Abstract
Purpose of the research
To identify distinct latent classes of individuals based on ratings of morning and evening fatigue; evaluate for differences in phenotypic characteristics, as well as symptom and quality of life scores, among these latent classes; and evaluate for an overlap in morning and evening fatigue class membership.
Patients and methods
In a sample of 167 oncology outpatients and 85 of their FCs, growth mixture modeling was used to identify distinct latent classes based on ratings of morning and evening fatigue obtained before, during, and after radiation therapy. Analyses of variance and Chi Square analyses were used to evaluate for differences among the morning and evening fatigue latent classes.
Results
Three distinct latent classes for morning fatigue were identified. Participants in the High Morning Fatigue class (47%) were younger and had lower functional status. Three distinct latent classes for evening fatigue were identified. Participants in the High Evening Fatigue class (61%) were younger, more likely to be female, more likely to have children at home, and more likely to be a FC. Only 10.3% of participants were classified in both the Very Low Morning and Low Evening Fatigue classes and 41.3% were classified in both the High Morning and High Evening Fatigue classes.
Conclusions
Different characteristics were associated with morning and evening fatigue, which suggests that morning and evening fatigue may be distinct but related symptoms. Additional research is needed to elucidate the mechanisms that may underlie diurnal variability in fatigue.
Keywords: Morning fatigue, Evening fatigue, Fatigue, Diurnal variability, Radiation therapy, Cancer treatment, Growth mixture modeling, Latent class analysis
Introduction
Fatigue is a frequent and disabling symptom (Lawrence et al., 2004) that occurs in approximately 80% of patients receiving radiation therapy (RT) (Henry et al., 2008; Hoffman et al., 2007) and in 24%–30% of family caregivers (FCs) (Swore Fletcher et al., 2008). The impact of fatigue on cancer patients and their FCs is significant in terms of inability to tolerate treatments, lost productivity, lost days from work, and decreased quality of life (QOL) (Dhrupad et al., 2012; Fletcher et al., 2009, 2008; Gupta et al., 2007; Vigilant et al., 1997). Some of the etiologies for fatigue may be similar or different between patients and FCs. However, the various physical (e.g., multiple co-morbid conditions, concurrent medical treatments) and psychological (e.g., stressor associated with cancer and its treatment) factors that contribute to the development and maintenance of fatigue in both patients and FCs merge into one or more common mechanistic pathways (e.g., inflammation).
While the majority of research on fatigue has reported mean changes in fatigue severity, prior work from our research team (Dhrupad et al., 2010; Fletcher et al., 2009; Miaskowski et al., 2008) and others (Dimsdale et al., 2007; Jim et al., 2011; Molassiotis and Chan, 2004) suggests that the severity of fatigue varies over the course of a day and varies substantially among individuals. In a previous study, we determined, using hierarchical linear modeling, that different demographic and clinical characteristics predicted inter-individual variability in the trajectories of morning and evening fatigue in both patients (Dhrupad et al., 2010; Miaskowski et al., 2008) and FCs (Fletcher et al., 2009). These findings provide support for the hypothesis that morning and evening fatigue are distinct but related symptoms. A careful evaluation of the phenotypic characteristics associated with diurnal variations in fatigue may provide new insights into modifiable risk factors, as well as the mechanisms that underlie this devastating symptom(s).
Growth mixture modeling (GMM) can be used to discover latent classes of individuals with distinct trajectories of fatigue that may not be identified using more conventional analytic techniques (Jung and Wickrama, 2008; Muthen, 2004). Only one study of oncology patients used GMM to identify two distinct groups of breast cancer survivors with low (n = 85) and high (n = 176) levels of fatigue (Donovan et al., 2007). Patients in the high fatigue class had a higher body mass index and higher fatigue catastrophizing scores. However, diurnal variability in fatigue was not examined. This study, as well as studies of different symptoms in oncology patients and their FCs (Dunn et al., 2012, 2011; Hou et al., 2010; Lam et al., 2012; Rose et al., 2009; Van Onselen et al., 2012), demonstrate that GMM is a useful technique to identify subgroups of individuals with distinct symptom phenotypes.
Given the paucity of research on diurnal variability in fatigue in oncology patients and their FCs, the purposes of this study were to: identify distinct latent classes of oncology patients and their FCs based on subjective reports of morning and evening fatigue; evaluate for differences in demographic and clinical characteristics, as well as symptom and quality of life (QOL) scores, among the latent classes for morning and evening fatigue; and evaluate for an overlap in class membership between the morning and evening fatigue latent classes.
Methods
Participants and settings
This longitudinal study evaluated multiple symptoms in patients who underwent primary or adjuvant RT and their FCs (Aouizerat et al., 2009; Carney et al., 2011; Dhrupad et al., 2012; Dunn et al., 2012; Miaskowski et al., 2010, 2011). Participants were recruited from two RT departments located in a Comprehensive Cancer Center and a community-based oncology program at the time of the patient’s simulation visit.
Patients were eligible to participate if they: were ≥18 years of age; were scheduled to receive primary or adjuvant RT for one of four cancer diagnoses (i.e., breast, prostate, lung, and brain); were able to read, write, and understand English; gave written informed consent; and had a Karnofsky Performance Status (KPS) score of ≥60. Patients were excluded if they had: metastatic disease; more than one cancer diagnosis; or a diagnosed sleep disorder. FCs were eligible to participate if they were ≥18 years of age; were able to read, write, and understand English; gave written informed consent; had a KPS score of ≥60; were living with the patient; and did not have a diagnosed sleep disorder.
Instruments
The demographic questionnaire obtained information on age, gender, marital status, education, ethnicity, employment status, and comorbidities. Medical records were reviewed for disease and treatment information.
The Lee Fatigue Scale (LFS) consists of 13 items designed to assess physical fatigue (Lee et al., 1991). Each item was rated on a 0 to 10 numeric rating scale (NRS). A total fatigue score was calculated as the mean of the 13 items with higher scores indicating greater fatigue severity. Participants were asked to rate each item based on how they felt “right now” within 30 min of awakening (morning fatigue) and prior to going to bed (evening fatigue). The LFS has been used with healthy individuals (Gay et al., 2004) and in patients with cancer and HIV disease (Miaskowski et al., 2006; Miaskowski and Lee, 1999; Miaskowski et al., 2008). Cut-off scores of ≥3.2 and ≥5.6 indicate high levels of morning and evening fatigue, respectively (Fletcher et al., 2008). The LFS was chosen for this study because it is relatively short, easy to administer, and has well established validity and reliability. In addition, the LFS is not a disease specific measure of cancer-related fatigue. It is a scale that can be used to measure fatigue in both patients and their FCs. In this study, Cronbach’s alphas for evening and morning fatigue at enrollment were 0.96 and 0.95 for patients and 0.95 and 0.96 for FCs, respectively.
The Center for Epidemiological Studies–Depression scale consists of 20 items selected to represent the major symptoms in the clinical syndrome of depression. Scores can range from 0 to 60, with scores of ≥16 indicating the need for individuals to seek clinical evaluation for major depression. The CES-D has well established concurrent and construct validity (Radloff, 1977; Sheehan et al., 1995). In the current study, the Cronbach’s alpha for the CES-D was 0.88 for patients and 0.84 for FCs.
The Spielberger State-Trait Anxiety Inventories (STAI-S, STAI-T) each consist of 20 items that are rated from 1 to 4. The scores for each scale are summed and can range from 20 to 80. A higher score indicates greater anxiety. The STAI-T measures an individual’s personality-related predisposition to anxiety. The STAI-S measures an individual’s transitory emotional response to a stressful situation. It evaluates the emotional responses of worry, nervousness, tension, and feelings of apprehension related to how a person feels “right now” in a stressful situation. Cutoff scores of ≥31.8 and ≥32.2 indicate high levels of trait and state anxiety, respectively. The STAIS and STAI-T inventories have well established criterion and construct validity and internal consistency reliability coefficients (Spielberger et al.,1983). In the current study, the Cronbach’s alphas for the STAI-T and STAI-S were 0.92 and 0.95 for patients and 0.89 and 0.93 for FCs, respectively.
The General Sleep Disturbance Scale (GSDS) consists of 21-items designed to assess the quality of sleep in the past week. Each item was rated on a 0 (never) to 7 (everyday) NRS. The GSDS total score can range from 0 (no disturbance) to 147 (extreme sleep disturbance). A higher total score indicates higher levels of sleep disturbance. A GSDS total score of ≥43 indicates a significant level of sleep disturbance (Fletcher et al., 2008). The GSDS has well-established validity and reliability in shift workers, pregnant women, and patients with cancer and HIV (Lee, 1992; Lee and DeJoseph, 1992; Miaskowski and Lee, 1999). In the current study, the Cronbach’s alpha for the GSDS total score was 0.84 for patients and 0.79 for FCs.
Occurrence of pain was evaluated using the Brief Pain Inventory (Daut et al., 1983). Participants who responded yes to the question of having pain were asked to indicate the cause of their pain and to rate its intensity (i.e., now, least, average, and worst) using 0 (no pain) to 10 (worst pain imaginable) NRS.
The Attentional Function Index (AFI) is a commonly used self-report measure of attentional function (Cimprich et al., 2011). It consists of 16-items that are rated on a 0 to 10 NRS. A higher mean score indicates greater capacity to direct attention (Cimprich, 1992; Cimprich et al., 2011). Scores are grouped into categories of attentional function (i.e., <5.0 low function, 5.0 to 7.5 moderate function, >7.5 high function) (Cimprich et al., 2005). The AFI has established reliability and validity (Cimprich, 1992; Jansen et al., 2008). In the current study, Cronbach’s alpha for the AFI was 0.95 for both patients and FCs.
QOL was measured using the QOL Scale-Patient version (QOL-PV) and the QOL Scale-Family version (QOL-FV) (Padilla et al., 1990, 1983). The QOL-PV is a 41-item instrument that measures four domains of QOL (i.e., physical well-being, psychological well-being, spiritual well-being, social well-being) as well as a total QOL score. Each item is rated on a 0 to 10 NRS with higher scores indicating better QOL. The QOL-PV has established validity and reliability (Ferrell et al., 1995a). In this study, the Cronbach’s alpha for the QOL-PV total score was 0.94. The QOL-FV is a 37-item instrument that measures the same four domains of QOL. Each item is rated on a 0 to 10 NRS with higher scores indicating better QOL. The QOL-FV has established validity and reliability (Ferrell, 1995; Ferrell et al., 1995b). In this study, the Cronbach’s alpha for the QOL-FV total score was 0.95. The total QOL (which is a mean of the 41 and 37 items) was used in subsequent analyses.
Study procedures
The study was approved by the Committee on Human Research at the University of California, San Francisco and at the second site. Approximately one week prior to the start of RT (i.e., simulation visit), patients were invited to participate in the study. If the FC was present, a research nurse explained the study protocol to both the patient and FC, determined eligibility, and obtained written informed consent. FCs who were not present were contacted by phone to determine their interest in participation. These FCs completed the enrollment procedures at home.
At enrollment, participants completed the self-report questionnaires. After the initiation of RT, participants completed the LFS at 4 weeks, at the end of RT, and at 4, 8, 12, and 16 weeks after the completion of RT (i.e., 7 assessments over 6 months). At each of these assessments, participants completed the LFS (Lee et al., 1991) before going to bed each night (i.e., evening fatigue) and upon arising each morning (i.e., morning fatigue) for 2 consecutive days. Mean morning and evening fatigue scores were calculated.
Data analysis
Data were analyzed using SPSS Version 19 (SPSS, 2010) and Mplus Version 6.11 (Muthen and Muthen, 1998–2010). Descriptive statistics and frequency distributions were generated on sample characteristics and fatigue severity scores. Analyses of variance and Chi-square analyses were done to evaluate for differences among the fatigue classes identified using GMM.
GMM with robust maximum likelihood estimation was used to identify latent classes (i.e., subgroups of participants) with distinct morning and evening fatigue trajectories over the 6 months of the study (Muthen, 2004). Because 65% of the participants were in patient-caregiver dyads, models were estimated with “dyad” as a clustering variable to ensure that any dependency between the morning and evening fatigue scores for patients and FCs in the same dyad were “controlled for” in the GMM analysis. After taking any dependency within dyads into account, no significant differences were found, between patients and FCs, in the parameter estimates for the various morning and evening fatigue GMM trajectories that were identified in the initial analysis.
The GMM methods are described in detail elsewhere (Dunn et al., 2012). In brief, a single growth curve that represented the “average” change trajectory was estimated for the total sample. Then the number of latent growth classes that best fit the data were identified using published guidelines (Jung and Wickrama, 2008; Nylund et al., 2007; Tofighi and Enders, 2008). Separate GMM analyses were done for morning and evening fatigue.
Adjustments were not made for missing data in comparisons of the classes identified with the GMM. Therefore, the cohort for each analysis was dependent on the largest set of available data across groups. A p-value of <.05 was considered statistically significant. Post hoc contrasts were done using the Bonferroni procedure to control the overall family alpha level of the three possible pairwise contrasts for the three GMM fatigue classes at .05. For any one of the three pairwise contrasts, a p-value of .017 (.05/3) was considered statistically significant.
Results
Participant characteristics
The total sample, which was 46.2% male and 53.8% female, consisted of 167 oncology outpatients and 85 FCs. The majority of the participants were well-educated (i.e., at least two years of college) and Caucasian with a mean age of 61.5 years. Mean KPS score was 92 and the average participant had greater than four co-morbid conditions. No differences were found between the patients and FCs in their KPS scores (91.1 ± 11.9 versus 93.7 ± 10.6, respectively) or number of co-morbid conditions (4.8 ± 2.6 versus 4.2 ± 2.9, respectively). Approximately 49% of the patients had prostate, 38% had breast, 7% had brain, and 6% had lung cancer. At enrollment, no significant differences were found between patients’ and FCs’ ratings of morning (2.3 ± 2.0 versus 2.3 ± 1.9) and evening (4.2 ± 2.0 versus 4.5 ± 2.0) fatigue.
Identification of latent classes using GMM
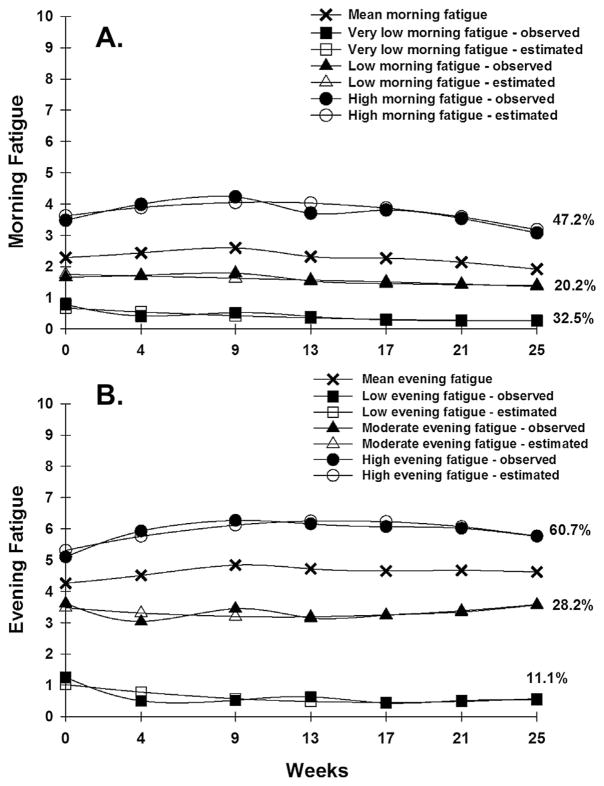
For both morning (Fig. 1A) and evening (Fig. 1B) fatigue, a three class solution provided the best model fit because the Bayesian Information Criterion was smaller than for the two-class and four-class models (Table 1) and each class had a reasonable size and interpretability (Jung and Wickrama, 2008).
Fig. 1.
(A) – Observed and estimated morning Lee Fatigue Scale (LFS) trajectories for participants in each of the latent classes and the mean morning LFS scores for the total sample. (B) – Observed and estimated evening Lee Fatigue Scale (LFS) trajectories for participants in each of the latent classes and the mean evening LFS scores for the total sample.
Table 1.
Fit Indices for morning and evening growth mixture model solutions over seven assessments, with dyad as a clustering variable.
| GMM | LL | AIC | BIC | Entropy | VLMRc |
|---|---|---|---|---|---|
| Morning Fatigue | |||||
| 1-Classa | −2729.675 | 5491.349 | 5547.820 | n/a | n/a |
| 2-Class | −2667.797 | 5377.594 | 5451.712 | 0.775 | 123.755n.s. |
| 3-Classb | −2641.862 | 5335.724 | 5427.489 | 0.811 | 51.870** |
| 4-Class | −2629.867 | 5321.734 | 5431.146 | 0.843 | 23.990n.s. |
| Evening Fatigue | |||||
| 1-Classd | −2837.094 | 5706.188 | 5762.659 | n/a | n/a |
| 2-Class | −2808.314 | 5662.627 | 5743.804 | 0.662 | 57.561** |
| 3-Classe | −2793.976 | 5643.951 | 5742.775 | 0.716 | 28.116* |
| 4-Class | −2779.614 | 5627.227 | 5747.228 | 0.731 | 31.791n.s. |
p < .05,
p < .01,
p > .05.
Abbreviations: AIC = Akaike Information Criteria; BIC = Bayesian Information Criterion; CFI = comparative fit index; GMM = Growth mixture model; LL = log likelihood; n/a = not applicable; n.s. = not significant; RMSEA = root mean square error of approximation; VLMR = Vuong-Lo-Mendell-Rubin likelihood ratio test.
Random coefficients latent growth curve model with linear and quadratic components; Chi2 = 60.528, 26 df, p = .0001, CFI = 0.962, RMSEA = 0.073.
3-class model was selected, based on its having the smallest BIC and a significant VLMR. Further, the VLMR is not significant for the 4-class model, and the 4-class model estimated a class with only 1.5% of the sample – a class size that is unlikely to be reliable.
This number is the Chi2 statistic for the VLMR for morning and evening fatigue. When significant, the VLMR test provides evidence that the K-class model fits the data better than the K-1-class model.
Random coefficients latent growth curve model with linear and quadratic components; Chi2 = 78.126, 26 df, p < .00005, CFI = 0.965, RMSEA = 0.089.
3-class model was selected, based on its having the smallest BIC and a significant VLMR. Further, the VLMR is not significant for the 4-class model.
Parameter estimates for the three classes for morning and evening fatigue are provided in Table 2. The largest percentages of participants were classified in the High Morning Fatigue (47.2%) and High Evening Fatigue (60.7%) classes. At enrollment, High Morning and High Evening Fatigue class participants had a mean LFS score of 3.6 and 5.3, respectively. Both of these scores increased slightly during RT and then decreased slightly after the completion of RT.
Table 2.
Parameter estimates for Morning and Evening Fatigue latent classa solutions over seven assessments, with dyad as a clustering variable.
| Parameter estimatesb | Very Low Morning Fatigue (1)
|
Low Morning Fatigue (2)
|
High Morning Fatigue (3)
|
Low Evening Fatigue (1)
|
Moderate Evening Fatigue (2)
|
High Evening Fatigue (3)
|
|---|---|---|---|---|---|---|
| n = 82 (32.5%) | n = 51 (20.2%) | n = 119 (47.2%) | n = 28 (11.1%) | n = 71 (28.2%) | n = 153 (60.7%) | |
| Means | Mean (S.E.) | Mean (S.E.) | ||||
| Intercept | 0.699***(0.136) | 1.768*** (0.204) | 3.647***(0.203) | 0.968*** (0.250) | 3.424*** (0.337) | 5.267***(0.224) |
| Linear slope | −0.143 (0.086) | −0.057 (0.144) | 0.325*(0.130) | −0.247 (0.129) | −0.180 (0.136) | 0.539***(0.089) |
| Quadratic slope | 0.016 (0.012) | 0.002 (0.020) | −0.060*** (0.019) | 0.032 (0.017) | 0.036 (0.021) | −0.071*** (0.014) |
| Variances | Variance (S.E.) | Variance (S.E.) | ||||
| Intercept | 0c | 0c | 1.580***(0.357) | 0c | 1.342*** (0.367) | 1.484***(0.394) |
| Linear slope | 0c | 0c | 0.052***(0.013) | 0c | 0.059**(0.020) | 0.008 (0.005) |
Abbreviations: S.E. = standard error.
p < .05,
p < .01,
p < .001.
Trajectory group sizes are for classification of individuals based on their most likely latent class probabilities.
Growth mixture model estimates were obtained with robust maximum likelihood, with dyad as a clustering variable to account for dependency between patients and family caregivers within the same dyad. Quadratic slope variances were fixed at zero to improve estimation.
Fixed at zero.
Differences in demographic and clinical characteristics among the morning and evening fatigue classes
Participants in the High Morning Fatigue class were significantly younger than the Low and Very Low Morning Fatigue classes and had a significantly lower KPS score than the Very Low Morning Fatigue class (Table 3).
Table 3.
Differences in baseline demographic and clinical characteristics among the three latent classes for Morning and Evening Fatigue.
| Characteristic | Very Low Morning Fatigue (1) 82 (32.5%)
|
Low Morning Fatigue (2) 51 (20.2%)
|
High Morning Fatigue (3) 119 (47.2%)
|
Statistics and post hoc comparisons for Morning Fatigue | Low Evening Fatigue (1) 28 (11.1%)
|
Moderate Evening Fatigue (2) 71 (28.2%)
|
High Evening Fatigue (3) 153 (60.7%)
|
Statistics and post hoc comparisons for Evening Fatigue | |
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||||
| Age (years) | 64.9 (10.6) | 63.8 (10.1) | 58.2 (11.3) |
F(2,505) = 10.6 p < .001 1 and 2 > 3 |
66.5 (9.0) | 62.5 (12.4) | 60.1 (10.8) | F(1,071) = 4.3, p = .014; 1 > 3 | |
| Education (years) | 15.8 (3.0) | 16.1 (3.4) | 16.0 (2.9) | ns | 15.4 (3.2) | 15.5 (2.8) | 16.2 (3.0) | ns | |
| Number of comorbid conditions | 4.1 (2.7) | 4.7 (2.6) | 4.9 (2.8) | ns | 3.9 (2.9) | 4.7 (2.5) | 4.7 (2.7) | ns | |
| Weight (pounds) | 178.5 (34.1) | 175.7 (37.0) | 172.0 (42.0) | ns | 180.0 (36.3) | 181.0 (35.2) | 171.2 (40.2) | ns | |
| KPS Score | 96.5 (7.9) | 92.2 (9.9) | 88.7 (13.2) |
F(2,823) = 11.6 p < .001 1 > 3 |
95.7 (8.4) | 93.6 (11.9) | 90.4 (11.7) | ns | |
|
| |||||||||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||||
|
| |||||||||
| Gender (% female) | 39 (47.6) | 26 (51.0) | 70 (51.9) | ns | 11 (39.3) | 32 (45.1) | 92 (60.1) |
X2 = 7.00 p = .030 3 > 1 and 2 |
|
| Ethnicity (% White) | 66 (80.5) | 41 (80.4) | 81 (68.1) | ns | 19 (67.9) | 52 (73.2) | 116 (76.3) | ns | |
| Lives Alone (% yes) | 14 (26.4) | 12 (40.0) | 27 (32.1) | ns | 10 (52.6) | 16 (28.1) | 27 (29.7) | ns | |
| Married or partnered (% yes) | 61 (74.4) | 34 (66.7) | 79 (67.5) | ns | 17 (60.7) | 47 (66.2) | 110 (72.8) | ns | |
| Children at home (% yes) | 9 (12.9) | 6 (13.6) | 21 (21.6) | ns | 0 (0.0) | 6 (9.7) | 30 (23.8) |
X2 = 11.18 p = .004 3 > 1 and 2 |
|
| Older adult at home (% yes) | 3 (4.3) | 0 (0.0) | 4 (4.0) | ns | 2 (8.7) | 1 (1.6) | 4 (3.1) | ns | |
| Work for pay (% yes) | 34 (42.0) | 23 (46.0) | 58 (50.0) | ns | 10 (35.7) | 34 (48.6) | 71 (47.7) | ns | |
| Patient/FC (% patient) | 53 (64.6) | 30 (58.8) | 84 (70.6) | ns | 19 (67.9) | 57 (80.3) | 91 (59.5) |
X2 = 9.43 p = .009 3 < 2 |
|
Abbreviations: FC = family caregiver; KPS = Karnofsky Performance Status; ns = not significant; SD = standard deviation.
Participants in the High Evening Fatigue class were significantly younger than those in the Low Evening Fatigue class. Compared to the Low and Moderate Evening Fatigue classes, participants in the High Evening Fatigue class were more likely to be female and more likely to have children at home. Compared to the Moderate Evening Fatigue class, participants in the High Evening Fatigue class were more likely to be a FC (Table 3).
Differences in symptom and QOL scores among the morning and evening fatigue classes
As shown in Table 4, significant differences were found among both the morning and evening fatigue classes for all of the symptom and QOL scores. However, differences in the percentages of participants who reported pain at enrollment were found only among the morning fatigue classes.
Table 4.
Differences in baseline symptom severity and quality of life scores among the three latent classes for Morning and Evening Fatigue.
| Characteristic | Very Low Morning Fatigue (1) 82 (32.5%)
|
Low Morning Fatigue (2) 51 (20.2%)
|
High Morning Fatigue (3) 119 (47.2%)
|
Statistics and post hoc comparisons for Morning Fatigue | Low Evening Fatigue (1) 28 (11.1%)
|
Moderate Evening Fatigue (2) 71 (28.2%)
|
High Evening Fatigue (3) 153 (60.7%)
|
Statistics and post hoc comparisons for Evening Fatigue |
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| Depression | 3.8 (4.6) | 7.2 (6.1) | 12.9 (8.8) |
F(2,242) = 39.97 p < .0001 1 < 2 < 3 |
3.0 (2.9) | 5.0 (5.2) | 11.6 (8.7) |
F(2,242) = 28.26 p < .0001 |
| Trait anxiety | 27.7 (7.7) | 33.6 (8.2) | 38.6 (9.6) |
F(2,246) = 37.52 p < .0001 1 < 2 < 3 |
26.0 (5.5) | 30.8 (8.9) | 37.1 (9.7) |
F(2,246) = 23.66 p < .0001 1 and 2 < 3 |
| State anxiety | 25.2 (8.3) | 29.3 (8.3) | 35.4 (11.3) |
F(2,243) = 25.81 p < .0001 1 and 2 < 3 |
23.6 (5.0) | 27.7 (8.9) | 33.8 (11.3) |
F(2,243) = 16.55 p < .0001 1 and 2 < 3 |
| Sleep disturbance | 27.1 (12.5) | 37.3 (16.7) | 47.7 (18.4) |
F(2,247) = 38.57 p < .0001 1 < 2<3 |
25.7 (12.9) | 32.4 (15.3) | 44.3 (18.8) |
F(2,247) = 20.56 p < .0001 1 and 2 < 3 |
| Attentional function | 8.2 (1.3) | 7.4 (1.4) | 6.4 (1.8) |
F(2,244) = 33.54 p < .0001 1 > 2>3 |
8.6 (1.0) | 7.7 (1.5) | 6.7 (1.8) |
F(2,244) = 20.15 p < .0001 1 > 2>3 |
| Quality of life | 7.8 (1.0) | 7.4 (1.0) | 6.2 (1.6) |
F(2,243) = 39.63 p < .0001 1 and 2 > 3 |
8.1 (0.7) 1 > 2>3 |
7.3 (1.3) | 6.5 (1.5) |
F(2,243) = 18.92 p < .0001 |
|
| ||||||||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||
|
| ||||||||
| Pain (% yes) | 26 (31.7) | 21 (41.2) | 73 (61.3) | χ2 = 18.16 p < .0001 1 < 3 | 0 (0.0) | 18 (51.4) | 76 (57.14) | ns |
Abbreviations: ns = not significant; SD = standard deviation.
Overlap in membership between morning and evening fatigue latent classes
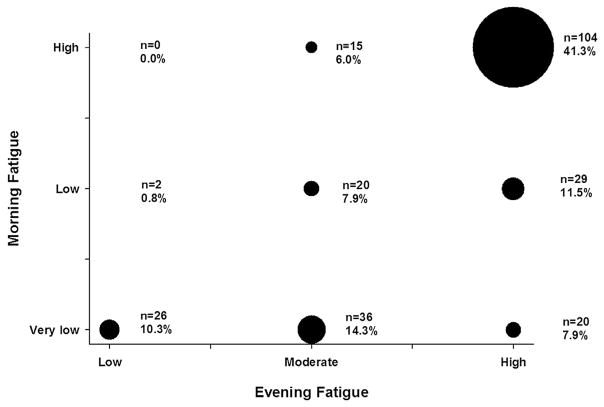
Fig. 2 illustrates the overlap in membership between the morning and evening fatigue latent classes. Of the 252 participants enrolled in this study, 41.3% were classified in both the High Morning and High Evening Fatigue latent classes. In addition, 10.3% of the participants were classified in both the Very Low Morning and Low Evening Fatigue latent classes.
Fig. 2.
Bubble plot of the overlap in latent class membership between the morning and evening fatigue latent classes.
Discussion
This study is the first to use GMM to identify distinct subgroups of participants based on their self-report ratings of morning and evening fatigue. While three distinct latent classes were identified for both morning and evening fatigue, different demographic and clinical characteristics predicted latent class membership. In addition, some differences in symptom severity and QOL scores were found between the morning and evening fatigue latent classes. Finally, only about 50% of the participants were classified in both the Very Low Morning and Low Evening or High Morning and High Evening Fatigue latent classes. These findings support our hypothesis that morning and evening fatigue may be distinct but related symptoms.
While some researchers might disagree with the approach of combining data from patients and FCs, in this study, using a generic rather than a disease-specific fatigue measure, no differences were found in patients’ and FCs’ ratings of the severity of morning or evening fatigue. In addition, compared to the Moderate Evening Fatigue class, participants in the High Evening Fatigue class were more likely to be an FC. This finding is consistent with previous reports from our research group (Carney et al., 2011; Fletcher et al., 2008) and others (Given et al., 1993) that demonstrate that FCs report symptoms of similar or greater severity than the patient. While some of the etiologies for fatigue may be different for patients and FCs, additional research is warranted to elucidate the common biological mechanisms and final common pathways that underlie morning and evening fatigue in both oncology patients and their FCs.
Findings from the GMM analysis for morning fatigue suggest that over 50% of the patients and FCs awoke from sleep, with low levels of morning fatigue and that this trajectory persisted for the entire 6 months of the study. In contrast, 47.2% of the participants reported morning fatigue scores that were above the cutpoint for clinically meaningful levels of fatigue (i.e., ≥3.2). Consistent with previous reports of mean fatigue severity scores (Hickok et al., 2005; Irvine et al., 1998; Jereczek-Fossa et al., 2002; Wratten et al., 2004), this fatigue trajectory was quadratic with a slight increase during RT followed by a slight decrease after RT. Of note, clinically meaningful levels of fatigue persisted in patients and FCs, in this study, for four months after the completion of RT. Younger age and poorer functional status were the only characteristics that distinguished between the High and Very Low Morning Fatigue classes. These same characteristics were found to be associated with higher mean fatigue severity scores in several studies (Curt et al., 2000; Dhrupad et al., 2010; Fletcher et al., 2008; Jereczek-Fossa et al., 2007; Miaskowski et al., 2008).
Findings from the GMM analysis for evening fatigue suggest that 60.7% of participants reported evening fatigue scores that were above the clinically meaningful cutpoint of ≥5.6 for most of the study. Like the High Morning Fatigue class, the trajectory for the High Evening Fatigue class was quadratic with a slight increase during RT followed by a slight decrease after RT. The trajectories for the other two evening fatigue classes were relatively flat over the six months of the study.
Younger age was the only demographic characteristic that was associated with membership in both the highest morning and evening fatigue classes. This association is seen consistently not only with fatigue (Janz et al., 2007; Jereczek-Fossa et al., 2007) but with other common symptoms in patients and FCs (Dunn et al., 2012; Miaskowski et al., 2012a, 2012b). While findings regarding gender differences in symptom severity are inconsistent (Baldwin et al., 2009; Cheung et al., 2011; Hickok et al., 2005), in our study, a higher percentage of female participants were classified into the High Evening Fatigue class. This risk factor may be related to two other characteristics that were associated with membership in the High Evening Fatigue class, namely caring for children at home and being a FC. In the total sample, while equal numbers of males and females were caring for children at home (17.0%), a significantly higher percentage of the FCs were female (i.e., 71.8%). These findings suggest that the added responsibilities associated with the provision of care increase the severity and duration of evening fatigue. Fatigue severity might decrease if education and resources were available to assist patients and FCs to modify caregiving routines and responsibilities.
Previous studies have demonstrated associations between fatigue and other common symptoms (Clevenger et al., 2012; Garrett et al., 2011; Liu et al., 2012), as well as QOL outcomes (Cheng and Lee, 2011; Fletcher et al., 2008), in oncology patients and their FCs. As shown in Table 4, for the morning fatigue GMM analysis, for all of the symptoms except state anxiety and pain, symptom severity scores differed significantly among all three latent classes. Post hoc contrasts demonstrated that the High Morning Fatigue class had significantly higher symptom severity scores than the Low Morning Fatigue class. In addition, the Low Morning Fatigue Class had significantly higher symptom severity scores than the Very Low Morning Fatigue class. In contrast, the findings from the evening fatigue GMM analysis found that depression, trait anxiety, state anxiety, and sleep disturbance scores were similar for the Low and Moderate Evening fatigue classes but significantly lower than for the High Evening Fatigue class. These findings suggest that the associations between common symptoms and morning fatigue differ from evening fatigue and may be related to differences in the underlying mechanisms for morning and evening fatigue (e.g., diurnal variations in the release of cytokines (Bower et al., 2011; Collado-Hidalgo et al., 2006)).
In order to further support our hypothesis that morning and evening fatigue may be distinct but related symptoms, we evaluated the percentages of participants who reported low and high levels of both symptoms. If these two symptoms had identical etiologies and/or underlying mechanisms, one would hypothesize that participants would self-report either high or low levels of both symptoms. As illustrated in Fig. 2, only 10.3% of participants were classified as having low levels of both morning and evening fatigue and 41.3% of participants were classified as having high levels of both symptoms. However, 48.4% of the participants were classified into distinct morning and evening fatigue classes. Along with differences in phenotypic characteristics between the morning and evening fatigue classes, this lack of clear overlap in membership in morning and evening fatigue classes with similar severity ratings lends additional support to our working hypothesis. Additional research is warranted to determine the specific phenotypic and genotypic characteristics that underlie the development and maintenance of higher levels of morning and evening fatigue.
Several limitations need to be acknowledged. While the sample size for GMM analysis was adequate (Tofighi and Enders, 2008), larger samples may identify additional latent classes. In addition, the predictors associated with latent class membership for both morning and evening fatigue must be interpreted with caution until they are replicated in future studies. Ideally future studies should be done with sample sizes that allow for better differentiation of morning and evening fatigue in terms of both phenotypic predictors and underlying molecular mechanisms. Finally, participants were assessed for only six months. Future studies need to evaluate for phenotypic and molecular characteristics that contribute to the maintenance of persistent fatigue in oncology patients and their FCs.
Findings from this study suggest that morning and evening fatigue are distinct but related symptoms that warrant confirmation in future studies. The lack of attention to the unique features of morning and evening fatigue may explain why fatigue remains a refractory and challenging symptom for both oncology patients and their FCs. Future studies need to evaluate for phenotypic and molecular characteristics that distinguish between individuals who report low levels of both morning and evening fatigue and high levels of both symptoms. If distinguishing characteristics are identified they may guide the development and testing of different interventions for morning and evening fatigue.
Acknowledgments
This research was supported by a grant from the National Institute of Nursing Research (NINR; NR04835). Dr. Miaskowski is funded by the American Cancer Society (ACS) as a Clinical Research Professor. Dr. Dhrupad is funded through NIH Mentored Patient-Oriented Research Career Development Award (K23 AT005340). Dr. Aouizerat received funding through the National Institutes of Health Roadmap for Medical Research Grant (KL2 RR624130). Dr. Dunn received funding from the Mount Zion Health Fund and the UCSF Academic Senate. Dr. Langford is supported by a Department of Defense Breast Cancer Research Program Postdoctoral Fellowship. Mr. Merriman is supported by an NINR fellowship (F31 NR012604), an ACS Doctoral Degree Scholarship (DSCN-10-087), an Oncology Nursing Society Doctoral Scholarship, and a UCSF Nursing Alumni Association Scholarship. Dr. Baggott is supported by an ACS Mentored Research Scholar Grant (MRSG-12-01-PCSM). Dr. Leutwyler is funded by the KL2 Program (RR624130).
Footnotes
Conflict of interest
None declared.
Disclosures
Dr. Cataldo was supported by an Oncology Nursing Society Fellowship. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Aouizerat BE, Dodd M, Lee K, West C, Paul SM, Cooper BA, et al. Preliminary evidence of a genetic association between tumor necrosis factor alpha and the severity of sleep disturbance and morning fatigue. Biological Research for Nursing. 2009;11:27–41. doi: 10.1177/1099800409333871. [DOI] [PubMed] [Google Scholar]
- Baldwin CM, Grant M, Wendel C, Hornbrook MC, Herrinton LJ, McMullen C, et al. Gender differences in sleep disruption and fatigue on quality of life among persons with ostomies. Journal of Clinical Sleep Medicine. 2009;5:335–343. [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Arevalo JM, Cole SW. Fatigue and gene expression in human leukocytes: increased NF-kappaB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behavior and Immunity. 2011;25:147–150. doi: 10.1016/j.bbi.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney S, Koetters T, Cho M, West C, Paul SM, Dunn L, et al. Differences in sleep disturbance parameters between oncology outpatients and their family caregivers. Journal of Clinical Oncology. 2011;29:1001–1006. doi: 10.1200/JCO.2010.30.9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KK, Lee DT. Effects of pain, fatigue, insomnia, and mood disturbance on functional status and quality of life of elderly patients with cancer. Critical Reviews in Oncology/Hematology. 2011;78:127–137. doi: 10.1016/j.critrevonc.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Cheung WY, Le LW, Gagliese L, Zimmermann C. Age and gender differences in symptom intensity and symptom clusters among patients with metastatic cancer. Supportive Care in Cancer. 2011;19:417–423. doi: 10.1007/s00520-010-0865-2. [DOI] [PubMed] [Google Scholar]
- Cimprich B. Attentional fatigue following breast cancer surgery. Research in Nursing and Health. 1992;15:199–207. doi: 10.1002/nur.4770150306. [DOI] [PubMed] [Google Scholar]
- Cimprich B, So H, Ronis DL, Trask C. Pre-treatment factors related to cognitive functioning in women newly diagnosed with breast cancer. Psychooncology. 2005;14:70–78. doi: 10.1002/pon.821. [DOI] [PubMed] [Google Scholar]
- Cimprich B, Visovatti M, Ronis DL. The attentional function index - a self-report cognitive measure. Psychooncology. 2011;20:194–202. doi: 10.1002/pon.1729. [DOI] [PubMed] [Google Scholar]
- Clevenger L, Schrepf A, Christensen D, DeGeest K, Bender D, Ahmed A, et al. Sleep disturbance, cytokines, and fatigue in women with ovarian cancer. Brain Behavior and Immunity. 2012;26:1037–1044. doi: 10.1016/j.bbi.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clinical Cancer Research. 2006;12:2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the fatigue coalition. Oncologist. 2000;5:353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin brief pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- Dhrupad A, Dodd M, Paul SM, Cooper BA, Lee K, West C, et al. Trajectories of fatigue in patients with breast cancer before, during, and after radiation therapy. Cancer Nursing. 2010;33:201–212. doi: 10.1097/NCC.0b013e3181c75f2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhrupad A, Lee K, Paul SM, West C, Dunn L, Dodd M, et al. Sleep-wake circadian activity rhythms and fatigue in family caregivers of oncology patients. Cancer Nursing. 2012;35:70–81. doi: 10.1097/NCC.0b013e3182194a25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimsdale JE, Ancoli-Israel S, Ayalon L, Elsmore TF, Gruen W. Taking fatigue seriously, II: variability in fatigue levels in cancer patients. Psychosomatics. 2007;48:247–252. doi: 10.1176/appi.psy.48.3.247. [DOI] [PubMed] [Google Scholar]
- Donovan KA, Small BJ, Andrykowski MA, Munster P, Jacobsen PB. Utility of a cognitive-behavioral model to predict fatigue following breast cancer treatment. Health Psychology. 2007;26:464–472. doi: 10.1037/0278-6133.26.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LB, Aouizerat BE, Cooper BA, Dodd M, Lee K, West C, et al. Trajectories of anxiety in oncology patients and family caregivers during and after radiation therapy. European Journal of Oncology Nursing. 2012;16:1–9. doi: 10.1016/j.ejon.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LB, Cooper BA, Neuhaus J, West C, Paul S, Aouizerat B, et al. Identification of distinct depressive symptom trajectories in women following surgery for breast cancer. Health Psychology. 2011;30:683–692. doi: 10.1037/a0024366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell BR. The impact of pain on quality of life. A decade of research. Nursing Clinics of North America. 1995;30:609–624. [PubMed] [Google Scholar]
- Ferrell BR, Dow KH, Grant M. Measurement of the quality of life in cancer survivors. Quality of Life Research. 1995a;4:523–531. doi: 10.1007/BF00634747. [DOI] [PubMed] [Google Scholar]
- Ferrell BR, Dow KH, Leigh S, Ly J, Gulasekaram P. Quality of life in long-term cancer survivors. Oncology Nursing Forum. 1995b;22:915–922. [PubMed] [Google Scholar]
- Fletcher BA, Schumacher KL, Dodd M, Paul SM, Cooper BA, Lee K, et al. Trajectories of fatigue in family caregivers of patients undergoing radiation therapy for prostate cancer. Research in Nursing and Health. 2009;32:125–139. doi: 10.1002/nur.20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher BS, Paul SM, Dodd MJ, Schumacher K, West C, Cooper B, et al. Prevalence, severity, and impact of symptoms on female family caregivers of patients at the initiation of radiation therapy for prostate cancer. Journal of Clinical Oncology. 2008;26:599–605. doi: 10.1200/JCO.2007.12.2838. [DOI] [PubMed] [Google Scholar]
- Garrett K, Dhrupad A, Koetters T, West C, Paul SM, Dunn LB, et al. Differences in sleep disturbance and fatigue between patients with breast and prostate cancer at the initiation of radiation therapy. Journal of Pain and Symptom Management. 2011;42:239–250. doi: 10.1016/j.jpainsymman.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay CL, Lee KA, Lee SY. Sleep patterns and fatigue in new mothers and fathers. Biological Research for Nursing. 2004;5:311–318. doi: 10.1177/1099800403262142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Given CW, Stommel M, Given B, Osuch J, Kurtz ME, Kurtz JC. The influence of cancer patients’ symptoms and functional states on patients’ depression and family caregivers’ reaction and depression. Health Psychology. 1993;12:277–285. doi: 10.1037//0278-6133.12.4.277. [DOI] [PubMed] [Google Scholar]
- Gupta D, Lis CG, Grutsch JF. The relationship between cancer-related fatigue and patient satisfaction with quality of life in cancer. Journal of Pain and Symptom Management. 2007;34:40–47. doi: 10.1016/j.jpainsymman.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Henry DH, Viswanathan HN, Elkin EP, Traina S, Wade S, Cella D. Symptoms and treatment burden associated with cancer treatment: results from a cross-sectional national survey in the U.S. Supportive Care in Cancer. 2008;16:7791–7801. doi: 10.1007/s00520-007-0380-2. [DOI] [PubMed] [Google Scholar]
- Hickok JT, Roscoe JA, Morrow GR, Mustian K, Okunieff P, Bole CW. Frequency, severity, clinical course, and correlates of fatigue in 372 patients during 5 weeks of radiotherapy for cancer. Cancer. 2005;104:1772–1778. doi: 10.1002/cncr.21364. [DOI] [PubMed] [Google Scholar]
- Hoffman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12 (Suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- Hou WK, Law CC, Yin J, Fu YT. Resource loss, resource gain, and psychological resilience and dysfunction following cancer diagnosis: a growth mixture modeling approach. Health Psychology. 2010;29:484–495. doi: 10.1037/a0020809. [DOI] [PubMed] [Google Scholar]
- Irvine DM, Vincent L, Graydon JE, Bubela N. Fatigue in women with breast cancer receiving radiation therapy. Cancer Nursing. 1998;21:127–135. doi: 10.1097/00002820-199804000-00006. [DOI] [PubMed] [Google Scholar]
- Jansen CE, Dodd MJ, Miaskowski CA, Dowling GA, Kramer J. Preliminary results of a longitudinal study of changes in cognitive function in breast cancer patients undergoing chemotherapy with doxorubicin and cyclophosphamide. Psychooncology. 2008;17:1189–1195. doi: 10.1002/pon.1342. [DOI] [PubMed] [Google Scholar]
- Janz NK, Mujahid M, Chung LK, Lantz PM, Hawley ST, Morrow M, et al. Symptom experience and quality of life of women following breast cancer treatment. Journal of Womens Health. 2007;16:1348–1361. doi: 10.1089/jwh.2006.0255. [DOI] [PubMed] [Google Scholar]
- Jereczek-Fossa BA, Marsiglia HR, Orecchia R. Radiotherapy-related fatigue. Critical Reviews in Oncology/Hematology. 2002;41:317–325. doi: 10.1016/s1040-8428(01)00143-3. [DOI] [PubMed] [Google Scholar]
- Jereczek-Fossa BA, Santoro L, Alterio D, Franchi B, Fiore MR, Fossati P, et al. Fatigue during head-and-neck radiotherapy: prospective study on 117 consecutive patients. International Journal of Radiation Oncology, Biology, Physics. 2007;68:403–415. doi: 10.1016/j.ijrobp.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Jim HS, Small B, Faul LA, Franzen J, Apte S, Jacobsen PB. Fatigue, depression, sleep, and activity during chemotherapy: daily and intraday variation and relationships among symptom changes. Annals of Behavioral Medicine. 2011;42:321–333. doi: 10.1007/s12160-011-9294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T, Wickrama KAS. An introduction to latent class growth analysis and growth mixture modeling. Social and Personality Psychology Compass. 2008;2:302–317. [Google Scholar]
- Lam WW, Shing YT, Bonanno GA, Mancini AD, Fielding R. Distress trajectories at the first year diagnosis of breast cancer in relation to 6 years survivorship. Psychooncology. 2012;21:90–99. doi: 10.1002/pon.1876. [DOI] [PubMed] [Google Scholar]
- Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. Journal of the National Cancer Institute. 2004;32:40–50. doi: 10.1093/jncimonographs/lgh027. [DOI] [PubMed] [Google Scholar]
- Lee KA. Self-reported sleep disturbances in employed women. Sleep. 1992;15:493–498. doi: 10.1093/sleep/15.6.493. [DOI] [PubMed] [Google Scholar]
- Lee KA, DeJoseph JF. Sleep disturbances, vitality, and fatigue among a select group of employed childbearing women. Birth. 1992;19:208–213. doi: 10.1111/j.1523-536x.1992.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Research. 1991;36:291–298. doi: 10.1016/0165-1781(91)90027-m. [DOI] [PubMed] [Google Scholar]
- Liu L, Mills PJ, Rissling M, Fiorentino L, Natarajan L, Dimsdale JE, et al. Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain Behavior and Immunity. 2012;26:706–713. doi: 10.1016/j.bbi.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C, Cooper B, Paul SM, West C, Langford D, Levine JD, et al. Identification of patient subgroups and risk factors for persistent breast pain following breast cancer surgery. J Pain. 2012a;13:1172–1187. doi: 10.1016/j.jpain.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C, Cooper BA, Dhrupad A, Dunn LB, Langford DJ, Cataldo JK, et al. Evidence of associations between cytokine genes and subjective reports of sleep disturbance in oncology patients and their family caregivers. PLoS One. 2012b;7:e40560. doi: 10.1371/journal.pone.0040560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C, Cooper BA, Paul SM, Dodd M, Lee K, Aouizerat BE, et al. Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: a cluster analysis. Oncology Nursing Forum. 2006;33:E79–E89. doi: 10.1188/06.ONF.E79-E89. [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Dodd M, Lee K, West C, Paul SM, Cooper BA, et al. Preliminary evidence of an association between a functional interleukin-6 polymorphism and fatigue and sleep disturbance in oncology patients and their family caregivers. Journal of Pain and Symptom Management. 2010;40:531–544. doi: 10.1016/j.jpainsymman.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C, Lee K, Dunn L, Dodd M, Aouizerat BE, West C, et al. Sleep-wake circadian activity rhythm parameters and fatigue in oncology patients before the initiation of radiation therapy. Cancer Nursing. 2011;34:255–268. doi: 10.1097/NCC.0b013e3181f65d9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C, Lee KA. Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: a pilot study. Journal of Pain and Symptom Management. 1999;17:320–332. doi: 10.1016/s0885-3924(99)00008-1. [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Paul SM, Cooper BA, Lee K, Dodd M, West C, et al. Trajectories of fatigue in men with prostate cancer before, during, and after radiation therapy. Journal of Pain and Symptom Management. 2008;35:632–643. doi: 10.1016/j.jpainsymman.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molassiotis A, Chan CW. Fatigue patterns in Chinese patients receiving radiotherapy. European Journal of Oncology Nursing. 2004;8:334–340. doi: 10.1016/j.ejon.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Muthen BO. Latent variable analysis: growth mixture modeling and related techniques for longitudinal data. In: Kaplan DW, editor. Handbook of Quantitative Methodology for the Social Sciences. Sage Publications; Newbury Park, CA: 2004. pp. 345–368. [Google Scholar]
- Muthen LK, Muthen BO. Mplus User’s Guide. 6. Muthen & Muthen; Los Angeles, CA: 1998–2010. [Google Scholar]
- Nylund KL, Asparouhov T, Muthen BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Structural Equation Modeling. 2007;14:535–569. [Google Scholar]
- Padilla GV, Ferrell B, Grant MM, Rhiner M. Defining the content domain of quality of life for cancer patients with pain. Cancer Nursing. 1990;13:108–115. [PubMed] [Google Scholar]
- Padilla GV, Presant C, Grant MM, Metter G, Lipsett J, Heide F. Quality of life index for patients with cancer. Research in Nursing and Health. 1983;6:117–126. doi: 10.1002/nur.4770060305. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rose JH, Kypriotakis G, Bowman KF, Einstadter D, O’Toole EE, Mechekano R, et al. Patterns of adaptation in patients living long term with advanced cancer. Cancer. 2009;115:4298–4310. doi: 10.1002/cncr.24584. [DOI] [PubMed] [Google Scholar]
- Sheehan TJ, Fifield J, Reisine S, Tennen H. The measurement structure of the center for epidemiologic studies depression scale. Journal of Personality Assessment. 1995;64:507–521. doi: 10.1207/s15327752jpa6403_9. [DOI] [PubMed] [Google Scholar]
- Spielberger CG, Gorsuch RL, Suchene R, Vagg PR, Jacobs GA. Manual for the State-Anxiety (Form Y): Self Evaluation Questionnaire. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- SPSS. IBM SPSS for Windows (Version 19) SPSS, Inc; Chicago, Illinois: 2010. [Google Scholar]
- Swore Fletcher BA, Dodd MJ, Schumacher KL, Miaskowski C. Symptom experience of family caregivers of patients with cancer. Oncology Nursing Forum. 2008;35:E23–E44. doi: 10.1188/08.ONF.E23-E44. [DOI] [PubMed] [Google Scholar]
- Tofighi D, Enders CK. Identifying the Correct Number of Classes in Growth Mixture Models. Information Age Publishing; Charlotte, NC: 2008. [Google Scholar]
- Van Onselen C, Cooper BA, Lee K, Dunn L, Aouizerat BE, West C, et al. Identification of distinct subgroups of breast cancer patients based on self-reported changes in sleep disturbance. Supportive Care in Cancer. 2012;20:2611–2619. doi: 10.1007/s00520-012-1381-3. [DOI] [PubMed] [Google Scholar]
- Vigilant NJ, Breitbart W, Cella D, Curt GA, Groopman JE, Horning SJ, et al. Patient, caregiver, and oncologist perceptions of cancer-related fatigue: results of a tripart assessment survey. The Fatigue Coalition. Seminars in Hematology. 1997;34:4–12. [PubMed] [Google Scholar]
- Wratten C, Kilmurray J, Nash S, Seldon M, Hamilton CS, O’Brien PC. Fatigue during breast radiotherapy and its relationship to biological factors. International Journal of Radiation Oncology, Biology, Physics. 2004;59:160–167. doi: 10.1016/j.ijrobp.2003.10.008. [DOI] [PubMed] [Google Scholar]