SUMMARY
The mTORC1 kinase is a master growth regulator that senses numerous environmental cues, including amino acids. The Rag GTPases interact with mTORC1 and signal amino acid sufficiency by promoting the translocation of mTORC1 to the lysosomal surface, its site of activation. The Rags are unusual GTPases in that they function as obligate heterodimers, which consist of RagA or B bound to RagC or D. While the loading of RagA/B with GTP initiates amino acid signaling to mTORC1, the role of RagC/D is unknown. Here, we show that RagC/D is a key regulator of the interaction of mTORC1 with the Rag heterodimer and that, unexpectedly, RagC/D must be GDP-bound for the interaction to occur. We identify FLCN and its binding partners, FNIP1/2, as Rag-interacting proteins with GAP activity for RagC/D, but not RagA/B. Thus, we reveal a role for RagC/D in mTORC1 activation and a molecular function for the FLCN tumor suppressor.
INTRODUCTION
The mechanistic target of rapamycin complex 1 (mTORC1) protein kinase is a master regulator of growth. It senses a diverse set of signals, such as growth factors, nutrient and energy levels, to regulate many anabolic and catabolic processes, including protein, lipid, and nucleotide synthesis, as well as autophagy. Given that mTORC1 regulates a multitude of processes, it is not surprising that the pathway it anchors is deregulated in various common diseases, including cancer (reviewed in Howell et al., 2013; Kim et al., 2013; Yuan et al., 2013; Zoncu et al., 2011b).
The mechanisms through which mTORC1 senses and integrates stimuli have been of great interest over the last few years. One key upstream factor is the TSC1-TSC2 tumor suppressor, which suppresses mTORC1 in response to growth factor or energy deprivation (Brugarolas et al., 2004; Castro et al., 2003; Corradetti et al., 2005; Garami et al., 2003; Inoki et al., 2003a; Inoki et al., 2003b; Ma et al., 2005; Reiling and Hafen, 2004; Roux et al., 2004; Saucedo et al., 2003; Stocker et al., 2003; Tee et al., 2003a; Tee et al., 2002; Tee et al., 2003b; Zhang et al., 2003). TSC1-TSC2 does so by inhibiting Rheb, a GTP-binding protein that is an essential activator of the mTORC1 kinase activity (Long et al., 2005; Sancak et al., 2007).
mTORC1 is also acutely sensitive to drops in amino acid levels, but these nutrients do not appear to signal through TSC1-TSC2 (Nobukuni et al., 2005; Roccio et al., 2006; Smith et al., 2005). Instead, emerging evidence indicates that mTORC1 activation by amino acids requires a lysosome-associated machinery, comprised of the vacuolar adenosine triphosphatase (v-ATPase), the Ragulator, and the Rag GTPases (Kim et al., 2008; Sancak et al., 2010; Sancak et al., 2008; Zoncu et al., 2011a). Like Rheb, the Rags are members of the Ras-related GTP-binding superfamily of proteins, but they are unusual in that they function as obligate heterodimers of RagA or B (A/B) with RagC or D (C/D). RagA and RagB are highly homologous and redundant, as are RagC and RagD (Hirose et al., 1998; Sancak et al., 2008; Schürmann et al., 1995; Sekiguchi et al., 2001). We have proposed that amino acids signal from within the lysosomal lumen to Ragulator, in a v-ATPase-dependent fashion. In turn, Ragulator activates RagA/B through its guanine nucleotide exchange factor (GEF) activity. When RagA/B is loaded with GTP, the Rag heterodimer recruits mTORC1 to the lysosomal surface where it binds Rheb and becomes activated (Bar-Peled et al., 2013; Bar-Peled et al., 2012; Efeyan et al., 2012).
Whereas much attention has focused on RagA/B, the role of RagC/D in mTORC1 signaling has remained a mystery. Here, we make the surprising finding that GDP-loading of RagC is necessary for the binding of mTORC1 to the Rag heterodimer, and that the nucleotide state of RagC affects the activation of mTORC1 in response to amino acids. Moreover, we identified the FLCN-FNIP complex as a potent GTPase activating protein (GAP) for RagC/D that interacts with the Rag heterodimer in an amino acid-sensitive fashion and localizes to the lysosomal surface upon amino acid starvation. Thus, we provide a molecular function for FLCN, mutations in which cause the Birt-Hogg-Dubé hereditary cancer syndrome, and reveal a role for RagC/D in amino acid signaling to mTORC1.
RESULTS
The RagC Nucleotide State Determines mTORC1 Binding to the Rag Heterodimer
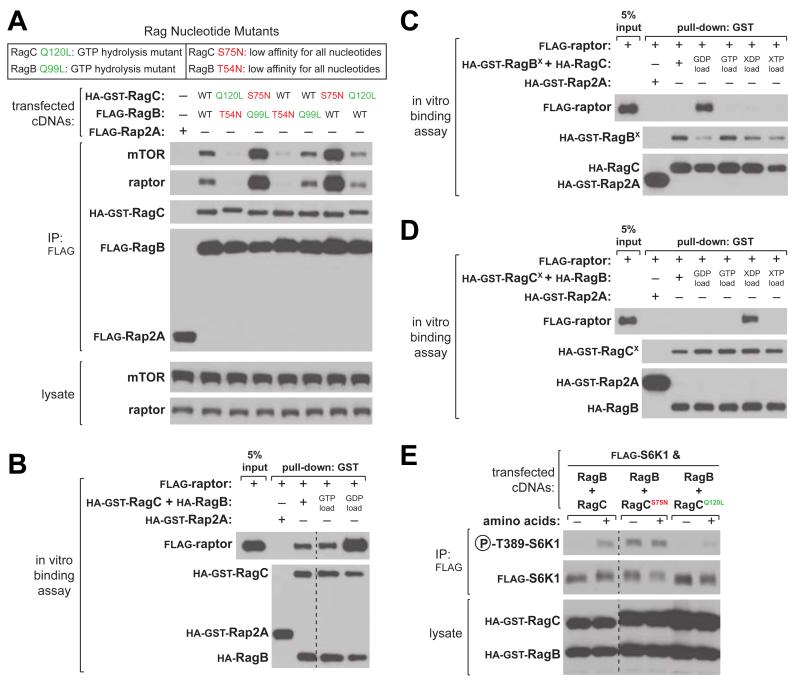
The binding of mTORC1 to the heterodimeric Rag GTPases in the presence of amino acids is a key event in the activation of mTORC1. Using two classes of Rag nucleotide binding mutants, we and others have shown that the interaction between the Rags and mTORC1 depends on the nucleotide configuration of the Rag heterodimer (Gong et al., 2011; Sancak et al., 2008). The first class of mutations (RagBQ99L and RagCQ120L) is analogous to the oncogenic H-RasQ61L mutant (Frech et al., 1994; Krengel et al., 1990) that abolishes GTPase activity and maintains RagB or RagC loaded with GTP (Bar-Peled et al., 2012; Sancak et al., 2008). Mutations of the second class (RagAT21N, RagBT54N, and RagCS75N) disrupt the coordination of the magnesium co-factor (Feig, 1999; Feig and Cooper, 1988; John et al., 1993), resulting in mutants with much lower affinity for all nucleotides but with likely preferential binding of GDP over GTP within cells (Bar-Peled et al., 2012; Sancak et al., 2008).
To test the contribution of each Rag to the binding of mTORC1, we expressed combinations of Rag nucleotide mutants in human embryonic kidney (HEK)-293T cells. Consistent with previous reports (Gong et al., 2011; Sancak et al., 2008), RagBQ99L–CS75N co-immunoprecipitated the largest amount of endogenous mTORC1 (Figure 1A). From these data, as well as the observation that RagAT21N- or RagBT54N-containing heterodimers do not co-immunoprecipitate mTORC1, it has been supposed that RagA/B nucleotide state is the major determinant for mTORC1 binding (Gong et al., 2011; Sancak et al., 2008). To re-examine which Rag heterodimer is responsible for mTORC1 binding, we immunoprecipitated single Rag nucleotide mutants paired with wild-type partners. Surprisingly, RagB-CS75N, but not RagBQ99L-C, was sufficient to recover large amounts of mTORC1 similar to that of RagBQ99L-CS75N, suggesting that the RagC nucleotide state determines mTORC1 binding (Figure 1A).
Figure 1. The RagC Nucleotide State Determines mTORC1 Binding to the Rag Heterodimer and Regulates Amino Acid Sensing by mTORC1.
(A) Rag heterodimers containing RagCS75N co-immunoprecipitate the largest amount of endogenous mTORC1. Anti-FLAG immunoprecipitates were prepared from HEK-293T cells expressing the indicated cDNAs. Cell lysates and immunoprecipitates were analyzed by immunoblotting for the indicated proteins.
(B) Raptor preferentially binds a GDP-loaded Rag heterodimer. In vitro binding assay in which recombinant HA-GST-tagged-RagB-RagC or -Rap2A were loaded with the indicated nucleotide and incubated with purified FLAG-tagged raptor protein. HA-GST precipitates were analyzed by immunoblotting for indicated proteins. Irrelevant lanes were removed and indicated by a dashed line.
(C) Raptor only binds to the RagBX-C heterodimer when RagC is GDP-loaded. In vitro binding assay in which recombinant HA-GST-tagged-RagBX-RagC or -Rap2A were loaded with the indicated nucleotide and incubated with purified FLAG-tagged raptor protein and analyzed as in (B).
(D) Raptor only binds to the RagB-CX heterodimer when RagCX is XDP-loaded. In vitro binding assay in which recombinant HA-GST-tagged-RagB-RagCX or -Rap2A were loaded with the indicated nucleotide and incubated with purified FLAG-tagged raptor protein and analyzed as in (B).
(E) Expression of RagCS75N or RagCQ120L renders the mTORC1 pathway insensitive to amino acid levels. HEK-293T cells expresssing the indicated cDNAs were analyzed as in (A). Irrelevant lanes were removed and indicated by a dashed line.
Because the behavior of these mutants may not reflect that of nucleotide-loaded, wild-type Rags, we developed an in vitro assay in which we load purified Rag heterodimers with specified nucleotides and monitor their ability to bind to raptor, the Rag-binding subunit of mTORC1. Unexpectedly, wild-type Rags loaded with GDP bound more raptor than Rags loaded with GTP (Figure 1B), suggesting that the GDP-bound state of one or both Rags promotes raptor binding. To determine which Rag in the heterodimer is responsible for this effect, we employed another class of Rag nucleotide mutants that has base specificity for xanthine rather than guanine nucleotides (Bar-Peled et al., 2012; Hoffenberg et al., 1995; Schmidt et al., 1996). We term these RagBX and RagCX (RagBD163N, RagCD181N), as they bind less than 2% of the amount of guanine nucleotides bound by their wild-type counterparts (Bar-Peled et al., 2012). Consistent with the results obtained in cells, the RagC nucleotide state determined raptor binding, as only GDP-loaded RagBX-C could bind raptor (Figure 1C). Importantly, RagB-CX loaded with XDP also recovered raptor, suggesting that the state induced by the nucleotide diphosphate loading of RagC promotes raptor binding (Figure 1D). Thus, unlike most GTPases, which activate their effectors in the GTP-bound state, it is the GDP-bound state of RagC that promotes raptor binding to the Rag heterodimer.
Given that the nucleotide state of RagC is important for mTORC1 binding, we reasoned that expression of RagC nucleotide mutants might alter the sensitivity of the mTORC1 pathway to amino acid levels. Indeed, expression of RagCS75N rendered mTORC1 activity resistant to amino acid starvation, as judged by phosphorylation of S6 kinase (S6K1), a canonical mTORC1 substrate (Figure 1E). Conversely, the GTP-bound mutant RagCQ120L blunted mTORC1 activity, even in the presence of amino acids. Thus, RagC plays a pivotal role in mediating the binding of mTORC1 to the Rag heterodimer and manipulating the nucleotide state of RagC affects mTORC1 pathway activity.
FLCN Interacts with the Rag GTPases in an Amino Acid-Sensitive Fashion
Because RagC is critical for mTORC1 activation, we sought to identify regulators of its nucleotide state. We employed proteomic approaches that have successfully identified other mTORC1 pathway components (see Experimental Procedures). Mass spectrometric analysis of anti-FLAG immunoprecipitates prepared from HEK-293T cells stably expressing FLAG-tagged RagA, −B, −C, or −D, but not control proteins, consistently identified peptides derived from Folliculin (FLCN) and its interacting partners, FNIP1 and FNIP2.
FLCN is evolutionarily conserved, yet its molecular function remains unknown (Schmidt, 2012; van Slegtenhorst et al., 2007). Loss-of-function mutations in FLCN cause a familial cancer syndrome called Birt-Hogg-Dubé (BHD), characterized by hamartomatous tumors of the hair follicle (fibrofolliculomas), kidney, and lung (BIRT et al., 1977; Nickerson et al., 2002). Given that TSC1/2, PTEN, and LKB1—genes linked to other hamartoma syndromes—are bona fide tumor suppressors that impinge on the mTORC1 pathway, FLCN is likely a fregulator of the pathway (Baba et al., 2006; Guertin and Sabatini, 2007). In addition, there is emerging evidence that FLCN plays a role in mTORC1 nutrient sensing, potentially implicating the involvement of the Rags. For example, deletions of the fission yeast orthologs of FLCN and TSC1/2 have opposite effects on the expression of amino acid metabolism genes (van Slegtenhorst et al., 2007). Furthermore, a chemical genomic screen revealed that the deletion mutants for the budding yeast orthologs of FLCN (LST7) and the Rags (GTR1, GTR2) exhibited similar growth sensitivities to various environmental and chemical insults (Figure S1) (Hillenmeyer et al., 2008). FLCN forms a complex with either FNIP1 or FNIP2, paralogs with 74% sequence similarity (Baba et al., 2006; Hasumi et al., 2008; Takagi et al., 2008). The FNIPs directly interact with AMP-activated protein kinase (AMPK), an energy-sensor that monitors the AMP/ATP ratio. Given these results, the possibility that the FLCN-FNIP complex interacts with the Rags was of great interest.
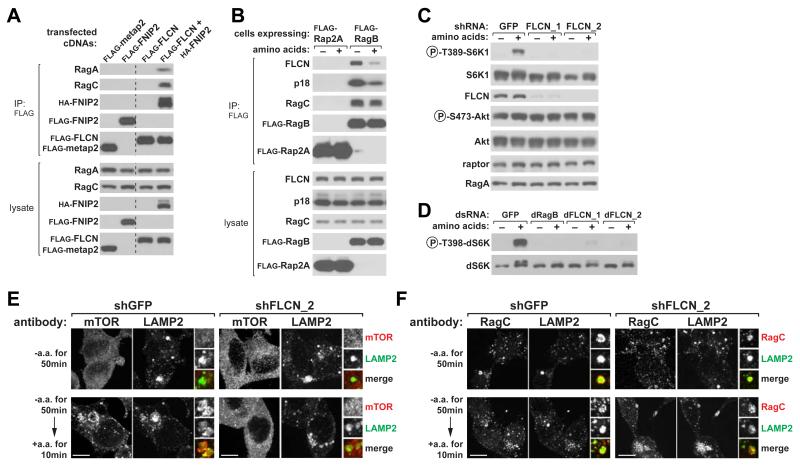
To begin to verify our mass spectrometric identification of FLCN and FNIPs as Rag-interacting proteins, we expressed them alone or in combination in HEK-293T cells. Endogenous RagA and RagC co-immunopreciptated with FLCN when it was co-expressed with FNIP2, but not with metap2, or when FLCN or FNIP2 were expressed alone (Figure 2A). This suggests that a FLCN-FNIP2 complex is required for either FLCN or FNIP2 to interact with the Rags.
Figure 2. FLCN-FNIP2 is a Rag-Interacting Complex and is Necessary for mTORC1 Activation by Amino Acids.
(A) Recombinant epitope-tagged FLCN-FNIP2 co-immunoprecipitates endogenous RagA and RagC. Anti-FLAG immunoprecipitates were prepared from HEK-293T cells expressing the indicated cDNAs in expression vectors and analyzed along with cell lysates by immunoblotting for indicated proteins. Irrelevant lanes were removed and indicated by a dashed line.
(B) Amino acid starvation increases the amount of endogenous FLCN that co-immunoprecipitates with recombinant RagB. HEK-293T cells stably expressing FLAG-RagB were starved for amino acids for 50 min, or starved and stimulated with amino acids for 10 min. Anti-FLAG immunoprecipitates were analyzed as in (A).
(C) FLCN is necessary for the activation of the mTORC1 pathway by amino acids. HEK-293T cells expressing a control shRNA or two distinct shRNAs targeting FLCN were starved for amino acids for 50 min, or starved and stimulated with amino acids for 10 min. Levels of indicated proteins and phosphorylation states were analyzed by immunobloting of cell lysates.
(D) FLCN function is conserved in Drosophila cells. Drosophila S2 cells were transfected with a control dsRNA, or dsRNAs targeting dRagB, or dFLCN, starved of amino acids for 90 min, or starved and re-stimulated with amino acids for 30 min and analyzed as in (C).
(E) Knockdown of FLCN prevents amino acid-induced translocation of mTOR to lysosomes. HEK-293T cells expressing the indicated shRNAs were starved or starved and re-stimulated with amino acids for the specified times before co-immunostaining for mTOR (red) and LAMP2 (green).
(F) FLCN is not required for the lysosomal localization of RagC. HEK-293T cells expressing the indicated shRNAs were treated and processed as described in (E). In all images, insets show selected fields that were magnified two times and their overlays. Scale bars represent 10 μm. See also Figure S1.
The Rags, Ragulator, and v-ATPase, established components of the mTORC1 nutrient-sensing machinery, all engage in nutrient-responsive interactions with each other (Bar-Peled et al., 2012; Efeyan et al., 2013; Zoncu et al., 2011a). Like that of Ragulator and the v-ATPase, the interaction between endogenous FLCN and the Rag heterodimer, isolated through stably expressed RagB, strengthened upon amino acid starvation (Figure 2B).
FLCN is Necessary for mTORC1 Activation and Localization to the Lysosomal Membranes
Studies investigating the role of FLCN in the mTORC1 pathway in mammalian systems have yielded equivocal results. While in most cell-based systems acute loss of FLCN inhibits mTORC1 activation (Bastola et al., 2013; Hartman et al., 2009; Hudon et al., 2010; Takagi et al., 2008; van Slegtenhorst et al., 2007), deletion of FLCN in tissues in vivo, causes mTORC1 hyperactivation (Baba et al., 2008; Baba et al., 2012; Chen et al., 2008; Hasumi et al., 2009) (see Discussion for more details). In the cell-based assays we have used to study other mTORC1 components, we find that FLCN is indeed necessary for mTORC1 activation by amino acids. In HEK-293T cells, short-hairpin RNAs (shRNAs) targeting FLCN suppressed mTORC1 activation by amino acids, as read out by the phosphorylation of S6K1 (Figure 2C). This phenotype was recapitulated in Drosophila S2 cells treated with double stranded RNAs (dsRNAs) targeting the ortholog of FLCN, indicating that the function of FLCN is conserved (Figure 2D). Collectively, these results show that FLCN interacts with the Rag GTPases in a nutrient-sensitive manner and is necessary for mTORC1 activation by amino acids.
A key event in the activation of mTORC1 by amino acids is its recruitment to the lysosomal surface by the Rag GTPases (Sancak et al., 2010). In HEK-293T cells expressing shRNAs targeting FLCN, mTOR failed to localize to LAMP2-positive lysosomes in response to amino acid stimulation (Figure 2E). Unlike Ragulator, the lysosomal scaffold for the Rags, FLCN was not required for Rag subcellular localization (Figure 2F). Thus, although the Rags localize appropriately to the lysosomal membranes in FLCN knockdown cells, mTORC1 is unable to be recruited there. These results are consistent with FLCN being required for mTORC1 activation by amino acids (Figure 2C).
FLCN Co-Localizes with the Rag GTPases on the Lysosomal Surface in an Amino Acid-Sensitive Fashion
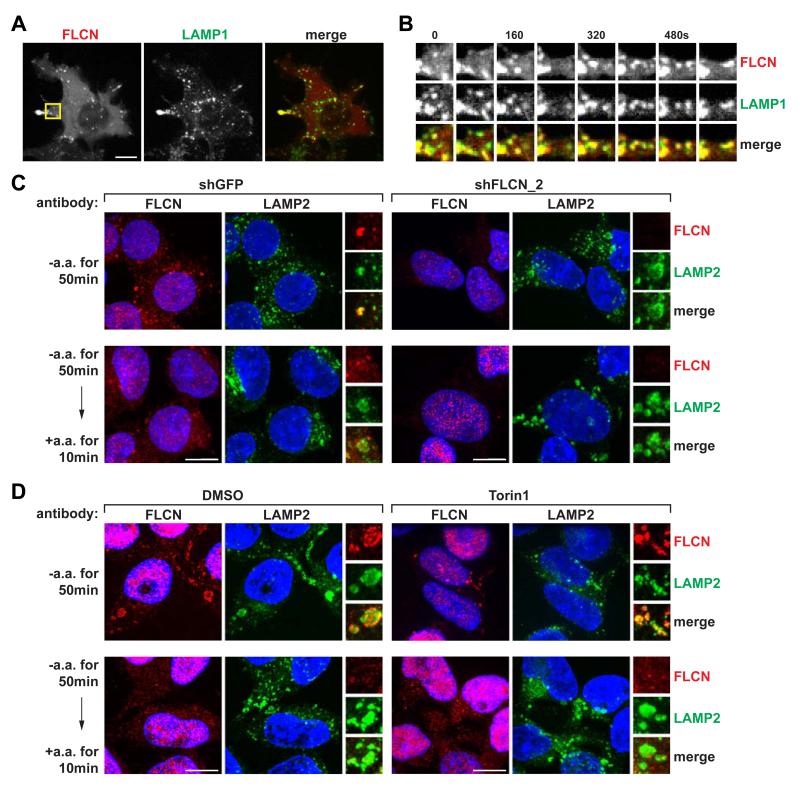
Given that FLCN and FNIPs are enriched in membranes (Takagi et al., 2008) and that FLCN interacts with the Rag GTPases, we tested the possibility that FLCN itself may localize to the lysosomal surface. Indeed, in HEK-293T cells co-expressing HAFNIP2, GFP-tagged FLCN co-localized with RFP-tagged LAMP1, a lysosomal marker (Figure 3A), and this association persisted over time as lysosomes trafficked within the cell (Figure 3B and Movie S1). Consistent with previous reports (Takagi et al., 2008), FLCN-GFP was found diffusely throughout the cell when FNIP2 was not co-expressed (Figure S2), suggesting that FNIP2 is required for the lysosomal localization of FLCN.
Figure 3. FLCN Localizes to the Lysosomal Surface in an Amino Acid-Sensitive Fashion.
(A) FLCN localizes to the lysosomal surface. Spinning disk confocal image of a HEK-293T cell co-expressing FLCN-GFP, HA-FNIP2, and mRFP-LAMP1 (pseudo-colored red and green in merge, respectively).
(B) FLCN associates with lysosomes as they traffic within cells. Time-lapse of FLCN- and LAMP1-positive lysosomes from the boxed region in (A) magnified by 2.5 times. Time intervals are in seconds.
(C) FLCN localizes to the lysosomal surface upon amino acid starvation. HEK-293T cells expressing the indicated shRNAs were starved or starved and re-stimulated with amino acids for the specified times before co-immunostaining for FLCN (red) and LAMP2 (green).
(D) Amino acid-sensitive localization of FLCN is independent of mTORC1 activity. HEK-293T cells treated with DMSO or Torin1 (250 nM) were starved or starved and re-stimulated with amino acids for the specified times before co-immunostaining for FLCN (red) and LAMP2 (green). In (C) and (D), insets show selected fields that were magnified two times and their overlays. All scale bars represent 10 μm.
See also Movie S1 and Figure S2.
Despite detecting amino acid-sensitive interactions between the Rags and FLCN in co-immunoprecipitation experiments, initial tests using transiently co-expressed FLCN and FNIP2 did not reveal appreciable nutrient-responsive changes in their localization. We reasoned that overexpression might overwhelm endogenous regulatory mechanisms; therefore, we sought to probe the localization of endogenous FLCN using an anti-FLCN antibody. Although knockdown of FLCN expression did not diminish the immunofluorescence signal of most anti-FLCN antibodies we tested, we did identify one antibody that showed both specific (lysosomal) and non-specific (nuclear) signals (Figure 3C). Using this antibody we found that, in HEK-293T cells, endogenous FLCN was enriched at the lysosomal surface during amino acid starvation and dispersed upon amino acid stimulation (Figure 3C). Furthermore, in HEK-293T cells treated with Torin1, an ATP-competitive inhibitor of mTOR (Thoreen et al., 2009), FLCN still dispersed from the lysosome upon amino acid stimulation (Figure 3D). Thus, FLCN localizes to the lysosomal surface during amino acid starvation, but leaves this site upon amino acid stimulation in an mTORC1 activity-independent manner. This regulated localization of FLCN to the lysosomes is consistent with the increased binding of FLCN to the Rags under amino acid starvation conditions (Figure 2B).
FLCN-FNIP2 is a GAP for RagC and RagD
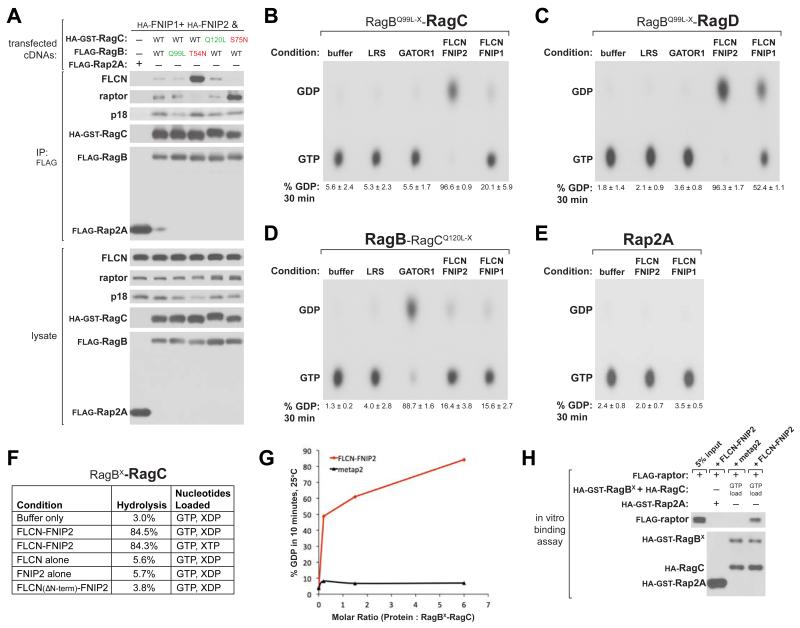
Regulators of GTP-binding proteins commonly associate with either the GTP- or GDP-bound form of their cognate GTPases (Takai et al., 2001). To investigate the molecular function of the FLCN-FNIP complex, we asked if it exhibits any preferential binding to the Rag GTPase mutants. Different combinations of Rag mutants were co-expressed with FNIPs in HEK-293T cells. Interestingly, Rag heterodimers containing low affinity nucleotide mutants behaved in opposite ways; large amounts of endogenous FLCN co-purified with the RagBT54N-C heterodimer, whereas little FLCN was recovered with RagB-CS75N (Figure 4A). In contrast, RagB-CS75N was able to interact with mTORC1 and Ragulator, as detected through their raptor and p18 subunits, respectively.
Figure 4. FLCN-FNIP is a GTPase-Activating Protein Complex for RagC and RagD.
(A) Rag heterodimers containing RagBT54N, but not RagCS75N, co-immunoprecipitate endogenous FLCN. Anti-FLAG immunoprecipitates were prepared from HEK-293T cells transfected with indicated cDNAs in expression vectors. Cell lysates and immunoprecipitates were analyzed by immunoblotting of indicated proteins.
(B) FLCN-FNIP stimulates GTP hydrolysis by RagC. 5 pmol of RagBQ99L-X-RagC was loaded with [α-32P]GTP and incubated with indicated proteins (20 pmol). GTP hydrolysis was determined by thin-layer chromatography (see Experimental Procedures). Each value represents the mean ± SD (n=3).
(C) FLCN-FNIP stimulates GTP hydrolysis by RagD. GAP assay was performed with RagBQ99L-X RagD as described in (B).
(D) GATOR1, but not FLCN-FNIP1/2, stimulates GTP hydrolysis by RagB. GAP assay was performed with RagB-RagCQ120L-X as described in (B).
(E) FLCN-FNIP does not stimulate GTP hydrolysis by Rap2A. GAP assay was performed with the Rap2A control GTPase as described in (B).
(F) Nucleotide state of RagB does not affect FLCN-FNIP2 GAP activity towards RagC, FLCNFNIP2 complex is required for GAP activity, and N-terminal region of FLCN is required for GAP activity. GAP assays were performed as described in (B) with 10 min incubations of indicated proteins. Values represent an average from at least 2 experiments.
(G) FLCN-FNIP2 stimulates GTP hydrolysis by RagC in a dose-dependent manner. GAP assay was performed as described in (B) with indicated molar ratios of FLCN-FNIP2 or control metap2 to RagBX-RagC.
(H) In vitro, the FLCN-FNIP2 GAP activity is sufficient to cause raptor to bind to the Rags. In vitro binding assay in which recombinant HA-GST-tagged-RagBX-RagC or -Rap2A were loaded with the indicated nucleotide and incubated with purified FLAG-tagged raptor along with FLCN-FNIP2 or metap2 control protein. HA-GST precipitates were analyzed by immunoblotting for indicated proteins.
See also Figure S3.
These binding properties are consistent with several possible functions for the FLCN-FNIP complex. The robust binding to RagBT54N suggests that the FLCN-FNIP complex might be a guanine nucleotide exchange factor (GEF) or GDP dissociation inhibitor (GDI) for RagB (Bos et al., 2007; DerMardirossian and Bokoch, 2005). Intriguingly, a recent report of the crystal structure of the FLCN C-terminal domain revealed that despite having almost no sequence similarity, it shares structural similarity with the DENN domain, which has GEF activity towards the Rab GTPases (Nookala et al., 2012). Alternatively, although not mutually exclusively, the inability of FLCN to interact with RagCS75N indicates that the FLCN-FNIP complex prefers binding to RagC in its GTP-bound state, a property shared by many GTPase activating proteins (GAPs) (Bos et al., 2007; Takai et al., 2001).
Because the Rags function as obligate heterodimers, it was necessary to monitor the nucleotide state of one Rag at a time. To accomplish this, we assembled Rag heterodimers composed of a wild-type Rag with its appropriate RagX partner. Thus, loading with radiolabeled guanine and unlabeled xanthine nucleotides allowed us to selectively monitor the nucleotide state of the wild-type Rag, as previously described (Bar-Peled et al., 2012). Purified FLCN-FNIP complex did not stimulate or inhibit the dissociation of GDP from RagB or RagC when coupled with either a RagX or wild-type partner (Figures S3A-S3C). These results suggest that FLCN-FNIP does not have GEF or GDI activities towards the Rags. Instead, the strong binding to RagBT54N might indicate that RagB serves as a docking site for FLCN-FNIP on the Rag heterodimer. As RagB is GDP-bound during amino acid starvation, this behavior would be consistent with both the increased binding to the Rag heterodimer and lysosomal localization of FLCN under this condition (Figures 2B and 3C).
We pursued the possibility that the FLCN-FNIP complex may be a GAP for RagC/D. To assay GTPase activating activity towards one Rag at a time, we prepared Rag heterodimers with a wild-type Rag partnered with a doubly mutated Rag that binds xanthine nucleotides but lacks GTPase activity, termed RagBQ99L-X and RagCQ120L-X. As expected, purified GATOR1, a GAP for RagA/B (Bar-Peled et al., 2013), strongly stimulated the GTPase activity of RagB, but not RagC or RagD (Figures 4B-4D). Conversely, purified FLCN-FNIP2 potently stimulated GTP hydrolysis by RagC and RagD, but not RagA, RagB, or Rap2A (a control GTPase), in a time- and dose-dependent manner (Figures 4B-4E, 4G, S3D). Similar degrees of GAP activity were observed toward RagC when RagBX was loaded with either XTP or XDP, suggesting that the GAP activity towards RagC/D is not dependent on the nucleotide state of RagA/B (Figure 4F). The FLCN-FNIP1 complex was overall less active than FLCNFNIP2 and appears to prefer RagD instead of RagC (Figures 4B and 4C).
The observed GTP hydrolysis was not due to contaminating phosphatases because addition of purified FLCN-FNIP2 to free GTP showed minimal hydrolysis (Figure S3E). Furthermore, an FLCN-FNIP2 complex with FLCN lacking its N-terminal region (Nookala et al., 2012), but that still interacts with the Rags, did not exhibit GAP activity, suggesting that this region is required for the GAP activity (Figure 4F). In contrast to a recent report that proposed that the leucyl-tRNA synthetase (LRS) acts as a GAP for RagD (Han et al., 2012), purified LRS did not increase basal GTPase activity of any of the Rags in any condition tested (Figures 4B-D). Lastly, purified FLCN or FNIP2 alone did not have GAP activity for the Rags, suggesting that an intact complex is required (Figure 4F).
As RagC-GDP is required for mTORC1 binding to the Rag heterodimer (Figure 1), we asked if FLCN-FNIP2 GAP activity was sufficient to promote binding of mTORC1 to the Rags in vitro. Indeed, addition of FLCN-FNIP2, but not a control protein, caused RagBX-C loaded with GTP to bind raptor (Figure 4H). Together, these results indicate that FLCN-FNIP2 acts as a positive component of the mTORC1 pathway by promoting the binding of mTORC1 to the Rag heterodimer via its GAP activity for RagC and RagD.
DISCUSSION
A growing body of evidence indicates that the Rags and Rheb are key components of a coincidence detector mechanism that ensures mTORC1 is active only in the appropriate growth conditions (reviewed in Dibble and Manning, 2013; Efeyan et al., 2012; Kim et al., 2013; Yuan et al., 2013). Through a Rag-mediated pathway, amino acids recruit mTORC1 to the lysosomal surface, where it can encounter Rheb. If growth factors and energy levels are sufficient, Rheb then binds to and activates the kinase activity of mTORC1. Our new findings support the idea that the Ragulator-Rag complex is a nutrient-regulated docking site for mTORC1 on lysosomes, in which the nucleotide state of RagC, and likely RagD, is the key determinant of mTORC1 binding.
Our work raises a number of intriguing questions. First, given the importance of RagC in the binding of the Rag heterodimer to mTORC1, what is the role of RagA/B? It is clear that RagA/B plays a dominant role in mTORC1 activation as expression of a RagA/B mutant that is bound constitutively to GTP makes the mTORC1 pathway completely insensitive to amino acid starvation (Efeyan et al., 2013; Kim et al., 2008; Sancak et al., 2008). A likely possibility is that RagA/B controls a process that has not been recognized but is critical for mTORC1 signaling. For example, RagA/B may regulate the subcellular localization of the heterodimer, thereby controlling its access to mTORC1. While the Rag proteins appear to be constitutively localized to the lysosomal surface, there may be a pool of Rag heterodimers that cycle on and off lysosomes upon amino acid stimulation, enabling them to find and retrieve mTORC1 from its nonlysosomal location in amino acid-starved cells. This cycling could be controlled by the RagA/B nucleotide state, which would be consistent with the observation that the loading of RagA/B with GDP greatly strengthens the binding of the Rag heterodimer to Ragulator, its lysosomal scaffold (Bar-Peled et al., 2012).
Such a model could also help address a second conundrum. As mTORC1 and the Rags reside on the lysosomal surface in the presence of amino acids, why is FLCN found diffusely in the cytoplasm under this same condition? A possibility consistent with the above model is that FLCN activates RagC/D in Rag heterodimers that have come off the lysosomes upon amino acid stimulation and are on route to recruiting mTORC1. Alternatively, in amino acid starved cells FLCN might be poised at the lysosomal surface to activate RagC/D upon the restoration of amino acid levels, but such a scenario would require a mechanism to regulate the FLCN-FNIP GAP activity.
A third question is why FLCN is a tumor suppressor and yet in most studies in cultured cells and whole organisms, including ours, it scores as a positive component of the TORC1 pathway (Baba et al., 2006; Bastola et al., 2013; Hartman et al., 2009; Hudon et al., 2010; Liu et al., 2013; Takagi et al., 2008; van Slegtenhorst et al., 2007). It is possible that in response to the suppression of mTORC1 signaling caused by FLCN loss, cells overdrive other pathways that more than compensate for mTORC1 inhibition. Indeed, in FLCN-null kidney tumors and cysts, as well as embryonic stem cells, the Ras-MAPK, Akt, and mTORC1 pathways all appear hyperactive (Baba et al., 2008; Cash et al., 2011; Chen et al., 2008; Hasumi et al., 2009), although this could reflect the proliferative state of the cells. Furthermore, in FLCN-null tumors there must be a mechanism to reactivate mTORC1, suggesting that there may be proteins that can compensate for FLCN loss. Candidates for such a role include C9orf72 and SMCR8, which have very little sequence homology with FLCN, but like it, are predicted to have DENN-like domains (Levine et al., 2013; Zhang et al., 2012). Lastly, it is likely that future studies will reveal that the FLCN-FNIP complex funnels so far unidentified regulatory signals to the Rag pathway so as to modulate amino acid sensing by mTORC1.
EXPERIMENTAL PROCEDURES
Cell Lysis and Immunoprecipitation
HEK-293T cells were rinsed once with ice-cold PBS and lysed with Triton lysis buffer (1% Triton X-100, 10 mM β-glycerol phosphate, 10 mM pyrophosphate, 40 mM Hepes pH 7.4, 2.5 mM MgCl2 and 1 tablet of EDTA-free protease inhibitor (per 25 ml)). When amino acid-sensitive interactions were interrogated, cells were lysed in CHAPS lysis buffer (0.3% CHAPS, 10 mM β-glycerol phosphate, 10 mM pyrophosphate, 40 mM Hepes pH 7.4, 2.5 mM MgCl2 and 1 tablet of EDTA-free protease inhibitor (per 25 ml)). The soluble fractions of cell lysates were isolated by centrifugation at 13,000 rpm in a refrigerated microcentrifuge for 10 minutes. For anti-FLAG-immunoprecipitations, the FLAG-M2 affinity gel was washed with lysis buffer 3 times and 50 μl of a 50% slurry of the affinity gel was then added to cleared cell lysates and incubated with rotation for 3 hours at 4°C. The beads were washed 3 times with lysis buffer containing 150 mM NaCl. Immunoprecipitated proteins were denatured by the addition of 50 μl of sample buffer and boiling for 5 minutes as described (Kim et al., 2002), resolved by 8%–16% SDS-PAGE, and analyzed by immunoblotting.
For co-transfection experiments, 2,000,000 HEK-293T cells were plated in 10 cm culture dishes. Twenty-four hours later, cells were transfected using XtremeGene 9 transection reagent with the pRK5-based cDNA expression plasmids indicated in the Figures in the following amounts: 100 ng HA-RagB; 100 ng HA- or HA-GST-RagC; 300 ng HA-GST-RagBQ99L or 300 ng HA-GST-RagBT54N; 300 ng HA-GST-RagCS75N or 300 ng HA-GST-RagCQ120L; 100 ng FLAG-Rap2A; 300 ng of FLAG-metap2, 300 ng of FLAG-FLCN, 300 ng of HA- or FLAG-FNIP2, and 5 ng Flag-S6K. The total amount of plasmid DNA in each transfection was normalized to 2 μg with empty pRK5. Thirty-six hours after transfection, cells were lysed as described above.
Amino acid Starvation of Cells
HEK-293T cells in culture dishes or coated glass cover slips were rinsed with and incubated in amino acid-free RPMI for 50 minutes and stimulated with amino acids for 10-15 minutes. After stimulation, the final concentration of amino acids in the media was the same as in RPMI. A 10× amino acid mixture used to stimulate cells, which was prepared from individual powders of amino acids. When Torin1 was used, cells were incubated with 250 nM of Torin1 or DMSO during the 50 minute starvation period and the 10 minute stimulation period.
RNAi in Mammalian Cells
Lentiviral shRNAs targeting FLCN and non-targeting controls (Sancak et al., 2008) were obtained from the TRC. The TRC number for each shRNA is as follows:
Human FLCN shRNA_1: TRCN0000237882
Human FLCN shRNA_2: TRCN0000237885
shRNA-encoding plasmids were co-transfected with the Delta VPR envelope and CMV VSV-G packaging plasmids into actively growing HEK-293T cells using XtremeGene 9 transfection reagent as previously described (Sarbassov et al., 2005). Virus-containing supernatants were collected 48 hours after transfection and passed through a 0.45 um filter to eliminate cells. Target cells were infected in the presence of 8 μg/ml polybrene. 24 hours later, cells were selected with puromycin and analyzed on the 3rd day after selection.
Immunofluorescence Assays
Immunofluorescence assays were performed as described in (Sancak et al., 2010). Briefly, 400,000 of the indicated HEK-293T cells were seeded on fibronectin-coated glass coverslips in 6-well tissue culture plates. Twenty-four hours later, the slides were starved or stimulated with amino acids as described above and fixed for 15 min with 4% paraformaldehyde in PBS at room temperature. The slides were rinsed twice with PBS and cells were permeabilized with 0.05% Triton X-100 in PBS for 5 min. After rinsing twice with PBS, the slides were incubated with primary antibody in 5% normal donkey serum for 1 hr at room temperature, rinsed four times with PBS, and incubated with secondary antibodies produced in donkey (diluted 1:400 in 5% normal donkey serum) for 40 min at room temperature and washed four times with PBS. Slides were mounted on glass coverslips using Vectashield containing DAPI (Vector Laboratories) and imaged on a spinning disk confocal system (Perkin Elmer).
Live Cell Imaging
300,000 HEK-293T cells were seeded on fibronectin-coated glass bottom 35 mm dishes (MatTek Corp.). The next day, cells were co-transfected using XtremeGene 9 with the following plasmids: 90 ng FLCN-GFP (Clontech), 10 ng HA-FNIP2, 100 ng mRFP-LAMP1, 300 ng empty pRK5. The following day, cells were imaged on a spinning disk confocal microscope (Andor Technology) with a 488-nm and a 568-nm laser through a 60× objective.
Purification of Recombinant Proteins for GAP and In Vitro Binding Assays
To produce protein complexes used for GAP assays, 4,000,000 HEK-293T cells were plated in 15 cm culture dishes. Forty-eight hours later, cells were transfected with the following combination of constructs (all cDNAs were expressed from the pRK5 expression plasmid). For RagB-RagCQ120L-X: 16 μg HA-RagB and 8 μg Flag-RagCQ120L-D181N; for RagBQ99L-X-RagC: 8 μg FLAG-RagBQ99L-D163N and 16 μg HA-RagC; for RagBQ99L-X-RagD: 8 μg FLAG-RagBQ99L-D163N and 16 μg HA-RagD; for RagBX-RagC: 8 μg FLAG-RagBD163N and 16 μg HA-RagC. For Rags used in in-vitro binding: 8 μg HA-GSTRagBD163N and 16 μg HA-RagC; 8 μg HA-GST-RagCD181N and 16 μg HA-RagB; 8 μg HAGST-RagC and 16 μg HA-RagB; GATOR1: 4 μg FLAG-DEPDC5 and 8 μg myc-NPRL2 and 8 μg myc-NPRL3 (Bar-Peled et al., 2013); FLCN-FNIP1: 8 μg FLAG-FNIP1 and 16 μg HA-FLCN; FLCN-FNIP2: 8 μg FLAG-FNIP2 and 16 μg HA-FLCN; FLCN(ΔN-term)-FNIP2: 8 μg FLAG-FNIP2 and 16 μg HA-FLCN(ΔN-term) (Nookala et al., 2012). For FLCN-FNIP purifications, it is crucial to immunoprecipitate through the FNIP component to obtain stoichiometric complexes with high activities. For individual proteins: 10 μg Flag- or HA-GST-Rap2A, 15 μg of FLAG-Leucyl tRNA synthetase (LRS), or 10 μg Flag-Metap2, 16 μg FLAG-FLCN, 16 μg FLAG-FNIP2.
Thirty-six hours post transfection cell lysates were prepared as described above, with the exception that for all FLCN or FNIP containing purifications, EDTA-free protease inhibitor tablet was added to prevent degradation and for all GATOR purifications cells were lysed in 0.3% CHAPS buffer without MgCl2. 200 μl of a 50% slurry of FLAG-M2 affinity gel or immobilized glutathione beads were added to lysates from cells expressing FLAG-tagged proteins or HA-GST tagged proteins, respectively. Recombinant proteins were immunoprecipitated for 3 hours at 4°C. Each sample was washed once with Triton lysis buffer, followed by 3 washes with Triton lysis buffer supplemented with 500 mM NaCl and finally, 4 washes with the CHAPS buffer. FLCN-FNIP complexes were rotated at 4°C in the last Triton salt wash for 30 minutes for a cleaner purification. FLAG-tagged proteins were eluted from the FLAG-M2 affinity gel with a competing FLAG peptide for 1 hour as described above. All proteins were stored in CHAPS buffer supplemented with 10% glycerol, snap frozen with liquid nitrogen and stored at −80°C.
To remove the FLAG peptide, proteins were subsequently purified on a HiLoad 16/60 Superdex 200 FPLC column (GE) pre-equilibrated with CHAPS buffer supplemented with 150 mM salt. For FLCN-FNIP purifications, 4 protease inhibitor tablets were supplemented per 400mL of CHAPS buffer. The peak corresponding to the desired complex was concentrated in 10,000 MW CO columns (Amicon), snap frozen in CHAPS buffer supplemented with 10% glycerol and stored at −80°C. All proteins were verified by Coomasie staining on a 4-16% gel.
Rag GTP Hydrolysis Assays
GAP assays were performed essentially as described in (Bar-Peled et al., 2013). In brief, the indicated GTPases were bound at 4°C to FLAG-M2 affinity gel. The resin was then washed to remove unbound protein, and the GTPases were loaded with XDP (or XTP where indicated) and [α-32P]GTP at room temperature followed by an incubation with MgCl2 to stabilize the nucleotide. The GTPases were subsequently washed to remove unbound nucleotide and eluted from the affinity gel with competing FLAG peptide. Protein concentrations were determined prior to use.
For the TLC-based GTP hydrolysis assay, 5 pmoles of the indicated Rag heterodimer or Rap2a loaded with xanthine nucleotides and [α-32P]GTP were added to 20 pmoles of purified LRS, GATOR1, or FLCN-FNIP in 45 μl of GTPase wash buffer. The reaction was incubated at 25°C for the indicated times and eluted samples were spotted on PEI Cellulose plates and developed for 2.5 hours in 0.5 M KH2PO4 pH 3.4. Plates were exposed to film and spot densities were quantified with ImageJ.
Supplementary Material
HIGHLIGHTS.
RagC/D nucleotide state is a key determinant of mTORC1 binding to the Rag GTPases
FLCN-FNIP complex interacts with the Rag GTPases in an amino-acid sensitive fashion
FLCN is necessary for mTORC1 activation by amino acids
FLCN-FNIP complex is a GTPase activating protein (GAP) for RagC/D
ACKNOWLEDGEMENTS
We thank Shuyu Wang, Larry Schweitzer, Mounir Koussa, Molly Plovanich, and Rich Possemato for critical review of the manuscript and all members of the Sabatini Lab for helpful suggestions. This work was supported by grants from NIH (CA103866 and AI47389) and Department of Defense (W81XWH-07-0448) to D.M.S., fellowship support from the NCI (F30CA180754) to Z.T., David H. Koch Graduate Fellowship Fund to L.B.-P., National Science Foundation to L.C. and T.W., Jane Coffin Childs Memorial Fund for Medical Research to R.Z., and support from Howard Hughes Medical Institute (HHMI) to C.K. D.M.S. is an investigator of the HHMI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Baba M, Furihata M, Hong S-B, Tessarollo L, Haines DC, Southon E, Patel V, Igarashi P, Alvord WG, Leighty R, et al. Kidney-targeted Birt-Hogg-Dube gene inactivation in a mouse model: Erk1/2 and Akt-mTOR activation, cell hyperproliferation, and polycystic kidneys. J Natl Cancer I. 2008;100:140–154. doi: 10.1093/jnci/djm288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M, Hong S-B, Sharma N, Warren MB, Nickerson ML, Iwamatsu A, Esposito D, Gillette WK, Hopkins RF, Hartley JL, et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. P Natl Acad Sci Usa. 2006;103:15552–15557. doi: 10.1073/pnas.0603781103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M, Keller JR, Sun H-W, Resch W, Kuchen S, Suh HC, Hasumi H, Hasumi Y, Kieffer-Kwon K-R, Gonzalez CG, et al. The Folliculin-FNIP1 pathway deleted in human Birt-Hogg-Dube syndrome is required for mouse B cell development. Blood. 2012 doi: 10.1182/blood-2012-02-410407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Peled L, Chantranupong L, Cherniack AD, Chen WW, Ottina KA, Grabiner BC, Spear ED, Carter SL, Meyerson M, Sabatini DM. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science. 2013;340:1100–1106. doi: 10.1126/science.1232044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell. 2012;150:1196–1208. doi: 10.1016/j.cell.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastola P, Stratton Y, Kellner E, Mikhaylova O, Yi Y, Sartor MA, Medvedovic M, Biesiada J, Meller J, Czyzyk-Krzeska MF. Folliculin Contributes to VHL Tumor Suppressing Activity in Renal Cancer through Regulation of Autophagy. PLoS ONE. 2013;8:e70030. doi: 10.1371/journal.pone.0070030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birt A, Hogg G, Dube W. Hereditary Multiple Fibrofolliculomas with Trichodiscomas and Acrochordons. Arch Dermatol. 1977;113:1674–1677. [PubMed] [Google Scholar]
- Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: Critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Gene Dev. 2004;18:2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash TP, Gruber JJ, Hartman TR, Henske EP, Simon MC. Loss of the Birt-Hogg-Dube tumor suppressor results in apoptotic resistance due to aberrant TGF beta-mediated transcription. Oncogene. 2011;30:2534–2546. doi: 10.1038/onc.2010.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro AF, Rebhun JF, Clark GJ, Quilliam LA. Rheb binds tuberous sclerosis complex 2 (TSC2) and promotes S6 kinase activation in a rapamycin- and farnesylation-dependent manner. The Journal of biological chemistry. 2003;278:32493–32496. doi: 10.1074/jbc.C300226200. [DOI] [PubMed] [Google Scholar]
- Chen J, Futami K, Petillo D, Peng J, Wang P, Knol J, Li Y, Khoo S-K, Huang D, Qian C-N, et al. Deficiency of FLCN in Mouse Kidney Led to Development of Polycystic Kidneys and Renal Neoplasia. Plos One. 2008;3:e3581. doi: 10.1371/journal.pone.0003581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradetti MN, Inoki K, Guan K-L. The stress-inducted proteins RTP801 and RTP801L are negative regulators of the mammalian target of rapamycin pathway. The Journal of biological chemistry. 2005;280:9769–9772. doi: 10.1074/jbc.C400557200. [DOI] [PubMed] [Google Scholar]
- DerMardirossian C, Bokoch G. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15:356–363. doi: 10.1016/j.tcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Dibble CC, Manning BD. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat Cell Biol. 2013;15:555–564. doi: 10.1038/ncb2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efeyan A, Zoncu R, Chang S, Gumper I, Snitkin H, Wolfson RL, Kirak O, Sabatini DD, Sabatini DM. Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival. Nature. 2013;493:679–683. doi: 10.1038/nature11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efeyan A, Zoncu R, Sabatini DM. Amino acids and mTORC1: from lysosomes to disease. Trends in molecular medicine. 2012 doi: 10.1016/j.molmed.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig LA. Tools of the trade: use of dominant-inhibitory mutants of Ras-family GTPases. Nat Cell Biol. 1999;1:E25–27. doi: 10.1038/10018. [DOI] [PubMed] [Google Scholar]
- Feig LA, Cooper GM. Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol Cell Biol. 1988;8:3235–3243. doi: 10.1128/mcb.8.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frech M, Darden TA, Pedersen LG, Foley CK, Charifson PS, Anderson MW, Wittinghofer A. Role of glutamine-61 in the hydrolysis of GTP by p21H-ras: an experimental and theoretical study. Biochemistry. 1994;33:3237–3244. doi: 10.1021/bi00177a014. [DOI] [PubMed] [Google Scholar]
- Garami A, Zwartkruis FJT, Nobukuni T, Joaquin M, Roccio M, Stocker H, Kozma SC, Hafen E, Bos JL, Thomas G. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell. 2003;11:1457–1466. doi: 10.1016/s1097-2765(03)00220-x. [DOI] [PubMed] [Google Scholar]
- Gong R, Li L, Liu Y, Wang P, Yang H, Wang L, Cheng J, Guan K-L, Xu Y. Crystal structure of the Gtr1p-Gtr2p complex reveals new insights into the amino acid-induced TORC1 activation. Gene Dev. 2011;25:1668–1673. doi: 10.1101/gad.16968011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell. 2012;149:410–424. doi: 10.1016/j.cell.2012.02.044. [DOI] [PubMed] [Google Scholar]
- Hartman TR, Nicolas E, Klein-Szanto A, Al-Saleem T, Cash TP, Simon MC, Henske EP. The role of the Birt-Hogg-Dube protein in mTOR activation and renal tumorigenesis. Oncogene. 2009;28:1594–1604. doi: 10.1038/onc.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasumi H, Baba M, Hong S-B, Hasumi Y, Huang Y, Yao M, Valera VA, Linehan WM, Schmidt LS. Identification and characterization of a novel folliculin-interacting protein FNIP2. Gene. 2008;415:60–67. doi: 10.1016/j.gene.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasumi Y, Baba M, Ajima R, Hasumi H, Valera VA, Klein ME, Haines DC, Merino MJ, Hong S-B, Yamaguchi TP, et al. Homozygous loss of BHD causes early embryonic lethality and kidney tumor development with activation of mTORC1 and mTORC2. P Natl Acad Sci Usa. 2009;106:18722–18727. doi: 10.1073/pnas.0908853106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillenmeyer ME, Fung E, Wildenhain J, Pierce SE, Hoon S, Lee W, Proctor M, St Onge RP, Tyers M, Koller D, et al. The chemical genomic portrait of yeast: Uncovering a phenotype for all genes. Science. 2008;320:362–365. doi: 10.1126/science.1150021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose E, Nakashima N, Sekiguchi T, Nishimoto T. RagA is a functional homologue of S. cerevisiae Gtr1p involved in the Ran/Gsp1-GTPase pathway. J Cell Sci. 1998;111(Pt 1):11–21. doi: 10.1242/jcs.111.1.11. [DOI] [PubMed] [Google Scholar]
- Hoffenberg S, Nikolova L, Pan JY, Daniel DS, Wessling-Resnick M, Knoll BJ, Dickey BF. Functional and structural interactions of the Rab5 D136N mutant with xanthine nucleotides. Biochem Bioph Res Co. 1995;215:241–249. doi: 10.1006/bbrc.1995.2459. [DOI] [PubMed] [Google Scholar]
- Howell JJ, Ricoult SJH, Ben-Sahra I, Manning BD. A growing role for mTOR in promoting anabolic metabolism. Biochem Soc T. 2013;41:906–912. doi: 10.1042/BST20130041. [DOI] [PubMed] [Google Scholar]
- Hudon V, Sabourin S, Dydensborg AB, Kottis V, Ghazi A, Paquet M, Crosby K, Pomerleau V, Uetani N, Pause A. Renal tumour suppressor function of the Birt-Hogg-Dube syndrome gene product folliculin. J Med Genet. 2010;47:182–189. doi: 10.1136/jmg.2009.072009. [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Xu T, Guan K. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Gene Dev. 2003a;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan K. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003b;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- John J, Rensland H, Schlichting I, Vetter I, Borasio GD, Goody RS, Wittinghofer A. Kinetic and structural analysis of the Mg(2+)-binding site of the guanine nucleotide-binding protein p21H-ras. J Biol Chem. 1993;268:923–929. [PubMed] [Google Scholar]
- Kim D, Sarbassov D, Ali S, King J, Latek R, Erdjument-Bromage H, TEMPST P, Sabatini D. MTOR interacts with Raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan K-L. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol. 2008;10:935–945. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SG, Buel GR, Blenis J. Nutrient regulation of the mTOR complex 1 signaling pathway. Mol Cells. 2013;35:463–473. doi: 10.1007/s10059-013-0138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krengel U, Schlichting I, Scherer A, Schumann R, Frech M, John J, Kabsch W, Pai EF, Wittinghofer A. Three-dimensional structures of H-ras p21 mutants: molecular basis for their inability to function as signal switch molecules. Cell. 1990;62:539–548. doi: 10.1016/0092-8674(90)90018-a. [DOI] [PubMed] [Google Scholar]
- Levine TP, Daniels RD, Gatta AT, Wong LH, Hayes MJ. The product of C9orf72, a gene strongly implicated in neurodegeneration, is structurally related to DENN Rab-GEFs. Bioinformatics. 2013 doi: 10.1093/bioinformatics/bts725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Chen Z, Ma Y, Wu X, Jin Y, Hou S. Genetic Characterization of the Drosophila Birt-Hogg-Dubé Syndrome Gene. PLoS ONE. 2013;8:e65869. doi: 10.1371/journal.pone.0065869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol. 2005;15:702–713. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Nickerson M, Warren M, Toro J, Matrosova V, Glenn G, Turner M, Duray P, Merino M, Choyke P, Pavlovich C, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dube syndrome. Cancer Cell. 2002;2:157–164. doi: 10.1016/s1535-6108(02)00104-6. [DOI] [PubMed] [Google Scholar]
- Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, Gulati P, Byfield MP, Backer JM, Natt F, Bos JL, et al. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. P Natl Acad Sci Usa. 2005;102:14238–14243. doi: 10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nookala RK, Langemeyer L, Pacitto A, Ochoa-Montaño B, Donaldson JC, Blaszczyk BK, Chirgadze DY, Barr FA, Bazan JF, Blundell TL. Crystal structure of folliculin reveals a hidDENN function in genetically inherited renal cancer. Open Biol. 2012;2:120071. doi: 10.1098/rsob.120071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiling JH, Hafen E. The hypoxia-induced paralogs Scylla and Charybdis inhibit growth by down-regulating S6K activity upstream of TSC in Drosophila. Gene Dev. 2004;18:2879–2892. doi: 10.1101/gad.322704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roccio M, Bos JL, Zwartkruis FJT. Regulation of the small GTPase Rheb by amino acids. Oncogene. 2006;25:657–664. doi: 10.1038/sj.onc.1209106. [DOI] [PubMed] [Google Scholar]
- Roux PP, Ballif BA, Anjum R, Gygi SP, Blenis J. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. P Natl Acad Sci Usa. 2004;101:13489–13494. doi: 10.1073/pnas.0405659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag Complex Targets mTORC1 to the Lysosomal Surface and Is Necessary for Its Activation by Amino Acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Sarbassov D, Guertin D, Ali S, Sabatini D. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Saucedo L, Gao X, Chiarelli D, Li L, Pan D, Edgar B. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat Cell Biol. 2003;5:566–571. doi: 10.1038/ncb996. [DOI] [PubMed] [Google Scholar]
- Schmidt G, Lenzen C, Simon I, Deuter R, Cool RH, Goody RS, Wittinghofer A. Biochemical and biological consequences of changing the specificity of p21ras from guanosine to xanthosine nucleotides. Oncogene. 1996;12:87–96. [PubMed] [Google Scholar]
- Schmidt LS. Birt-Hogg-Dubé syndrome: from gene discovery to molecularly targeted therapies. Fam Cancer. 2012 doi: 10.1007/s10689-012-9574-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürmann A, Brauers A, Massmann S, Becker W, Joost HG. Cloning of a novel family of mammalian GTP-binding proteins (RagA, RagBs, RagB1) with remote similarity to the Ras-related GTPases. The Journal of biological chemistry. 1995;270:28982–28988. doi: 10.1074/jbc.270.48.28982. [DOI] [PubMed] [Google Scholar]
- Sekiguchi T, Hirose E, Nakashima N, Ii M, Nishimoto T. Novel G proteins, Rag C and Rag D, interact with GTP-binding proteins, Rag A and Rag B. J Biol Chem. 2001;276:7246–7257. doi: 10.1074/jbc.M004389200. [DOI] [PubMed] [Google Scholar]
- Smith EM, Finn SG, Tee AR, Browne GJ, Proud CG. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. The Journal of biological chemistry. 2005;280:18717–18727. doi: 10.1074/jbc.M414499200. [DOI] [PubMed] [Google Scholar]
- Stocker H, Radimerski T, Schindelholz B, Wittwer F, Belawat P, Daram P, Breuer S, Thomas G, Hafen E. Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nat Cell Biol. 2003;5:559–565. doi: 10.1038/ncb995. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Kobayashi T, Shiono M, Wang L, Piao X, Sun G, Zhang D, Abe M, Hagiwara Y, Takahashi K, et al. Interaction of folliculin (Birt-Hogg-Dube gene product) with a novel Fnip1-like (FnipL/Fnip2) protein. Oncogene. 2008;27:5339–5347. doi: 10.1038/onc.2008.261. [DOI] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- Tee AR, Anjum R, Blenis J. Inactivation of the tuberous sclerosis complex-1 and -2 gene products occurs by phosphoinositide 3-kinase/Akt-dependent and -independent phosphorylation of tuberin. The Journal of biological chemistry. 2003a;278:37288–37296. doi: 10.1074/jbc.M303257200. [DOI] [PubMed] [Google Scholar]
- Tee AR, Fingar DC, Manning BD, Kwiatkowski DJ, Cantley LC, Blenis J. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. P Natl Acad Sci Usa. 2002;99:13571–13576. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003b;13:1259–1268. doi: 10.1016/s0960-9822(03)00506-2. [DOI] [PubMed] [Google Scholar]
- Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. The Journal of biological chemistry. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Slegtenhorst M, Khabibullin D, Hartman TR, Nicolas E, Kruger WD, Henske EP. The Birt-Hogg-Dube and tuberous sclerosis complex homologs have opposing roles in amino acid homeostasis in Schizosaccharomyces pombe. J Biol Chem. 2007;282:24583–24590. doi: 10.1074/jbc.M700857200. [DOI] [PubMed] [Google Scholar]
- Yuan H-X, Xiong Y, Guan K-L. Nutrient sensing, metabolism, and cell growth control. Mol Cell. 2013;49:379–387. doi: 10.1016/j.molcel.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Iyer LM, He F, Aravind L. Discovery of Novel DENN Proteins: Implications for the Evolution of Eukaryotic Intracellular Membrane Structures and Human Disease. Front Genet. 2012;3:283. doi: 10.3389/fgene.2012.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003;5:578–581. doi: 10.1038/ncb999. [DOI] [PubMed] [Google Scholar]
- Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 Senses Lysosomal Amino Acids Through an Inside-Out Mechanism That Requires the Vacuolar H+-ATPase. Science. 2011a;334:678–683. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Bio. 2011b;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.