Abstract
Aortic aneurysms are life-threatening and often associated with defects in connective tissues and mutations in smooth muscle cell (SMC) contractile proteins. Despite recent advances in understanding altered signaling in aneurysms of Marfan syndrome, the underlying mechanisms and options for pharmacological treatment for other forms of aneurysms are still under investigation. We previously showed in mice that deficiency in the fibulin-4 gene in vascular SMCs (Fbln4SMKO) leads to loss of the SMC contractile phenotype, hyperproliferation and ascending aortic aneurysms. Here, we report that abnormal upregulation of angiotensin converting enzyme (ACE) in SMCs and subsequent activation of angiotensin II (AngII) signaling is involved in the onset of aortic aneurysms in Fbln4SMKO mice. In this model, aneurysm formation was completely prevented by inhibition of the AngII pathway with losartan or captopril within a narrow therapeutic window during the first month of life, even though the altered mechanical properties of blood vessel walls were not reversed by the pharmacological treatment. The therapeutic effects of losartan in Fbln4SMKO mice do not require the AngII receptor type 2 (Agtr2) but likely require both type 1a (Agtr1a) and 1b (Agtr1b) receptors. The results indicate that fibulin-4 is a vascular matrix component required for regulation of local angiotensin signaling, aortic aneurysms, and development and maintenance of the SMC phenotype.
Introduction
Aortic aneurysms are associated with extrinsic or intrinsic defects of the blood vessel wall, including compromised vessel integrity and alterations of contractile proteins in vascular smooth muscle cells (SMCs) (reviewed in (1,2)). Dysregulation of TGFß has been shown to be a central mechanism of the pathology in various forms of thoracic aortic aneurysms in humans (3-5), a mouse model of Marfan syndrome (Fbn1C1039G/+) and Fbln4 hypomorphic mice (6,7). Inhibition of the angiotensin II (AngII) receptor type 1 (Agtr1) by angiotensin receptor blockade (ARB) was suggested to inhibit transcription of TGFß in Marfan mice (8) or lower blood pressure in Fbln4 hypomorphic mice (9), and was effective in attenuation of the aneurysm phenotype in both mouse models (8,9). However, a fundamental question remains whether the endogenous angiotensin system plays a causal role in aneurysm formation and whether ARB therapy can be applied to all congenital aortic aneurysms as a universal therapy.
Fibulin-4 localizes to microfibrils of elastic fibers and possesses multiple functions pertaining to vascular development, including binding to elastin and elastin cross-linking enzymes to assist in the formation of elastic fibers and maintenance of a SMC contractile phenotype (10-13). Mutations in Fbln4 in humans cause cutis laxa with various systemic vascular defects, including arterial tortuosity, aneurysms and stenosis (14-16). We have previously shown that proliferation of SMCs precedes aneurysm formation, with a marked increase of phosphorylated (p-) ERK1/2 in the ascending aorta of mice that have a deletion of Fbln4 specifically in vascular SMCs (termed Fbln4SMKO) (13), suggesting that soluble factor(s) may be involved in the initiation and/or progression of aneurysmal lesions. Here, we used the Fbln4SMKO mouse model to further explore mechanisms underlying aneurysm development associated with defects in vascular matrix proteins.
Results
ACE is upregulated in vascular SMCs of the aneurysmal wall in Fbln4SMKO mice
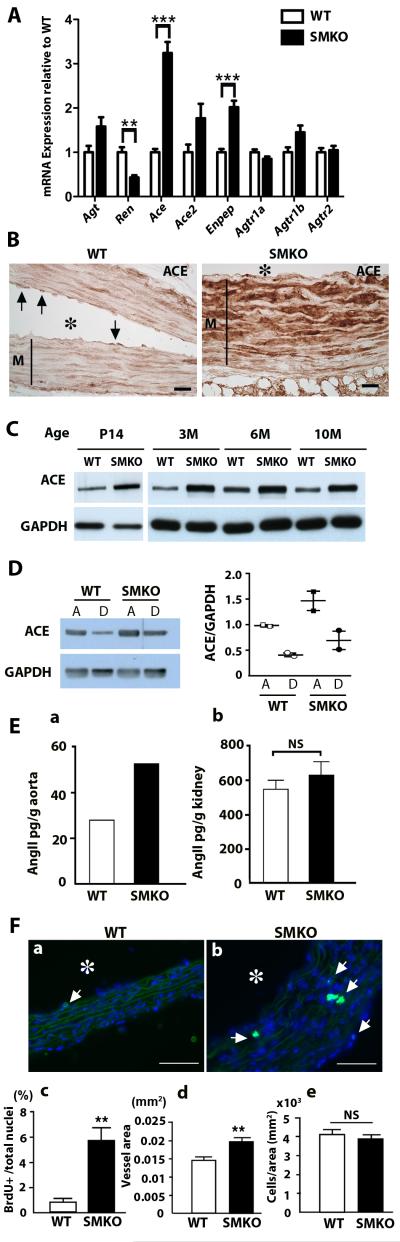
To search for signaling pathways involved in proliferative changes of SMCs with elevated p-ERK1/2 in aneurysms of Fbln4SMKO mice, we performed qPCR analyses on Fbln4SMKO aortas at 1 month of age. We found that transcripts of angiotensin converting enzyme (Ace) and endopeptidase (Enpep) were increased 2.5-3 times (P =0.0001 and P =0.0008 for Ace and Enpep, respectively) whereas renin (Ren) was decreased by half in the Fbln4SMKO ascending aorta (P=0.0039, Fig. 1A). Ace and Enpep were also upregulated in the descending aorta where aneurysms did not develop (Fig. S1). No differences in Ace expression between wild-type and mutants were detected in the lung, but a 1.5-fold increase in Ace expression was observed in the kidney of Fbln4SMKO mice (Fig. S2). Amounts of angiotensinogen (Agt), a precursor for angiotensin I and substrate for renin, were comparable between the liver of Fbln4SMKO and wild-type (Fig. S2). Consistent with qPCR results, high amounts of ACE were detected by immunostaining of aneurysmal wall SMCs in mutant aorta, whereas in the wild-type aorta, ACE staining was confined to endothelial cells (Fig. 1B). Upregulation of ACE was apparent in 14-day-old Fbln4SMKO aorta and it persisted throughout adult life (Fig. 1C, Fig. S3). ACE was higher in ascending aorta compared to descending aorta in wild-type and Fbln4SMKO mice (Fig. 1D). ACE expression in primary SMCs from Fbln4SMKO aortas, however, was comparable to that of SMCs from wild type aortas, suggesting that loss of Fbln4 was not sufficient to induce ACE in vitro (Fig. S4), and that additional mechanical or soluble factors from other cell types or disruption of cell-matrix connections may need to be present.
Fig. 1.
Upregulation of ACE in the ascending aorta of Fbln4SMKO (SMKO) mice. (A) qPCR analysis of mRNA from aortas of wild-type (WT, n=4) and SMKO (n=4) mice at 1 month of age. Two-tailed Student t-test. Bars are mean ± SEM. ** P < 0.01, *** P < 0.001 (B) ACE immunostaining of the aorta from WT and SMKO mice at 3 months of age. Arrows indicate ACE expressed in endothelial cells in the WT aorta. Asterisk indicates lumen and M indicates medial layer. Bar is 20 μm. (C) Western blot showing ACE upregulation in the mutant mice throughout postnatal and adult life. (D) Western blot showing ACE expression in the ascending (A) and descending (D) aortas of WT and SMKO mice (1-3 month). Experiment was repeated with a different set of animals and quantified (right). (E) Tissue Ang II concentration determined by enzyme immunoassay. a, Entire thoracic aortas from WT (n=16) and SMKO (n=14) mice were pooled and analyzed. b, Kidneys from WT (n=6) and SMKO (n=6) mice were separately analyzed. Two-tailed Student t-test. Bars are mean ± SEM. NS: not significant. (F) BrdU proliferation assay. a-b, Cross sections of WT (a, n=4) and SMKO (b, n=4) aortas were examined. Arrows indicate BrdU-positive cells. Asterisk indicates lumen. Bar is 50 μm. c, Percentage of BrdU+cells per total nuclei. d-e, Comparison of vessel area (d) and cell numbers per vessel area (e). Two-tailed Student t-test. Bars are mean ± SEM.** P < 0.01.
We asked if the local upregulation of ACE resulted in upregulation of Ang II in the mutant aorta. Ang II levels were increased 2-fold in Fbln4SMKO aorta relative to wild-type aorta (Fig. 1Ea), whereas in the kidney, Ang II levels were comparable between genotypes (Fig. 1Eb). This result also indicated that upregulation of Ace resulted in a net increase in local AngII levels despite upregulation of Enpep, which cleaves AngII to Ang III. Because we previously reported that the vessel wall of Fbln4SMKO aorta was thicker and contained immature SMCs (13), we performed proliferation assays in 1-month Fbln4SMKO aorta using bromodeoxyuridine (BrdU). In the mutant aorta, nearly 6% of cells were positive for BrdU compared to less than 1% in the wild-type aorta (P=0.0039, Figs. 1Fa-c). Vessel area was increased in Fbln4SMKO aorta compared to wild-type (P=0.0063, Fig. 1Fd), and cell number per vessel area (mm2) was comparable between genotypes (Fig. 1Fe), indicating that the increase in vessel area reflects an increase in cell number in the mutant aortic wall (Fig. 1Fd-e). These data suggest that local production of AngII due to increased ACE expression in SMCs causes upregulation of local AngII-mediated signaling (e.g., ERK1/2 (17)) leading to hyperproliferation of SMCs in the Fbln4SMKO ascending aorta.
Losartan and captopril effectively prevent the aneurysm phenotype in Fbln4SMKO mice
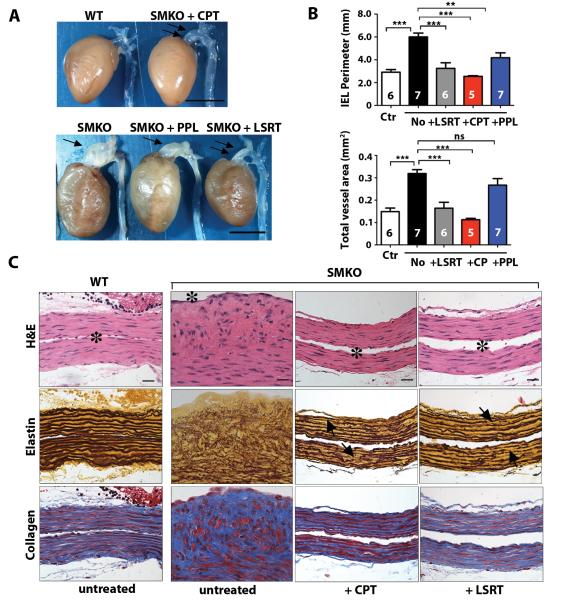
To examine the possible cause-effect relationship of the AngII pathway and aneurysm formation in Fbln4SMKO mice, we performed pharmacological experiments with captopril (ACE inhibitor, 75 mg/l in drinking water ad libitum), losartan (ARB, 0.6 g/l ad libitum) and propranolol (β-adrenergic receptor blocker, 0.5 g/l ad libitum) as previously described (8). Each drug was administered from mid-gestation by giving it to pregnant females, and continued until 3 months of age, when all animals were sacrificed and analyzed. Large aneurysms observed in untreated Fbln4SMKO mice were completely prevented by either losartan or captopril, whereas propranolol showed only modest inhibitory effects on aneurysm formation (Fig. 2A). Similarly, internal elastic lamina (IEL) perimeter and total vessel area were completely normalized with losartan or captopril, whereas propranolol treatment only resulted in mild reduction of IEL perimeter and no effects on the total vessel area (Fig. 2B, Table S1). Histologically, hyperproliferation and disarray of SMCs in the aortic wall were also completely prevented by losartan or captopril (Fig. 2C). Morphologically, SMCs in these treated animals displayed a more normal spindle cell shape with flattened nuclei, and vessel wall thickness was decreased and similar to control. Although the amount of collagen was decreased, elastic fibers remained irregular, and numerous disruptions were observed even after drug treatment (arrows in Fig. 2C). These findings indicate that ligand-dependent activation of Agtr1 is responsible for hyperproliferation of SMCs, and inhibition of AngII signaling by ACE inhibitor or ARB is equivalent and sufficient to prevent aneurysms, but without complete reversal of elastic fiber defects in the aneurysmal wall.
Fig. 2.
Pharmacological prevention of aneurysms in SMKO mice. (A) Gross morphology of the ascending aorta in WT, untreated SMKO, and SMKO mice prenatally treated with captopril (CPT), propranolol (PPL) or losartan (LSRT). A large aneurysm (single arrows) is detected in SMKO and PPL-treated SMKO aorta. CPT or LSRT treatment prevents aneurysm formation (double arrows). Bars are 5 mm. (B) Morphometric analysis of cross sections of the ascending aorta from control (Ctr), untreated, LSRT, CPT or PPL-treated SMKO mice. Internal elastic lamina (IEL) perimeter and total vessel area are shown. One-way ANOVA. Bars are mean ± SEM. * P < 0.05, *** P < 0.001. Number of animals is indicated on each bar. (C) Histological images of the ascending aorta from WT, untreated SMKO, CPT or LSRT-treated SMKO mice stained with H&E, Hart’s stain (for elastin) and Masson-Trichrome stain (for collagen). Lumen is indicated by asterisk. Fuzzy appearance and disruption of elastic fibers is detectable even after CPT or LSRT treatment (arrows). Bars are 20 μm.
Postnatal days 7 to 30 are a critical time window for therapeutic intervention in mice
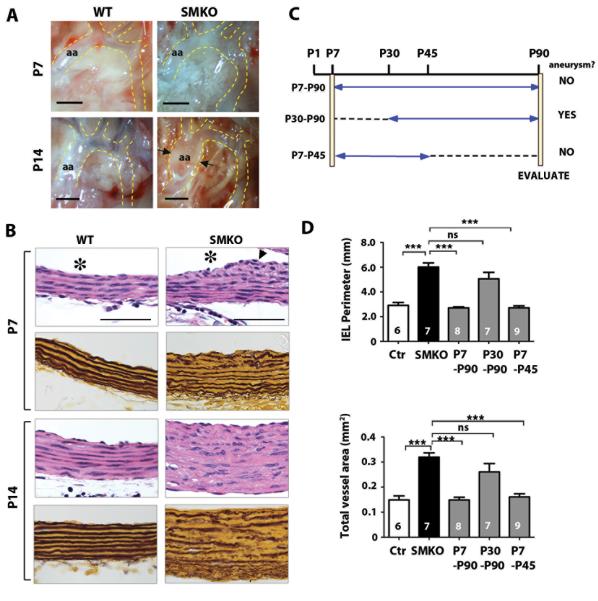
To determine the critical time window for the therapeutic effect of AngII inhibition, we used losartan to further examine the time course for prevention of the aneurysmal phenotype. We first examined early pathological changes in the Fbln4SMKO aorta at postnatal days (P)1, P7, and P14. The ascending aorta was macroscopically indistinguishable between mutant and wild-type at P1 and P7 (Fig. S5A, Fig. 3A), whereas slight dilatation was noted at P14 (arrows in Fig. 3A). The aorta remained histologically normal at P1 in the mutants (Fig. S5B), but cellularity increased by P7, especially in the subendothelial layer and near the adventitia (Fig. 3B, arrowhead). By P14, SMCs were morphologically abnormal, and the proliferation of SMCs extended throughout the medial layer in the mutant aorta (Fig. 3B). These data indicated that the microscopic changes began around P7, and suggested that P7 may be a target time point for postnatal drug treatment.
Fig. 3.
Determining a critical time window for therapeutic intervention. (A) Macroscopic changes in the SMKO ascending aorta at early neonatal stages. Arrows indicate aneurysm. aa; ascending aorta. Bars are 1 mm. (B) Cross sections of ascending aorta at P7 and P14 stained with H&E and Hart’s (elastin). Bars are 50 μm. Lumen is indicated by asterisk. (C) Postnatal losartan administration protocol. All animals were evaluated at 3 months of age. (D) Morphometric analysis of aortas from SMKO mice treated with LSRT at various time points according to the above protocol. One-way ANOVA. Bars are mean ± SEM. *** P < 0.001.
We set up drug treatment protocols (Fig. 3C) in which losartan was administered from P7 to P90, P30 to P90, or P7 to P45, and the ascending aorta was evaluated at P90. Aneurysms were completely prevented if losartan was administered starting at P7, even if treatment stopped at P45 (Fig. 3C and 3D). In contrast, administration of losartan from P30 to P90 did not prevent aneurysm development. These data indicate that the optimal therapeutic window is between P7 and P30, and that transient inhibition of AngII signaling during the early postnatal period is sufficient to prevent aneurysm development at least until 90 days of age.
Blood pressure and vessel mechanical properties are not completely normalized by losartan treatment in Fbln4SMKO mice
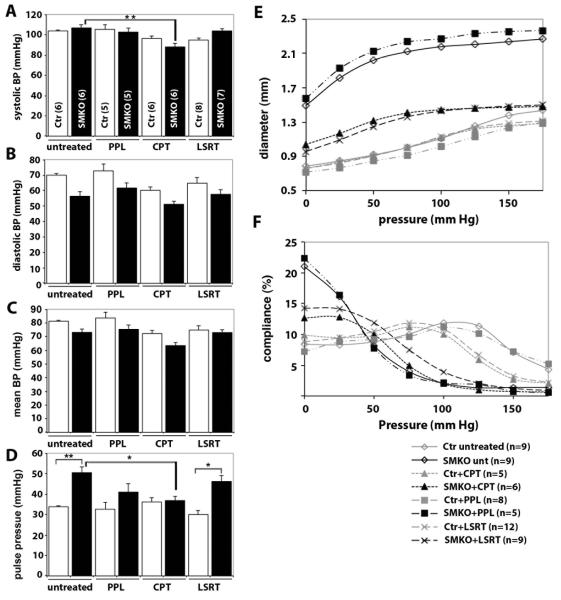
Hemodynamic changes occur during the transition from embryonic to adult circulation, and growth-related increases in blood pressure and body weight are prominent between P7 and P30 during mouse development (18). It is plausible that intrinsic defects in SMCs and the resultant altered response to increasing hemodynamic stress in the elevated AngII microenvironment trigger aneurysm formation in Fbln4SMKO mice. To gain insights into hemodynamic contributions to aneurysm formation, we measured blood pressure in untreated animals and those treated with losartan, propranolol or captopril from P7 to P43 ± 2 (Fig. 4A-D, Table S2). Average arterial systolic pressures were similar in untreated control (104 ± 1 (SEM) mmHg) and Fbln4SMKO mice (107 ± 3 (SEM) mmHg, Fig. 4A); however, there was a trend toward lower average diastolic pressures in Fbln4SMKO mice (Fig. 4B), and thus pulse pressures were increased 50% in untreated Fbln4SMKO mice compared to control (P=0.004, Fig. 4D). This marked increase in pulse pressure in the mutants was similar to what has been observed in Fbln5-null (19) and Fbln4 hypomorphic mice (9), in which elastic laminae are severely disrupted. Captopril decreased systolic blood pressures about 20% in Fbln4SMKO mice (88 ± 3 (SEM) mmHg, n=5, P=0.006, Fig. 4A) and eliminated the increase in pulse pressure observed in untreated Fbln4SMKO mice (Fig. 4D). Losartan did not affect systolic pressures in Fbln4SMKO mice (104 ± 2 (SEM) mmHg, n=7, Fig. 4A), thus pulse pressures remained higher in losartan-treated Fbln4SMKO mice. Propranolol showed no effects on blood pressures in control or Fbln4SMKO mice (Fig 4A-D). Because both captopril and losartan prevented the aneurysm phenotype, but only captopril had significant effects on systolic and pulse pressure in Fbln4SMKO mice, these data suggest that hemodynamic changes are not required to prevent aneurysms in Fbln4SMKO mice.
Fig. 4.
Blood pressure and vessel biomechanics in SMKO mice. (A-D) Average systolic blood pressures (BP), diastolic blood pressures, mean blood pressures and pulse pressures in untreated or propranolol (PPL), captopril (CPT), or losartan (LSRT)-treated animals. Control (Ctr, white bars) and SMKO (black bars) are shown. Number of animals tested is indicated in parentheses. One-way ANOVA. Bars are mean ± SEM. * P < 0.05, ** P < 0.005. (E) Pressure-diameter curves for the ascending aorta in control (gray symbols) and SMKO (black symbols) untreated and drug-treated animals. (F) Pressure-compliance curves for the ascending aorta in control (gray symbols) and SMKO (black symbols) untreated and drug-treated animals. In E and F, oneway ANOVA. Error bars are not included for clarity.
The mechanical stress on the vessel wall is determined both by hemodynamic forces, such as blood pressure, and the mechanical properties of the wall, which are illustrated by pressure-diameter and pressure-compliance relationships. Pressure-diameter curves showed large starting diameters in Fbln4SMKO aorta (~ 2-fold greater than control at zero pressure), indicating the presence of aneurysms in Fbln4SMKO ascending aorta. These diameters were significantly larger than control animals at all pressures tested (P < 0.001, Fig. 4E, Table S3). Compliance of the Fbln4SMKO ascending aorta, defined as the percent change in outer diameter with each 25 mmHg pressure step, was decreased 60-80% in the mid- to high pressure range (75-175 mmHg) compared to the control aorta (P < 0.001, Fig. 4F, Table S3), indicating that the vessel wall is stiffer, similar to elastin heterozygous mutants (20). Thus, the compliance or stiffness of Fbln4SMKO vessels is similar to that of other mouse models with mild to severe elastic fiber defects that do not develop aortic aneurysms, suggesting that mechanical changes alone are not sufficient to induce aneurysm formation.
We then examined whether drug treatment altered the mechanical properties of the ascending aortas. Pressure-diameter curves were almost identical in control vessels regardless of drug treatment. Propranolol-treated Fbln4SMKO aortas showed starting diameters about 2-fold larger than control aortas, similar to untreated Fbln4SMKO animals, indicating the presence of aneurysms (Fig. 4E). Losartan or captopril-treated Fbln4SMKO aortas showed approximately a 20% increase in starting diameter compared to controls but were smaller than untreated and propranolol treated Fbln4SMKO aortas for every pressure tested (P < 0.001) and did not have aneurysms. Compliance of losartan- or captopril-treated Fbln4SMKO aortas was between that of control and untreated or propranolol-treated Fbln4SMKO in the low pressure range (0-50 mmHg); however, all the mutant aortas regardless of drug treatment showed reduced compliance in the high-pressure range (100-175 mmHg) compared to controls (P < 0.001, Fig. 4F). These data indicate that increased stiffness of the vessel wall is not completely reversed pharmacologically. Combined with the blood pressure data, these results show that changes in mechanical stress on the vessel wall are not the primary mechanism for aneurysm prevention in losartan and captopril-treated Fbln4SMKO mice.
Elevated p-ERK1/2 and decreased expression of SMC differentiation markers are reversed by losartan
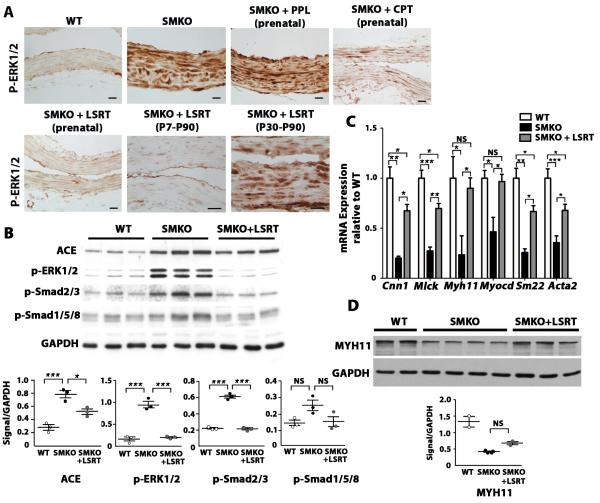
Recently, it was shown in Fbn1C1039G/+ mice that elevated p-ERK1/2 driven by non-canonical TGFß-signaling is responsible for aneurysm development (6). We therefore examined the effect of inhibiting the AngII-Agtr1 pathway on p-ERK1/2 levels in aneurysm lesions of the Fbln4SMKO aorta. Strong upregulation of p-ERK1/2 was observed in untreated Fbln4SMKO aorta, whereas marked downregulation of p-ERK1/2 was found in prenatal or postnatal losartan-treated groups and the prenatal captopril-treated group (Fig. 5A). In contrast, propranolol treated- or delayed losartan-treated groups, in which modest or no preventative effects were detected, showed persistently high levels of p-ERK1/2. These data suggest that increased p-ERK1/2 driven by strong AngII-Agtr1 signaling is associated with the development of aneurysms in Fbln4SMKO mice (Fig. 5A).
Fig. 5.
The effects of AngII-Agtr1 inhibition on SMCs in aneurysmal lesions. (A) Changes in p-ERK1/2 concentration in aneurysmal lesions after pharmacological intervention for the indicated time periods. Bars are 20 μm. (B) Western blot showing the effect of prenatal treatment with LSRT on the concentrations of p-ERK1/2, p-Smad2/3, p-Smad1/5/8, and ACE in mutant aortas. Quantification is shown below. One-way ANOVA. Bars are mean ± SEM. * P < 0.05, ***P < 0.001. (C) qPCR analysis of SMC differentiation marker genes in ascending aortas of wild type (n=3) and SMKO aortas (n=2-3), with and without prenatal losartan treatment. One-way ANOVA. Bars are mean ± SEM. * P < 0.05, ** P < 0.005, ***P < 0.001. (D) Western blot comparing MHY11 levels in WT and mutant aorta with and without LSRT treatment. Housekeeping protein GAPDH is shown as a loading control. Quantification is shown below. One-way ANOVA. Bars are mean ± SEM. Animals were analyzed at 3 months of age.
To compare signaling pathways implicated in aneurysm formation in congenital aortic aneurysm models, we examined p-Smad2/3, p-Smad1/5/8 and p-ERK1/2 by Western blot analysis. Our previous studies failed to detect increased Smad2/3 signaling in the aneurysmal wall (13); however, improved preparation of the aortic samples by complete removal of periaortic adipose tissues enabled us to detect elevation of p-Smad2/3 in aneurysm lesions of Fbln4SMKO mice. Losartan treatment effectively suppressed p-ERK1/2 and p-Smad2/3 levels to those of wild-type aorta (Fig. 5B), but the change in p-Smad1/5/8 levels was less prominent. qPCR analysis revealed that expression of SMC contractile genes was dramatically increased by losartan treatment compared to the untreated group (Fig. 5C). Among all contractile genes, Myocd, a master regulator of SMC differentiation, and Myh11, a marker of terminal SMC differentiation, returned to normal levels with losartan treatment (Fig. 5C). MYH11 protein levels showed a trend for increase, although not statistically significant, in losartan-treated animals (Fig. 5D). ACE protein was minimally decreased in losartan-treated animals (Fig. 5B), consistent with ACE being upstream of the AngII-Agtr1 pathway.
Genetic ablation of Agtr1a alone is insufficient for prevention of ascending aortic aneurysms in Fbln4SMKO mice
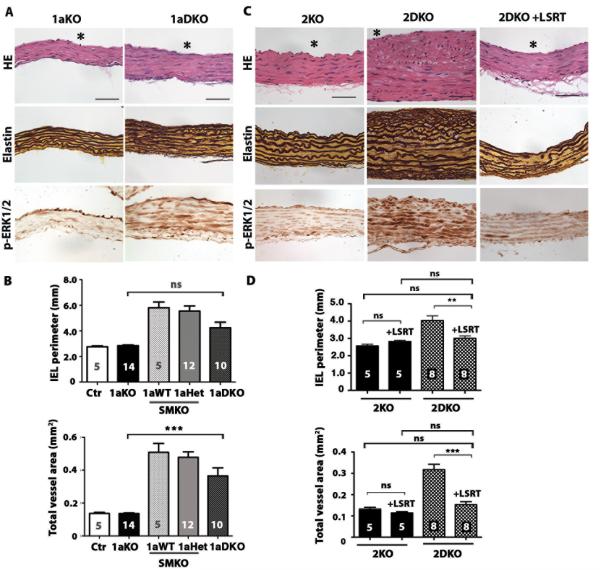
We used a genetic approach to examine whether Agtr1-mediated pathways are essential for aneurysm formation in Fbln4SMKO mice. AngII is known to bind two types of G-protein coupled receptors, Agtr1 and Agtr2. In mice, two subtypes of Agtr1, Agtr1a and Agtr1b, are generated by duplication of Agtr1. Since Agtr1a is the predominant receptor in adult tissues (21), we used mice null for Agtr1a (Agtr1a−/− termed 1aKO) and crossed them with Fbln4SMKO mice to generate Agtr1a−/−;Fbln4SMKO (termed 1aDKO), then examined aneurysm formation at 3 months compared with their littermates. Morphology of SMCs and elastic fibers in 1aDKO aorta was comparable to that of 1aKO aorta; however, the aortic wall was thicker in 1aDKO mice compared to 1aKO (Fig. 6A). Morphometric analysis showed that although 2 out of 10 1aDKO aortas had large IEL perimeters (> 0.6 mm2), average IEL perimeter was improved compared to Fbln4SMKO animals (Fig. 6B). Nevertheless, the total vessel areas of 1aDKO aortas were larger than control or 1aKO. This result was in stark contrast to losartan-treated animals in which aneurysm formation was prevented completely (Fig. 2A), suggesting that Agtr1b may also contribute to aneurysm formation in 1aDKO mice. This suggestion is supported by persistent elevation of p-ERK1/2 in the 1aDKO aorta (Fig. 6A). These results indicate that the therapeutic effect of losartan was achieved by blocking both Agtr1a and Agtr1b.
Fig. 6.
Evaluation of the role of Agtr1a and Agtr2 in formation of aortic aneurysms. (A) Histological images of the ascending aorta from Agtr1a-null (1aKO) and Agtr1a-null;Fbln4SMKO (1aDKO) mice at 3 months of age stained with H&E, Hart’s (for elastin) and immunostained with anti-pERK1/2. Lumen is indicated by asterisk. Bars are 50 μm. (B) The effect of the loss of an Agtr1a allele in Fbln4SMKO mice on IEL perimeter and total vessel area. One-way ANOVA. Bars are mean ± SEM. *** P < 0.001. (C) Histological images of the ascending aorta from Agtr2-null (2KO) and Agtr2-null;Fbln4SMKO (2DKO) mice at 3 month of age stained with H&E, Hart’s and immunostained with anti-pERK1/2. Bars are 50 μm. (D) The effect of prenatal treatment with LSRT on IEL perimeter and total vessel area in Agtr2-null (2KO, black column) and Agtr2-null; Fbln4SMKO (2DKO, shaded column) mice evaluated at 3 months of age. One-way ANOVA. Bars are mean ± SEM. ** P < 0.005 and *** P < 0.001.
Agtr2 is not required for losartan-mediated prevention of ascending aortic aneurysms in Fbln4SMKO mice
In contrast to the defined intracellular signaling pathway and biological functions of Agtr1 in vivo, the role of Agtr2 has been elusive (22). Recently, deletion of Agtr2 in Fbn1C1039G mice was shown to exacerbate aortic root enlargement and losartan was ineffective at preventing aneurysm formation in Fbn1C1039G; Agtr2-null mice, suggesting that Agtr2 functions as a protective receptor and acts antagonistically to Agtr1 in vivo (23). We therefore asked if Agtr2 is required for the therapeutic effect of losartan by rendering the AngII signal to exert protective effects through Agtr2 on SMCs in Fbln4SMKO mice. Deletion of Agtr2 (Agtr2Y/−; termed as 2KO) in Fbln4SMKO mice (Fbln4SMKO;Agtr2Y/−; termed 2DKO) did not affect aneurysm formation (Fig. 6C). The media of the aortic wall was much thicker in the double mutants than in 2KO, with hyperproliferation and disarray of SMCs observed at 3 months of age (Fig. 6C). Administration of losartan completely prevented the aneurysm phenotype in 2DKO mice (Figs. 6C, D) and p-ERK1/2 concentrations were much lower in the losartan-treated group than in the untreated 2DKO group (Fig. 6C), indicating that the therapeutic effect of losartan does not require the action of Agtr2 on SMCs. These results suggest that Agtr2 is a by-stander receptor in the process of aneurysm formation, and the protective effect of AngII-Agtr1 inhibition is independent of Agtr2 action in Fbln4SMKO mice.
Discussion
In this study, we have shown that loss of one ECM protein produced by vascular SMCs is sufficient to drive upregulation of ACE in the aortic wall of Fbln4SMKO mice. Because angiotensinogen expression in the liver and ACE expression in the lung were comparable between controls and Fbln4SMKO mice and AngII concentration in the aorta was increased in Fbln4SMKO mice, it is likely that local conversion of AngI to AngII contributes to the cellular changes in the mutant aorta. This is consistent with the previous report showing that local ACE serves as a key rate-limiting enzyme for AngII-mediated vascular hypertrophy in the rat carotid artery overexpressing the Ace gene (24). It is noteworthy that Fbln4SMKO aorta shows marked proliferation and disarray of SMCs. We speculate that the absence of intact elastic laminae and thus loss of physical barriers within the vessel wall further facilitate migration of SMCs across the lamellar unit and expansion of immature SMCs in the Fbln4SMKO aorta.
Upregulation of ACE is associated with various pathological conditions, including hypertension, atherosclerosis and diabetic nephropathy (reviewed in (25,26)). Although the endothelium is a prominent source of ACE in the vasculature, ACE has also been shown to be upregulated in SMCs of diseased vessels in humans and animal models, such as neointima induced by wire denudation injury, percutaneous transluminal coronary angioplasty (27,28), and small pulmonary arteries in hypoxia-induced pulmonary hypertension (29). Interestingly, SMCs explanted from thoracic aneurysms of patients carrying MYH11 mutations show high levels of ACE transcripts (30). The exact mechanism of ACE upregulation in SMCs has not been completely understood; however, soluble factors such as bFGF and endothelin were suggested to mediate upregulation of ACE (31,32). Mechanical forces such as shear stress and pulsatile pressure, as well as hypoxia, can also increase expression of ACE in SMCs (33,34). Our study provides evidence that loss of fibulin-4 in the context of vessel wall is sufficient for upregulation of ACE in SMCs in vivo. The resulting activation of local AngII signaling caused ascending aortic aneurysms in Fbln4SMKO mice, as evidenced by the complete prevention of aneurysm formation through perturbation of this pathway either at the level of AngII production or receptor activation. The results also suggest that fibulin-4 is required to prevent overactivation of local AngII-induced pathways that result in loss of the SMC phenotype but may be largely overcome by early interruption of AngII signaling.
Our study allowed us to determine the critical therapeutic time window for prevention of aneurysm development in vivo. Inhibition of AngII-mediated pathway by losartan effectively suppressed local proliferative changes in SMCs if treatment was initiated at P7, and these effects persisted even after withdrawal of losartan at P45. In contrast, after P30, mutant SMCs did not respond to losartan and upregulation of p-ERK1/2 continued. Thus, a critical therapeutic time window is between P7 and P30, and proliferation of mutant SMCs is dependent on AngII signaling during this period. The mechanism underlying the irreversible change in SMCs after P30 was not examined in this study; however, it is possible that AngII activates alternative signaling pathways that independently sustain the abnormal SMC phenotype in Fbln4SMKO aorta and/or vessel microenvironment containing high AngII may induce chromatin modification to alter the response to losartan and/or suppress SMC differentiation markers.
Results from genetic studies with angiotensin II receptor-deficient mice demonstrated that inhibition of Agtr1a alone is not sufficient to achieve complete prevention of aneurysms in Fbln4SMKO mice. The fact that losartan completely prevented aneurysmal phenotypes indicated that Agtr1b also mediates pathological signals in the Fbln4SMKO aorta. Agtr1a is the dominant receptor in adult tissues in mice, and Agtr1b expression is limited to a few organs, including adrenal glands, testis and brain (21). Upregulation of Agtr1b, however, was shown to be responsible for augmenting glomerular lesions in the absence of Atgr1a in murine autoimmune nephritis (35). Therefore, it is possible that AngII-mediated signaling through Agtr1b participates in the development of aneurysms in 1aDKO aorta and that losartan effectively prevents the aneurysm formation by blocking both Agtr1a and Agtr1b in Fbln4SMKO mice. Generation of triple knockout mice for Agtr1a, Agtr1b, and Fbln4 in a SMC specific manner will provide information on the role of Agtr1 receptors in the development of aneurysms in SMKO mice. Our results also show that Agtr2 is not required for mediating the protective action of losartan in Fbln4SMKO mice, unlike its critical role described in Fbn1C1039G mice (23). Prevention of the aneurysm phenotype by captopril also confirms that blocking AngII signals mediated by both Agtr1 and Agtr2 is an effective strategy in prevention of aneurysm formation in Fbln4SMKO mice and provides evidence against an antagonistic interaction between Atgr1 and Agtr2 in the development of aortic aneurysms in Fbln4SMKO mice. This finding is of particular importance in designing a strategy to stimulate Agtr2 as a potential treatment for cardiovascular diseases (36). Recently, direct stimulation of Agtr2 by a non-peptide agonist Compound 21 (C21) was shown to reduce arterial stiffness independent of blood pressure in rats treated with nitric oxide synthase inhibitor (37). C21-mediated beneficial effects were also reported on scar size and cardiac function in rat myocardial infarction (MI) model (38), as well as on perivascular fibrotic changes in stroke-prone rats (39), while another study in a mouse MI model did not support a protective effect of Agtr2 stimulation (40). Our animal model will be useful to determine the effect of Agtr2 agonists in aneurysm progression in vivo.
A protective association between ACE inhibitors and risk of aneurysm rupture was reported in a large population-based case-control study in abdominal aortic aneurysm patients, in which an ACE inhibitor, but not ARB or other anti-hypertensive reagents, decreased the risk of aneurysm rupture independent of lowering blood pressures (41). In the study targeted to Marfan patients, an ACE inhibitor reduced arterial stiffness and aortic root diameters compared to placebo-treated group (42) or ß blocker (43). The clinical trails with a larger cohort are currently underway to test the effects of ARB on Marfan patients (44,45). Our in vivo results provide further support for the idea that inhibition of AngII-Agtr pathway by ACE inhibitors or ARBs are equally effective choices for the treatment of ascending aortic aneurysms during the early postnatal period in human ascending aortic aneurysm patients and warrant further investigation.
By combining hemodynamic and mechanical studies with pharmacological experiments, we were able to assess the relationship between mechanical stress on the vessel wall and aneurysm phenotypes. Although our data indicate that loss of integrity of the vessel wall is coupled with cellular changes in SMCs, the aneurysm phenotype can be prevented without completely reversing increased pulse pressures (for losartan-treated animals) or the altered mechanical properties of the aorta, including decreased compliance and increased stiffness. The absence of pressure changes in Fbln4SMKO mice with losartan treatment implies that signaling through Agtr2 is sufficient to maintain blood pressure in these mice and provides further evidence against the complete dependence on pressure changes for the aneurysm prevention. Our results show that aneurysm in our model is a disease affecting SMC phenotypes and ECM-cell interactions, rather than a condition resulting solely from increased hemodynamic forces and/or mechanical defects of the vessel wall, and that intervention to alter SMC phenotypes by captopril or losartan leads to effective prevention of the aneurysm formation.
Although fibulin-4 is expressed in both ascending and descending aorta, the aneurysms resulting from a lack of this protein are confined to the ascending aorta, suggesting that SMCs in this region may possess unique characteristics that confer susceptibility to external growth signals in addition to the direct influence of outflow pressure. One possibility is that SMCs with a diverse embryonic origin may contribute to the differential response to AngII signals in the vessel wall. SMCs are known to be derived from the neural crest in the ascending aorta (46) and are distinct from SMCs in the descending/ abdominal aorta derived from the somite (47). Our data indicating that ACE expression is higher in the ascending aorta than descending aorta support this possibility. Regional differences in ACE activity were also reported in chick embryos, demonstrating significantly increased ACE activity in the ascending aorta compared to the abdominal aorta (48). Furthermore, endothelial cells incubated with conditioned media harvested from neural crest-derived SMCs exhibited a marked increase in ACE activity compared with those from mesenchyme-derived SMCs. Therefore, basal ACE activity may be regulated in a cell lineage-dependent manner and may contribute to the regional difference in formation of aortic aneurysms.
In summary, our results highlight the AngII pathway as a cause of ascending aortic aneurysms in Fbln4SMKO mice and provide a basis for the effective prevention of aneurysms with postnatal administration of ACE inhibitor and/or ARB. Our study underscores differences in the pathological basis of aortic aneurysms and suggests that therapeutic regimens need to be tailored according to underlying disease mechanisms in human patients. The limitations of this study include a lack of observations of the aneurysm phenotype over an extended period of time after early losartan treatment, which would have enabled us to evaluate the long-term effects of losartan on aneurysm prevention in Fbln4SMKO mice. We recognize that our mouse model is a cell-type specific inactivation of Fbln4, whereas reported human patients have systemic inactivation of FBLN4 (14-16) and, as expected, symptoms are more severe than the present mouse model. Thus, additional information regarding tissue/serum levels of fibulin-4 in a large cohort of aortic aneurysms and the correlation with clinical features and severity of the disease will be valuable. In addition, results from this mouse model of aneurysm development indicate that further investigations regarding intracellular signaling pathways downstream of AngII-Agtr that are responsible for induction of proliferative changes in SMCs and identification of specific SMC populations activated by Ang II signals are needed.
Materials and Methods
Mice
Fbln4SMKO (Fbln4fl/KO containing SM22α-Cre transgene) and Agtr2 knockout mice were generated and described previously (13) (49). Agtr1a knockout mice were purchased from Jackson Laboratory (B6.129P2-Agtr1atm1Unc/J). Comparisons of the phenotype between animals were performed on the same genetic background, and wild-type littermates containing Cre transgenes were used as controls (Tables S1-S3). Both female and male mice were used in this study. All mice were kept on a 12 h/12 h light/dark cycle under specific pathogen free conditions, and all animal protocols were approved by the IACUC of the University of Texas Southwestern Medical Center.
Histology and immunohistochemistry
Aortas were harvested and perfusion fixed in 4% paraformaldehyde and embedded in paraffin. Five-μm sections were stained with Hematoxylin & Eosin (H&E) for routine histology and Masson-Trichrome and Hart’s staining for detection of collagen fibers and elastic fibers, respectively. Immunostaining was performed with antibodies for ACE (1:100, Santa Cruz) and p-ERK1/2 (1:100, Cell Signaling) as previously described (13). Minimum of 4 animals were analyzed in each experiment.
Western blot analysis
Aortas were harvested and perivascular adipose tissues were thoroughly removed. Wild-type aortas (n=2-6) and Fbln4SMKO aortas (n=1-4) were pooled in each experiment and minced in 2x lammelli buffer or RIPA buffer containing 1% protease inhibitor (Sigma) and 1% phosphatase inhibitor (Sigma), then boiled at 95°C for 10 min, and subjected to SDS-PAGE. SMCs were prepared from P45 wild-type and Fbln4SMKO aortas as previously described (13) and passage number 5 was used for protein extraction. Proteins were transferred to a Western PVDF membrane (Millipore, Inc.) and immunoblotted with antibodies to phosphorylated ERK1/2 (1:1000, Cell Signaling), ACE (1:1000, Santa Cruz), or GAPDH (1:3000, Cell Signaling). Membranes were then incubated with anti-mouse or anti-rabbit HRP-conjugated secondary antibody, as appropriate (1:3000, Bio-Rad) and visualized with a chemoluminescence kit (Santa Cruz Biotechnology, Inc.). Quantification of Western blots was performed with Scion NIH IMAGE Software (National Institutes of Health).
Quantitative PCR
RNA was isolated from aortas at the indicated ages with an RNeasy Plus Micro Kit (QIAGEN) and 1 μg of total RNA was subjected to reverse transcription reactions with superscript III reverse transcriptase (Invitrogen). SYBR Green was used for amplicon detection, and gene expression was normalized to the expression of housekeeping genes β2-microglobulin (β2M) and GAPDH. PCR reactions were carried out in triplicate in a CFX96 real-time PCR detection system (Bio-Rad) with one cycle of 3 min at 95°C, then 40 cycles of 10 sec at 95°C and 30 sec at 60°C. mRNA concentrations were determined with the ddCt method and expressed relative to WT controls. Wild-type aortas (n=4-6) and Fbln4SMKO aortas (n=1-3) were pooled and analyzed in each experiment. Primer sequences are provided in Table S4.
BrdU proliferation assay
Wild-type (n=4) and Fbln4SMKO (n=4) mice at 1 month of age were given 100 mg/kg 5-bromo-2′-deoxyuridine (BrdU) by IP injection once per day on four consecutive days. Aortas were harvested 1 hr after the final BrdU injection and embedded in OCT (Tissue-Tek). Tissue sections (10 μm thick) were fixed in 4% paraformaldehyde for 10 min and treated with 2N HCl containing 0.5% Triton-X-100 for 1 hr, followed by TBS pH 8.4 for 5 min to neutralize HCl. Sections were blocked for 30 min and incubated with rat anti-mouse BrdU antibody (Abcam, 1:100) overnight followed by Alexa 488-conjugated donkey anti-rat antibody (Invitrogen, 1:200) for 1 hr. Sections were mounted with DAPI and photographed using a Zeiss Axio Observer.Z1 microscope and Axiovision software. BrdU+ nuclei and total nuclei were counted in 4 high-powered fields (40x) for each tissue sample and expressed as a percentage of BrdU+ cells. Vessel areas were measured using ImageJ software (NIH).
Tissue AngII measurement
Aortas from 3-month old wild-type (n=16) and Fbln4SMKO mice (n=14) were pooled and processed for tissue extraction. Kidneys from 3-4 month old wild-type (n=6) and Fbln4SMKO (n=6) mice were individually processed. Tissues were boiled in 2 ml of water for 20 min to deactivate endogenous peptidases. The solution was then adjusted to 1N CH3COOH and homogenized. After centrifugation (2,600 g × 40 min), the supernatant was loaded onto OASIS HLB Extraction Cartridges (Waters), washed with water/0.1%TFA, and eluted with 100%CH3CN/0.1%TFA. The eluates were lyophilized and dissolved in the EIA buffer of the Angiotensin II ELISA kit (SPI-BIO). Angiotensin II levels were measured in duplicate according to the manufacturer’s instructions.
Pharmacological experiments
Losartan (0.6 g/l in drinking water, ad libitum, provided by Merck Inc.), captopril (75 mg/l in drinking water, ad libitum, Sigma-Aldrich) and propranolol (0.5 g/l in drinking water, ad libitum, Sigma-Aldrich) were administered during the indicated periods. The numbers of animals used in each group are provided in each graph. For prenatal treatment, drugs were administered to the pregnant mother and then given to the mother through the period of lactation until weaning.
Morphometric analysis
Cross sections of the aorta were stained with Hart’s stain, and images were digitally captured with a Leica DM2000 microscope. Morphometric analysis was performed with Scion NIH IMAGE Software (National Institutes of Health). At least three sections were taken from different levels of ascending and descending aorta, and measurements were done three times per section by a blinded examiner.
Blood pressure measurement
The mice used for this experiment were all male, except for one female included for losartan treatment both in control and Fbln4SMKO groups (6-8 weeks). For drug-treated animals, losartan (LSRT), captopril (CPT), or propranolol (PPL) was administered from P7 to P43 ± 2, then the drugs were removed and animals were acclimated for 5 days before blood pressure measurement. The animals were anesthetized with 1.5% isoflurane, and body temperature was maintained with a feedback controlled heating pad. Arterial pressure was measured with a catheter (1.2 F, Scisense, Inc.) inserted in the ascending aorta through the right common carotid artery.
Mechanical testing
Mechanical testing was performed on the ascending aorta with a pressure myograph (Danish Myotechnology), as previously described (18). Briefly, each aorta was mounted in the test system in physiologic saline at 37 °C, stretched to its approximate in vivo length and preconditioned. Each aorta was then pressurized for three cycles from 0 – 175 mmHg in steps of 25 mmHg with 12 sec/step. Compliance was defined as the percent change in outer diameter with each pressure step.
Statistical analysis
One-way ANOVA with the Bonferroni post hoc test was used for comparison of drug effects (Figs. 2B, 3D, 4, 5C) and Agtr1a allelic difference (Fig. 6B) on aneurysm phenotypes, and two-tailed Student t-test was used for the rest of the statistical analysis. Bars are mean ± SEM and P <0.05 was considered significant.
Supplementary Material
Fig. S1. qPCR analysis of the renin-angiotensin pathway in the descending aortas.
Fig. S2. qPCR analysis of the renin-angiotensin pathway in the lung, kidney and liver.
Fig. S3. Quantification of ACE in Fbln4SMKO (SMKO) mice throughout postnatal and adult life.
Fig. S4. ACE expression in primary Fbln4SMKO SMCs.
Fig. S5. Morphological analysis of Fbln4SMKO ascending aorta at P1.
Table S1. Animals used in morphometric analysis.
Table S2. Animals used in blood pressure measurement.
Table S3. Animals used in vessel biomechanics.
Table S4. qPCR primer sequences.
One Sentence Summary: Upregulation of angiotensin converting enzyme (ACE) in the aortic wall causes aneurysms in fibulin-4 deficient mice, and the aneurysm phenotype can be prevented by losartan or captopril without reversal of mechanical properties in the absence of angiotensin II receptor type 2 (Agtr2).
Acknowledgments
We thank Greg Urquhart, Mana Hiroshima, and the Pathology Core Laboratories for technical assistance, James Richardson for assistance in evaluation of histological sections. We also thank Eric Olson, Ann Word and Masashi Yanagisawa for critical reading of the manuscript. Funding: Supported by grants from NIH R01HL106305 (HY), R00HL087653 (JEW), R01HL105314 (JEW), R37HL58205 (TI), American Heart Association (Grant-In-Aid, 0855200F, HY), The National Marfan Foundation (HY426g, HY), and an NIH institutional Training in Cardiovascular Research grant 5T32HL007360-34 (CLP). HY is a recipient of the Established Investigator Award from the American Heart Association.
Footnotes
This manuscript has been accepted for publication in Science Translational Medicine. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencetranslationalmedicine.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.”
Author contributions: JH, YY, YI, CLP contributed to conception and design, acquired experimental data, were involved in analysis and interpretation of data, and drafted the manuscript. YL, MP, and VPL helped design, performed experiments, analyzed and interpretted the data. TI made contributions to this study by generating reagents, interpreting the data and intellectual input to the manuscript. JEW contributed to the design of experiments, analysis and interpretation of data, and writing the manuscript. HY is the senior author of this manuscript, provided funding and contributed to its conception and design, acquisition of experimental data and interpretation of data, and writing the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: Losartan was obtained from Merck & CO., Inc. under MTA 38648.
References and Notes
- 1.Milewicz DM, Guo DC, Tran-Fadulu V, Lafont AL, Papke CL, Inamoto S, Kwartler CS, Pannu H. Genetic basis of thoracic aortic aneurysms and dissections: focus on smooth muscle cell contractile dysfunction. Annu Rev Genomics Hum Genet. 2008;9:283–302. doi: 10.1146/annurev.genom.8.080706.092303. [DOI] [PubMed] [Google Scholar]
- 2.Jain D, Dietz HC, Oswald GL, Maleszewski JJ, Halushka MK. Causes and histopathology of ascending aortic disease in children and young adults. Cardiovasc Pathol. 2011;20:15–25. doi: 10.1016/j.carpath.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 4.van de Laar IM, Oldenburg RA, Pals G, Roos-Hesselink JW, de Graaf BM, Verhagen JM, Hoedemaekers YM, Willemsen R, Severijnen LA, Venselaar H, Vriend G, Pattynama PM, Collee M, Majoor-Krakauer D, Poldermans D, Frohn-Mulder IM, Micha D, Timmermans J, Hilhorst-Hofstee Y, Bierma-Zeinstra SM, Willems PJ, Kros JM, Oei EH, Oostra BA, Wessels MW, Bertoli-Avella AM. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat Genet. 2011;43:121–126. doi: 10.1038/ng.744. [DOI] [PubMed] [Google Scholar]
- 5.Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, Xu FL, Myers LA, Spevak PJ, Cameron DE, De Backer J, Hellemans J, Chen Y, Davis EC, Webb CL, Kress W, Coucke P, Rifkin DB, De Paepe AM, Dietz HC. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet. 2005;37:275–281. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- 6.Holm TM, Habashi JP, Doyle JJ, Bedja D, Chen Y, van Erp C, Lindsay ME, Kim D, Schoenhoff F, Cohn RD, Loeys BL, Thomas CJ, Patnaik S, Marugan JJ, Judge DP, Dietz HC. Noncanonical TGFbeta signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science. 2011;332:358–361. doi: 10.1126/science.1192149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanada K, Vermeij M, Garinis GA, de Waard MC, Kunen MG, Myers L, Maas A, Duncker DJ, Meijers C, Dietz HC, Kanaar R, Essers J. Perturbations of vascular homeostasis and aortic valve abnormalities in fibulin-4 deficient mice. Circ Res. 2007;100:738–746. doi: 10.1161/01.RES.0000260181.19449.95. [DOI] [PubMed] [Google Scholar]
- 8.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moltzer E, te Riet L, Swagemakers SM, van Heijningen PM, Vermeij M, van Veghel R, Bouhuizen AM, van Esch JH, Lankhorst S, Ramnath NW, de Waard MC, Duncker DJ, van der Spek PJ, Rouwet EV, Danser AH, Essers J. Impaired vascular contractility and aortic wall degeneration in fibulin-4 deficient mice: effect of angiotensin II type 1 (AT1) receptor blockade. PLoS One. 2011;6:e23411. doi: 10.1371/journal.pone.0023411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLaughlin PJ, Chen Q, Horiguchi M, Starcher BC, Stanton JB, Broekelmann TJ, Marmorstein AD, McKay B, Mecham R, Nakamura T, Marmorstein LY. Targeted disruption of fibulin-4 abolishes elastogenesis and causes perinatal lethality in mice. Mol Cell Biol. 2006;26:1700–1709. doi: 10.1128/MCB.26.5.1700-1709.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi N, Kostka G, Garbe JH, Keene DR, Bachinger HP, Hanisch FG, Markova D, Tsuda T, Timpl R, Chu ML, Sasaki T. A comparative analysis of the fibulin protein family. Biochemical characterization, binding interactions, and tissue localization. J Biol Chem. 2007;282:11805–11816. doi: 10.1074/jbc.M611029200. [DOI] [PubMed] [Google Scholar]
- 12.Horiguchi M, Inoue T, Ohbayashi T, Hirai M, Noda K, Marmorstein LY, Yabe D, Takagi K, Akama TO, Kita T, Kimura T, Nakamura T. Fibulin-4 conducts proper elastogenesis via interaction with cross-linking enzyme lysyl oxidase. Proc Natl Acad Sci U S A. 2009;106:19029–19034. doi: 10.1073/pnas.0908268106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang J, Davis EC, Chapman SL, Budatha M, Marmorstein LY, Word RA, Yanagisawa H. Fibulin-4 deficiency results in ascending aortic aneurysms: a potential link between abnormal smooth muscle cell phenotype and aneurysm progression. Circ Res. 2010;106:583–592. doi: 10.1161/CIRCRESAHA.109.207852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hucthagowder V, Sausgruber N, Kim KH, Angle B, Marmorstein LY, Urban Z. Fibulin-4: a novel gene for an autosomal recessive cutis laxa syndrome. Am J Hum Genet. 2006;78:1075–1080. doi: 10.1086/504304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dasouki M, Markova D, Garola R, Sasaki T, Charbonneau NL, Sakai LY, Chu ML. Compound heterozygous mutations in fibulin-4 causing neonatal lethal pulmonary artery occlusion, aortic aneurysm, arachnodactyly, and mild cutis laxa. Am J Med Genet A. 2007;143A:2635–2641. doi: 10.1002/ajmg.a.31980. [DOI] [PubMed] [Google Scholar]
- 16.Erickson LK, Opitz J, Zhou HH. Lethal Osteogenesis Imperfecta-Like Condition with Cutis Laxa and Arterial Tortuosity in MZ Twins due to a Homozygous Fibulin-4 Mutation. Pediatr Dev Pathol. 2011 doi: 10.2350/11-03-1010-CR.1. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz U, Berk BC. Angiotensin II signal transduction: Stimulation of multiple mitogen-activated protein kinase pathways. Trends Endocrinol Metab. 1997;8:261–266. doi: 10.1016/s1043-2760(97)00101-x. [DOI] [PubMed] [Google Scholar]
- 18.Le VP, Knutsen RH, Mecham RP, Wagenseil JE. Decreased aortic diameter and compliance precedes blood pressure increases in postnatal development of elastin-insufficient mice. Am J Physiol Heart Circ Physiol. 2011;301:H221–229. doi: 10.1152/ajpheart.00119.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanagisawa H, Davis EC, Starcher BC, Ouchi T, Yanagisawa M, Richardson JA, Olson EN. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415:168–171. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]
- 20.Wagenseil JE, Ciliberto CH, Knutsen RH, Levy MA, Kovacs A, Mecham RP. Reduced vessel elasticity alters cardiovascular structure and function in newborn mice. Circ Res. 2009;104:1217–1224. doi: 10.1161/CIRCRESAHA.108.192054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burson JM, Aguilera G, Gross KW, Sigmund CD. Differential expression of angiotensin receptor 1A and 1B in mouse. Am J Physiol. 1994;267:E260–267. doi: 10.1152/ajpendo.1994.267.2.E260. [DOI] [PubMed] [Google Scholar]
- 22.Stegbauer J, Coffman TM. New insights into angiotensin receptor actions: from blood pressure to aging. Curr Opin Nephrol Hypertens. 2011;20:84–88. doi: 10.1097/MNH.0b013e3283414d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Habashi JP, Doyle JJ, Holm TM, Aziz H, Schoenhoff F, Bedja D, Chen Y, Modiri AN, Judge DP, Dietz HC. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science. 2011;332:361–365. doi: 10.1126/science.1192152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morishita R, Gibbons GH, Ellison KE, Lee W, Zhang L, Yu H, Kaneda Y, Ogihara T, Dzau VJ. Evidence for direct local effect of angiotensin in vascular hypertrophy. In vivo gene transfer of angiotensin converting enzyme. J Clin Invest. 1994;94:978–984. doi: 10.1172/JCI117464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dzau VJ, Bernstein K, Celermajer D, Cohen J, Dahlof B, Deanfield J, Diez J, Drexler H, Ferrari R, van Gilst W, Hansson L, Hornig B, Husain A, Johnston C, Lazar H, Lonn E, Luscher T, Mancini J, Mimran A, Pepine C, Rabelink T, Remme W, Ruilope L, Ruzicka M, Schunkert H, Swedberg K, Unger T, Vaughan D, Weber M. The relevance of tissue angiotensin-converting enzyme: manifestations in mechanistic and endpoint data. Am J Cardiol. 2001;88:1L–20L. doi: 10.1016/s0002-9149(01)01878-1. [DOI] [PubMed] [Google Scholar]
- 26.Bakris G. Are there effects of renin-angiotensin system antagonists beyond blood pressure control? Am J Cardiol. 2010;105:21A–29A. doi: 10.1016/j.amjcard.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Rakugi H, Kim DK, Krieger JE, Wang DS, Dzau VJ, Pratt RE. Induction of angiotensin converting enzyme in the neointima after vascular injury. Possible role in restenosis. J Clin Invest. 1994;93:339–346. doi: 10.1172/JCI116965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohishi M, Ueda M, Rakugi H, Okamura A, Naruko T, Becker AE, Hiwada K, Kamitani A, Kamide K, Higaki J, Ogihara T. Upregulation of angiotensin-converting enzyme during the healing process after injury at the site of percutaneous transluminal coronary angioplasty in humans. Circulation. 1997;96:3328–3337. doi: 10.1161/01.cir.96.10.3328. [DOI] [PubMed] [Google Scholar]
- 29.de Man FS, Tu L, Handoko ML, Rain S, Ruiter G, Francois C, Schalij I, Dorfmuller P, Simonneau G, Fadel E, Perros F, Boonstra A, Postmus PE, van der Velden J, Vonk-Noordegraaf A, Humbert M, Eddahibi S, Guignabert C. Dysregulated Renin-Angiotensin-Aldosterone System Contributes to Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2012 doi: 10.1164/rccm.201203-0411OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pannu H, Tran-Fadulu V, Papke CL, Scherer S, Liu Y, Presley C, Guo D, Estrera AL, Safi HJ, Brasier AR, Vick GW, Marian AJ, Raman CS, Buja LM, Milewicz DM. MYH11 mutations result in a distinct vascular pathology driven by insulin-like growth factor 1 and angiotensin II. Hum Mol Genet. 2007;16:2453–2462. doi: 10.1093/hmg/ddm201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fishel RS, Thourani V, Eisenberg SJ, Shai SY, Corson MA, Nabel EG, Bernstein KE, Berk BC. Fibroblast growth factor stimulates angiotensin converting enzyme expression in vascular smooth muscle cells. Possible mediator of the response to vascular injury. J Clin Invest. 1995;95:377–387. doi: 10.1172/JCI117666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moroi M, Fukazawa M, Ishikawa M, Aikawa J, Namiki A, Yamaguchi T. Effect of endothelin on angiotensin converting enzyme activity in cultured vascular smooth muscle cells. Gen Pharmacol. 1996;27:463–465. doi: 10.1016/0306-3623(95)02038-1. [DOI] [PubMed] [Google Scholar]
- 33.Gosgnach W, Challah M, Coulet F, Michel JB, Battle T. Shear stress induces angiotensin converting enzyme expression in cultured smooth muscle cells: possible involvement of bFGF. Cardiovasc Res. 2000;45:486–492. doi: 10.1016/s0008-6363(99)00269-2. [DOI] [PubMed] [Google Scholar]
- 34.Iizuka K, Machida T, Kawaguchi H, Hirafuji M. Pulsatile mechanical pressure promotes Angiotensin-converting enzyme expression in aortic smooth muscle cells. Cardiovasc Drugs Ther. 2008;22:383–390. doi: 10.1007/s10557-008-6118-7. [DOI] [PubMed] [Google Scholar]
- 35.Crowley SD, Vasievich MP, Ruiz P, Gould SK, Parsons KK, Pazmino AK, Facemire C, Chen BJ, Kim HS, Tran TT, Pisetsky DS, Barisoni L, Prieto-Carrasquero MC, Jeansson M, Foster MH, Coffman TM. Glomerular type 1 angiotensin receptors augment kidney injury and inflammation in murine autoimmune nephritis. J Clin Invest. 2009;119:943–953. doi: 10.1172/JCI34862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steckelings UM, Larhed M, Hallberg A, Widdop RE, Jones ES, Wallinder C, Namsolleck P, Dahlof B, Unger T. Non-peptide AT2-receptor agonists. Curr Opin Pharmacol. 2011;11:187–192. doi: 10.1016/j.coph.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Paulis L, Becker ST, Lucht K, Schwengel K, Slavic S, Kaschina E, Thone-Reineke C, Dahlof B, Baulmann J, Unger T, Steckelings UM. Direct angiotensin II type 2 receptor stimulation in Nomega-nitro-L-arginine-methyl ester-induced hypertension: the effect on pulse wave velocity and aortic remodeling. Hypertension. 2012;59:485–492. doi: 10.1161/HYPERTENSIONAHA.111.185496. [DOI] [PubMed] [Google Scholar]
- 38.Kaschina E, Grzesiak A, Li J, Foryst-Ludwig A, Timm M, Rompe F, Sommerfeld M, Kemnitz UR, Curato C, Namsolleck P, Tschope C, Hallberg A, Alterman M, Hucko T, Paetsch I, Dietrich T, Schnackenburg B, Graf K, Dahlof B, Kintscher U, Unger T, Steckelings UM. Angiotensin II type 2 receptor stimulation: a novel option of therapeutic interference with the renin-angiotensin system in myocardial infarction? Circulation. 2008;118:2523–2532. doi: 10.1161/CIRCULATIONAHA.108.784868. [DOI] [PubMed] [Google Scholar]
- 39.Rehman A, Leibowitz A, Yamamoto N, Rautureau Y, Paradis P, Schiffrin EL. Angiotensin type 2 receptor agonist compound 21 reduces vascular injury and myocardial fibrosis in stroke-prone spontaneously hypertensive rats. Hypertension. 2012;59:291–299. doi: 10.1161/HYPERTENSIONAHA.111.180158. [DOI] [PubMed] [Google Scholar]
- 40.Jehle AB, Xu Y, Dimaria JM, French BA, Epstein FH, Berr SS, Roy RJ, Kemp BA, Carey RM, Kramer CM. A nonpeptide angiotensin II type 2 receptor agonist does not attenuate postmyocardial infarction left ventricular remodeling in mice. J Cardiovasc Pharmacol. 2012;59:363–368. doi: 10.1097/FJC.0b013e3182444110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hackam DG, Thiruchelvam D, Redelmeier DA. Angiotensin-converting enzyme inhibitors and aortic rupture: a population-based case-control study. Lancet. 2006;368:659–665. doi: 10.1016/S0140-6736(06)69250-7. [DOI] [PubMed] [Google Scholar]
- 42.Ahimastos AA, Aggarwal A, D’Orsa KM, Formosa MF, White AJ, Savarirayan R, Dart AM, Kingwell BA. Effect of perindopril on large artery stiffness and aortic root diameter in patients with Marfan syndrome: a randomized controlled trial. JAMA. 2007;298:1539–1547. doi: 10.1001/jama.298.13.1539. [DOI] [PubMed] [Google Scholar]
- 43.Yetman AT, Bornemeier RA, McCrindle BW. Usefulness of enalapril versus propranolol or atenolol for prevention of aortic dilation in patients with the Marfan syndrome. Am J Cardiol. 2005;95:1125–1127. doi: 10.1016/j.amjcard.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 44.Möberg K, De Nobele S, Devos D, Goetghebeur E, Segers P, Trachet B, Vervaet C, Renard M, Coucke P, Loeys B, De Paepe A, De Backer J. The Ghent Marfan Trial--a randomized, double-blind placebo controlled trial with losartan in Marfan patients treated with beta-blockers. Int J Cardiol. 2012;157:354–358. doi: 10.1016/j.ijcard.2010.12.070. [DOI] [PubMed] [Google Scholar]
- 45.Radonic T, de Witte P, Baars MJ, Zwinderman AH, Mulder BJ, Groenink M. Losartan therapy in adults with Marfan syndrome: study protocol of the multi-center randomized controlled COMPARE trial. Trials. 2010;11:3. doi: 10.1186/1745-6215-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- 47.Pouget C, Gautier R, Teillet MA, Jaffredo T. Somite-derived cells replace ventral aortic hemangioblasts and provide aortic smooth muscle cells of the trunk. Development. 2006;133:1013–1022. doi: 10.1242/dev.02269. [DOI] [PubMed] [Google Scholar]
- 48.Topouzis S, Catravas JD, Ryan JW, Rosenquist TH. Influence of vascular smooth muscle heterogeneity on angiotensin converting enzyme activity in chicken embryonic aorta and in endothelial cells in culture. Circ Res. 1992;71:923–931. doi: 10.1161/01.res.71.4.923. [DOI] [PubMed] [Google Scholar]
- 49.Ichiki T, Labosky PA, Shiota C, Okuyama S, Imagawa Y, Fogo A, Niimura F, Ichikawa I, Hogan BL, Inagami T. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature. 1995;377:748–750. doi: 10.1038/377748a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. qPCR analysis of the renin-angiotensin pathway in the descending aortas.
Fig. S2. qPCR analysis of the renin-angiotensin pathway in the lung, kidney and liver.
Fig. S3. Quantification of ACE in Fbln4SMKO (SMKO) mice throughout postnatal and adult life.
Fig. S4. ACE expression in primary Fbln4SMKO SMCs.
Fig. S5. Morphological analysis of Fbln4SMKO ascending aorta at P1.
Table S1. Animals used in morphometric analysis.
Table S2. Animals used in blood pressure measurement.
Table S3. Animals used in vessel biomechanics.
Table S4. qPCR primer sequences.