholar]
- 8.Buijs JT, Henriquez NV, van Overveld PG, et al. Bone morphogenetic protein 7 in the development and treatment of bone metastases from breast cancer. Cancer Res. 2007;67:8742–8751. doi: 10.1158/0008-5472.CAN-06-2490. [DOI] [PubMed] [Google Scholar]
- 9.Chao TY, Yu JC, Ku CH, et al. Tartrate-resistant acid phosphatase 5b is a useful serum marker for extensive bone metastasis in breast cancer patients. Clin Cancer Res. 2005;11:544–550. [PubMed] [Google Scholar]
- 10.Diel IJ, Solomayer EF, Seibel MJ, et al. Serum bone sialoprotein in patients with primary breast cancer is a prognostic marker for subsequent bone metastasis. Clin Cancer Res. 1999;5:3914–3919. [PubMed] [Google Scholar]
- 11.Wada N, Ishii S, Ikeda T, Enomoto K, Kitajima M. Serum tartrate resistant acid phosphatase as a potential marker of bone metastasis from breast cancer. Anticancer Res. 1999;19:4515–4521. [PubMed] [Google Scholar]
- 12.Wu YY, Janckila AJ, Ku CH, et al. Serum tartrate-resistant acid phosphatase 5b activity as a prognostic marker of survival in breast cancer with bone metastasis. BMC Cancer. 2010;10:158. doi: 10.1186/1471-2407-10-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipton A, Costa L, Ali SM, Demers LM. Bone markers in the management of metastatic bone disease. Cancer Treat Rev. 2001;27:181–185. doi: 10.1053/ctrv.2000.0212. [DOI] [PubMed] [Google Scholar]
- 14.Samoszuk M, Leuther M, Hoyle N. Role of serum P1NP measurement for monitoring treatment response in osteoporosis. Biomark Med. 2008;2:495–508. doi: 10.2217/17520363.2.5.495. [DOI] [PubMed] [Google Scholar]
- 15.Lipton A. Emerging role of bisphosphonates in the clinic-antitumor activity and prevention of metastasis to bone. Cancer Treat Rev. 2008;34(Suppl 1):S25–S30. doi: 10.1016/j.ctrv.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Nangia JR, Ma JD, Nguyen CM, Mendes MA, Trivedi MV. Denosumab for treatment of breast cancer bone metastases and beyond. Expert Opin Biol Ther. 2012;12:491–501. doi: 10.1517/14712598.2012.664634. [DOI] [PubMed] [Google Scholar]
- 17.Barton MK. Denosumab an option for patients with bone metastasis from breast cancer. CA Cancer J Clin. 2011;61:135–136. doi: 10.3322/caac.20116. [DOI] [PubMed] [Google Scholar]
- 18.Henry DH, Costa L, Goldwasser F, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29:1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 19.Lipton A, Steger GG, Figueroa J, et al. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol. 2007;25:4431–4437. doi: 10.1200/JCO.2007.11.8604. [DOI] [PubMed] [Google Scholar]
- 20.Andersen PK, Gill RD. Cox’s regression model for counting processes: a large sample study. Ann Stat. 1982;10:1100–1120. [Google Scholar]
- 21.Tawara K, Oxford JT, Jorcyk CL. Clinical significance of interleukin (IL)-6 in cancer metastasis to bone: potential of anti-IL-6 therapies. Cancer Manag Res. 2011;3:177–189. doi: 10.2147/CMR.S18101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koizumi M, Yonese J, Fukui I, Ogata E. The serum level of the amino-terminal propeptide of type I procollagen is a sensitive marker for prostate cancer metastasis to bone. BJU Int. 2001;87:348–351. doi: 10.1046/j.1464-410x.2001.00105.x. [DOI] [PubMed] [Google Scholar]
- 23.Thurairaja R, Iles RK, Jefferson K, McFarlane JP, Persad RA. Serum amino-terminal propeptide of type 1 procollagen (P1NP) in prostate cancer: a potential predictor of bone metastases and prognosticator for disease progression and survival. Urol Int. 2006;76:67–71. doi: 10.1159/000089738. [DOI] [PubMed] [Google Scholar]
- 24.Oremek G, Sauer-Eppel H, Klepzig M. Total procollagen type 1 amino-terminal propeptide (total P1NP) as a bone metastasis marker in gynecological carcinomas. Anticancer Res. 2007;27:1961–1962. [PubMed] [Google Scholar]
- 25.Pollmann D, Siepmann S, Geppert R, Wernecke KD, Possinger K, Luftner D. The amino-terminal propeptide (PINP) of type I collagen is a clinically valid indicator of bone turnover and extent of metastatic spread in osseous metastatic breast cancer. Anticancer Res. 2007;27:1853–1862. [PubMed] [Google Scholar]
- 26.Marin L, Koivula MK, Jukkola-Vuorinen A, Leino A, Risteli J. Comparison of total and intact aminoterminal propeptide of type I procollagen assays in patients with breast cancer with or without bone metastases. Ann Clin Biochem. 2011;48:447–451. doi: 10.1258/acb.2011.011040. [DOI] [PubMed] [Google Scholar]
- 27.Saarto T, Blomqvist C, Risteli J, Risteli L, Sarna S, Elomaa I. Aminoterminal propeptide of type I procollagen (PINP) correlates to bone loss and predicts the efficacy of antiresorptive therapy in pre- and post-menopausal non-metastatic breast cancer patients. Br J Cancer. 1998;78:240–245. doi: 10.1038/bjc.1998.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tahtela R, Tholix E. Serum concentrations of type I collagen carboxyterminal telopeptide (ICTP) and type I procollagen carboxy-and aminoterminal propeptides (PICP, PINP) as markers of metastatic bone disease in breast cancer. Anticancer Res. 1996;16:2289–2293. [PubMed] [Google Scholar]
- 29.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCloskey E, Paterson A, Kanis J, Tahtela R, Powles T. Effect of oral clodronate on bone mass, bone turnover and subsequent metastases in women with primary breast cancer. Eur J Cancer. 2010;46:558–565. doi: 10.1016/j.ejca.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Esteva FJ, Guo H, Zhang S, et al. PTEN, PIK3CA, p-AKT, and p-p70S6K status: association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am J Pathol. 2010;177:1647–1656. doi: 10.2353/ajpath.2010.090885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali SM, Esteva FJ, Hortobagyi G, et al. Safety and efficacy of bisphosphonates beyond 24 months in cancer patients. J Clin Oncol. 2001;19:3434–3437. doi: 10.1200/JCO.2001.19.14.3434. [DOI] [PubMed] [Google Scholar]
- 33.Esteva FJ, Valero V, Pusztai L, Boehnke-Michaud L, Buzdar AU, Hortobagyi GN. Chemotherapy of metastatic breast cancer: what to expect in 2001 and beyond. Oncologist. 2001;6:133–146. doi: 10.1634/theoncologist.6-2-133. [DOI] [PubMed] [Google Scholar]
- 34.Esteva FJ. Monoclonal antibodies, small molecules, and vaccines in the treatment of breast cancer. Oncologist. 2004;9:4–9. doi: 10.1634/theoncologist.9-suppl_3-4. [DOI] [PubMed] [Google Scholar]
- 35.Aapro MS. Denosumab for bone metastases from breast cancer: A new therapy option? J Clin Oncol. 2011;29:e419–e420. doi: 10.1200/JCO.2010.33.9150. author reply e421-e414. [DOI] [PubMed] [Google Scholar]
- 36.de Kluyver RL, Sayers TJ. Breast cancer bone metastases: combination therapy targeting cancer cells and the tumor microenvironment. Cancer Biol Ther. 2010;9:551–553. doi: 10.4161/cbt.9.7.11580. [DOI] [PubMed] [Google Scholar]
- 37.Fizazi K, Lipton A, Mariette X, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27:1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 38.Lee BL, Higgins MJ, Goss PE. Denosumab and the current status of bone-modifying drugs in breast cancer. Acta Oncol. 2012;51:157–167. doi: 10.3109/0284186X.2011.633555. [DOI] [PubMed] [Google Scholar]
- 39.van der Pluijm G. Breast cancer bone metastases: denosumab or zoledronic acid? Nat Rev Endocrinol. 2011;7:134–135. doi: 10.1038/nrendo.2011.18. [DOI] [PubMed] [Google Scholar]
- 40.Paterson AH, Anderson SJ, Lembersky BC, et al. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): a multicentre, placebo-controlled, randomised trial. Lancet Oncol. 2012;13:734–742. doi: 10.1016/S1470-2045(12)70226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Body JJ, Dumon JC, Gineyts E, Delmas PD. Comparative evaluation of markers of bone resorption in patients with breast cancer-induced osteolysis before and after bisphosphonate therapy. Br J Cancer. 1997;75:408–412. doi: 10.1038/bjc.1997.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fornander T, Rutqvist LE, Wilking N, Carlstrom K, von Schoultz B. Oestrogenic effects of adjuvant tamoxifen in postmenopausal breast cancer. Eur J Cancer. 1993;29A:497–500. doi: 10.1016/s0959-8049(05)80138-8. [DOI] [PubMed] [Google Scholar]
- 43.Kong QQ, Sun TW, Dou QY, et al. Beta-CTX and ICTP act as indicators of skeletal metastasis status in male patients with non-small cell lung cancer. Int J Biol Markers. 2007;22:214–220. doi: 10.1177/172460080702200309. [DOI] [PubMed] [Google Scholar]
- 44.Leeming DJ, Delling G, Koizumi M, et al. Alpha CTX as a biomarker of skeletal invasion of breast cancer: immunolocalization and the load dependency of urinary excretion. Cancer Epidemiol Biomark Prev. 2006;15:1392–1395. doi: 10.1158/1055-9965.EPI-05-0909. [DOI] [PubMed] [Google Scholar]
- 45.Brasso K, Christensen IJ, Johansen JS, et al. Prognostic value of PINP, bone alkaline phosphatase, CTX-I, and YKL-40 in patients with metastatic prostate carcinoma. Prostate. 2006;66:503–513. doi: 10.1002/pros.20311. [DOI] [PubMed] [Google Scholar]
- 46.Kim SW, Kim JS, Papadopoulos J, et al. Consistent interactions between tumor cell IL-6 and macrophage TNF-alpha enhance the growth of human prostate cancer cells in the bone of nude mouse. Int Immunopharmacol. 2011;11:862–872. doi: 10.1016/j.intimp.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang CH, Chuang JY, Fong YC, Maa MC, Way TD, Hung CH. Bone-derived SDF-1 stimulates IL-6 release via CXCR4, ERK and NF-kappaB pathways and promotes osteoclastogenesis in human oral cancer cells. Carcinogenesis. 2008;29:1483–1492. doi: 10.1093/carcin/bgn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsangari H, Findlay DM, Kuliwaba JS, Atkins GJ, Fazzalari NL. Increased expression of IL-6 and RANK mRNA in human trabecular bone from fragility fracture of the femoral neck. Bone. 2004;35:334–342. doi: 10.1016/j.bone.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Blair JM, Zhou H, Seibel MJ, Dunstan CR. Mechanisms of disease: roles of OPG, RANKL and RANK in the pathophysiology of skeletal metastasis. Nat Clin Pract Oncol. 2006;3:41–49. doi: 10.1038/ncponc0381. [DOI] [PubMed] [Google Scholar]
- 50.Wall KM, Nunez-Rocha GM, Salinas-Martinez AM, Sanchez-Pena SR. Determinants of the use of breast cancer screening among women workers in urban Mexico. Prev Chronic Dis. 2008;5:A50. [PMC free article] [PubMed] [Google Scholar]