Abstract
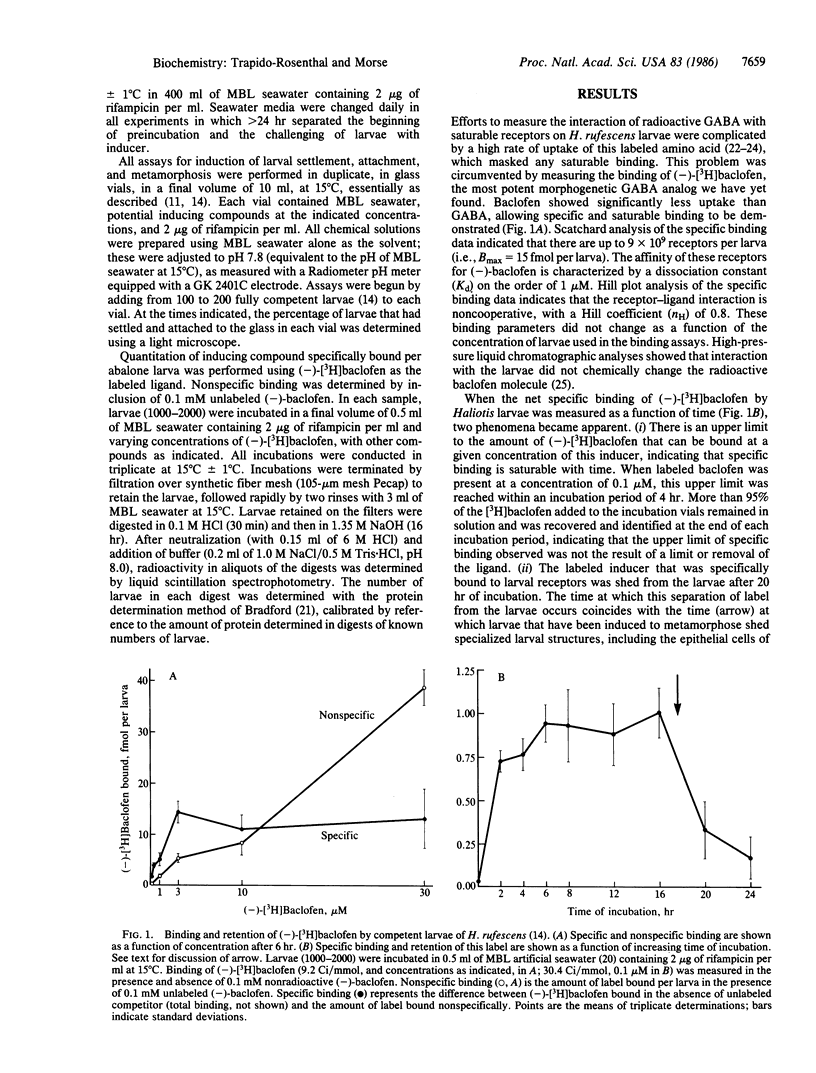
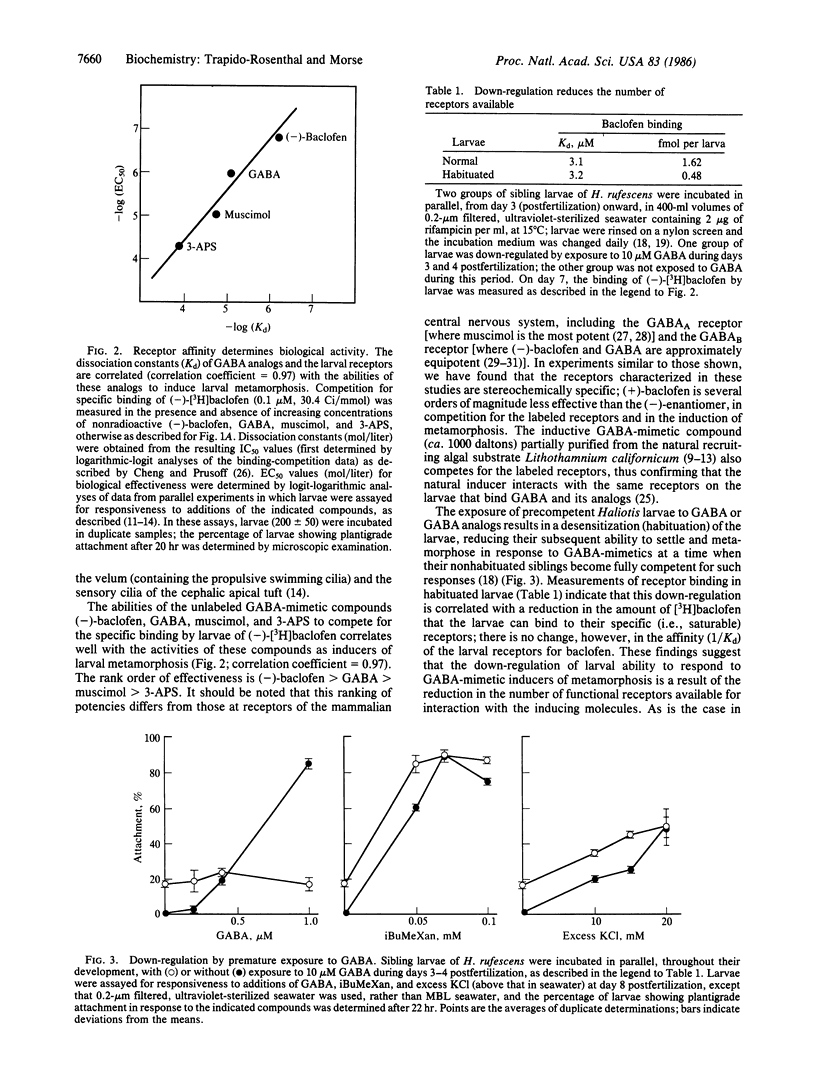
Larvae of the gastropod mollusc Haliotis rufescens are induced to settle from the plankton and metamorphose in response to exogenous gamma-aminobutyric acid (GABA) and a number of GABA-mimetic compounds, including a GABA-mimetic inducer uniquely associated with the surfaces of the naturally recruiting algae. Previous evidence has shown that recognition of these inducers is mediated by specialized chemosensory receptors on the larval epithelium and that transduction of the morphogenetic signal then is mediated by cAMP and excitatory depolarization. We demonstrate here the specific and saturable labeling of a population of larval receptors with the GABA analog beta-(p-chlorophenyl)-[3H]GABA ([3H]baclofen); identification of these labeled receptors with those controlling metamorphosis is suggested by four independent criteria: the effectiveness of GABA and its close structural analogs to induce metamorphosis is closely correlated with the effectiveness of these compounds to compete for binding to this receptor; the natural inducer purified from the recruiting algae competes for binding to this receptor; (-)-[3H]baclofen specifically bound to the receptors is shed from the larvae after approximately 20 hr, at the time corresponding to the metamorphic abscission and shedding of sensory cilia and other structures from the larvae; and the availability of the receptors for labeling and the ability of the larvae to respond to GABA and GABA analogs can be down-regulated in parallel by habituation of the larvae early in their development. These down-regulated larvae are fully capable of settlement and metamorphosis in response to agents that elevate intracellular cAMP or depolarize the chemosensory membrane, confirming that down-regulation is confined to the receptors, with no effect on the postreceptor pathway. The results reported here thus suggest that the sensitivity of marine invertebrate larvae to morphogenetic stimuli from the environment can be down-regulated by reduction in the number of chemosensory receptors available for interaction with the molecules that induce settlement and metamorphosis. In this respect, chemosensory receptors for environmental and morphogenetic signals are demonstrated biochemically to respond to habituation in a similar manner to neuronal and hormonal receptors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borden L. A., Czajkowski C., Chan C. Y., Farb D. H. Benzodiazepine receptor synthesis and degradation by neurons in culture. Science. 1984 Nov 16;226(4676):857–860. doi: 10.1126/science.6093257. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Doble A., Hill D. R., Hudson A. L., Shaw J. S., Turnbull M. J., Warrington R. Bicuculline-insensitive GABA receptors on peripheral autonomic nerve terminals. Eur J Pharmacol. 1981 Apr 24;71(1):53–70. doi: 10.1016/0014-2999(81)90386-1. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L. Characteristics of GABAB receptor binding sites on rat whole brain synaptic membranes. Br J Pharmacol. 1983 Jan;78(1):191–206. doi: 10.1111/j.1476-5381.1983.tb09380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burke R. D. Pheromonal Control of Metamorphosis in the Pacific Sand Dollar, Dendraster excentricus. Science. 1984 Jul 27;225(4660):442–443. doi: 10.1126/science.225.4660.442. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981 Mar 12;290(5802):149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Kopf G. S., Lewis C. A., Vacquier V. D. Regulation of abalone sperm cyclic AMP concentrations and the acrosome reaction by calcium and methylxanthines. Dev Biol. 1983 Jul;98(1):28–36. doi: 10.1016/0012-1606(83)90332-9. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr A response regulator model in a simple sensory system. Science. 1977 Jun 3;196(4294):1055–1063. doi: 10.1126/science.870969. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Goldbeter A., Stock J. B. Amplification and adaptation in regulatory and sensory systems. Science. 1982 Jul 16;217(4556):220–225. doi: 10.1126/science.7089556. [DOI] [PubMed] [Google Scholar]
- Manahan D. T., Wright S. H., Stephens G. C., Rice M. A. Transport of Dissolved Amino Acids by the Mussel, Mytilus edulis: Demonstration of Net Uptake from Natural Seawater. Science. 1982 Mar 5;215(4537):1253–1255. doi: 10.1126/science.215.4537.1253. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Duncan H., Hooker N., Baloun A., Young G. GABA induces behavioral and developmental metamorphosis in planktonic molluscan larvae. Fed Proc. 1980 Dec;39(14):3237–3241. [PubMed] [Google Scholar]
- Morse D. E., Hooker N., Duncan H., Jensen L. ggr-Aminobutyric Acid, a Neurotransmitter, Induces Planktonic Abalone Larvae to Settle and Begin Metamorphosis. Science. 1979 Apr 27;204(4391):407–410. doi: 10.1126/science.204.4391.407. [DOI] [PubMed] [Google Scholar]
- Posner B. I., Khan M. N., Bergeron J. J. Endocytosis of peptide hormones and other ligands. Endocr Rev. 1982 Summer;3(3):280–298. doi: 10.1210/edrv-3-3-280. [DOI] [PubMed] [Google Scholar]
- Rosenfeld R. G., Hintz R. L., Dollar L. A. Insulin-induced loss of insulin-like growth factor-I receptors on IM-9 lymphocytes. Diabetes. 1982 May;31(5 Pt 1):375–381. doi: 10.2337/diab.31.5.375. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Lefkowitz R. J. Molecular mechanisms of receptor desensitization using the beta-adrenergic receptor-coupled adenylate cyclase system as a model. Nature. 1985 Sep 12;317(6033):124–129. doi: 10.1038/317124a0. [DOI] [PubMed] [Google Scholar]
- Weissman A. M., Harford J. B., Svetlik P. B., Leonard W. L., Depper J. M., Waldmann T. A., Greene W. C., Klausner R. D. Only high-affinity receptors for interleukin 2 mediate internalization of ligand. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1463–1466. doi: 10.1073/pnas.83.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]



