Abstract
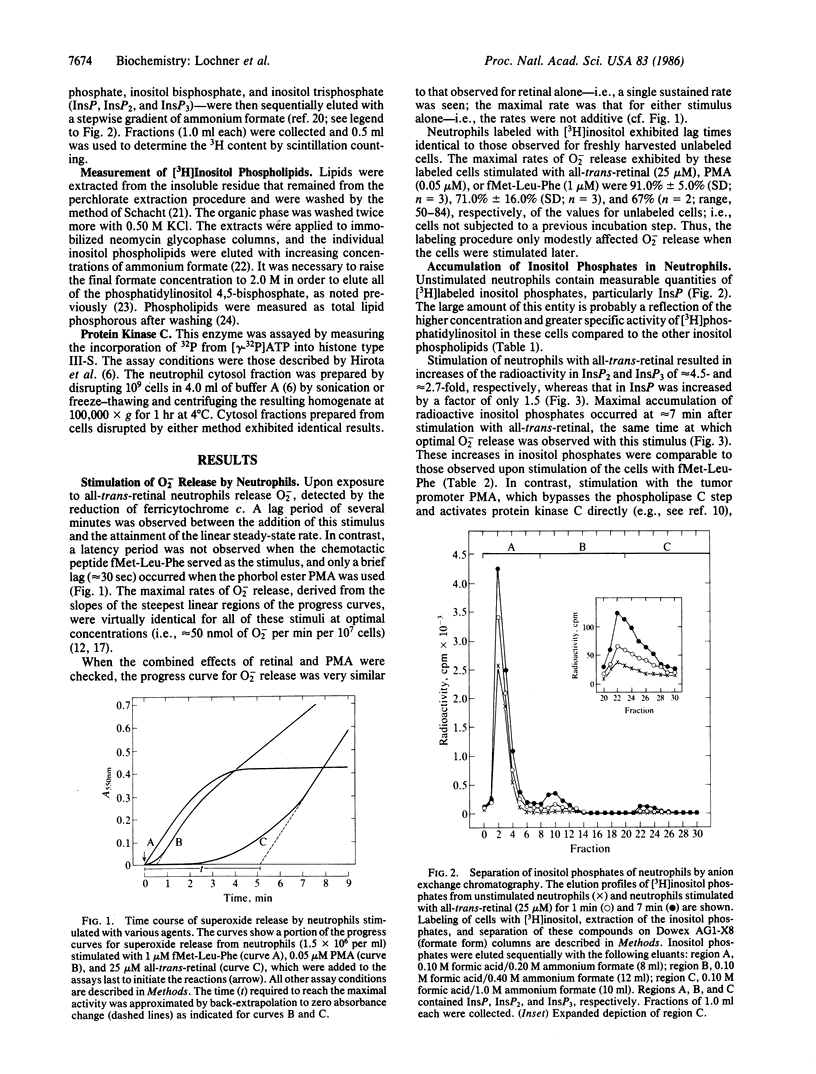
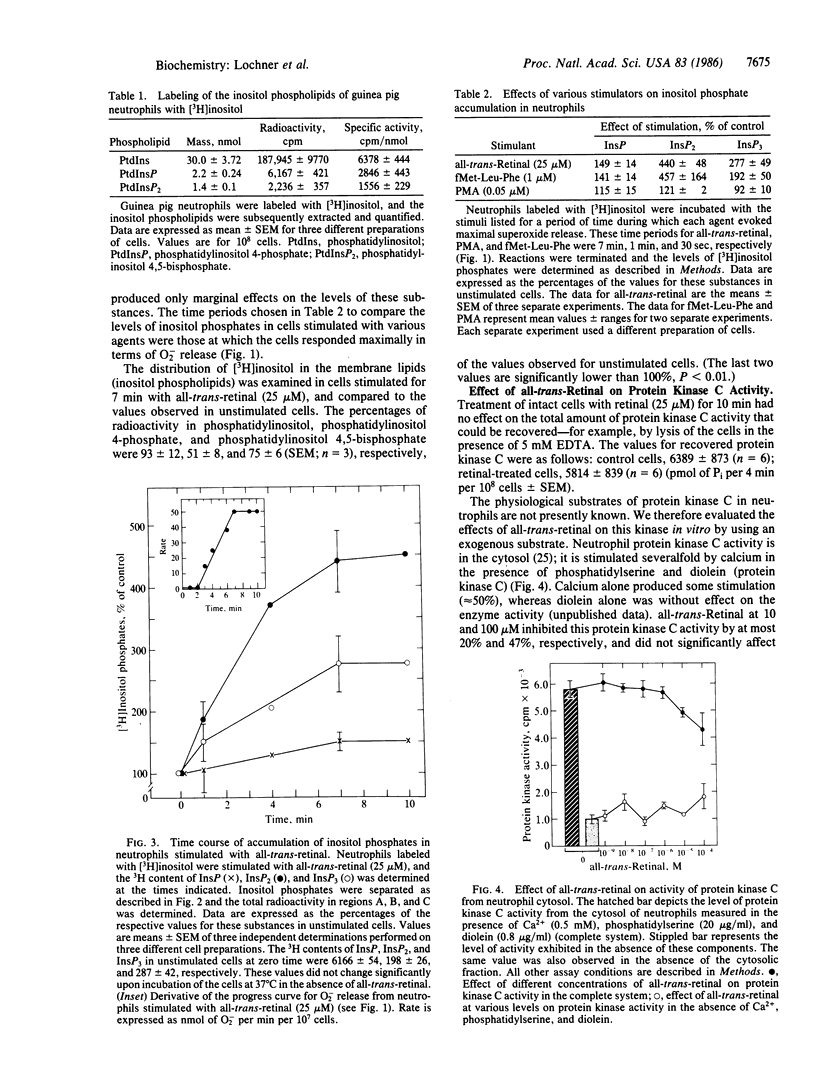
all-trans-Retinal was previously shown to stimulate high levels of superoxide release by guinea pig neutrophils. When the cells, previously labeled with [3H]inositol, are treated with all-trans-retinal, they exhibit a decrease in the levels of [3H]inositol phospholipids and an increase in the accumulation of [3H]inositol phosphates. The maximal accumulation of inositol phosphates and the optimal rate of superoxide release occurred together at approximately 7 min after stimulation. The levels of [3H]inositol phosphates accumulated were comparable to those observed when the cells were stimulated with a chemotactic peptide. In direct measurements, using concentrations that stimulate intact cells maximally, all-trans-retinal was found not to inhibit protein kinase C from the cytosol of neutrophils significantly. This contrasts with the situation with this kinase obtained from other sources. These observations represent additional effects of vitamin A on cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badwey J. A., Curnutte J. T., Robinson J. M., Berde C. B., Karnovsky M. J., Karnovsky M. L. Effects of free fatty acids on release of superoxide and on change of shape by human neutrophils. Reversibility by albumin. J Biol Chem. 1984 Jun 25;259(12):7870–7877. [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Production of superoxide by phagocytic leukocytes: a paradigm for stimulus-response phenomena. Curr Top Cell Regul. 1986;28:183–208. doi: 10.1016/b978-0-12-152828-7.50006-8. [DOI] [PubMed] [Google Scholar]
- Badwey J. A., Robinson J. M., Curnutte J. T., Karnovsky M. J., Karnovsky M. L. Retinoids stimulate the release of superoxide by neutrophils and change their morphology. J Cell Physiol. 1986 May;127(2):223–228. doi: 10.1002/jcp.1041270206. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bradford P. G., Rubin R. P. Characterization of formylmethionyl-leucyl-phenylalanine stimulation of inositol trisphosphate accumulation in rabbit neutrophils. Mol Pharmacol. 1985 Jan;27(1):74–78. [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- DePierre J. W., Karnovsky M. L. Ecto-enzymes of the guinea pig polymorphonuclear leukocyte. I. Evidence for an ecto-adenosine monophosphatase, adenosine triphosphatase, and -p-nitrophenyl phosphates. J Biol Chem. 1974 Nov 25;249(22):7111–7120. [PubMed] [Google Scholar]
- Dougherty R. W., Godfrey P. P., Hoyle P. C., Putney J. W., Jr, Freer R. J. Secretagogue-induced phosphoinositide metabolism in human leucocytes. Biochem J. 1984 Sep 1;222(2):307–314. doi: 10.1042/bj2220307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman D. S. Vitamin A and retinoids in health and disease. N Engl J Med. 1984 Apr 19;310(16):1023–1031. doi: 10.1056/NEJM198404193101605. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Bell R. M. Activation of protein kinase C by Triton X-100 mixed micelles containing diacylglycerol and phosphatidylserine. J Biol Chem. 1985 Aug 25;260(18):10039–10043. [PubMed] [Google Scholar]
- Hirota K., Hirota T., Aguilera G., Catt K. J. Hormone-induced redistribution of calcium-activated phospholipid-dependent protein kinase in pituitary gonadotrophs. J Biol Chem. 1985 Mar 25;260(6):3243–3246. [PubMed] [Google Scholar]
- Irvine R. F., Hemington N., Dawson R. M. The calcium-dependent phosphatidylinositol-phosphodiesterase of rat brain. Mechanisms of suppression and stimulation. Eur J Biochem. 1979 Sep;99(3):525–530. doi: 10.1111/j.1432-1033.1979.tb13284.x. [DOI] [PubMed] [Google Scholar]
- Kapoor C. L., Chader G. J. Endogenous phosphorylation of retinal photoreceptor outer segment proteins by calcium phospholipid-dependent protein kinase. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1397–1403. doi: 10.1016/0006-291x(84)91246-4. [DOI] [PubMed] [Google Scholar]
- Kensler T. W., Mueller G. C. Retinoic acid inhibition of the comitogenic action of mezerein and phorbol esters in bovine lymphocytes. Cancer Res. 1978 Mar;38(3):771–775. [PubMed] [Google Scholar]
- Klausner R. D., Kleinfeld A. M., Hoover R. L., Karnovsky M. J. Lipid domains in membranes. Evidence derived from structural perturbations induced by free fatty acids and lifetime heterogeneity analysis. J Biol Chem. 1980 Feb 25;255(4):1286–1295. [PubMed] [Google Scholar]
- Ohta H., Okajima F., Ui M. Inhibition by islet-activating protein of a chemotactic peptide-induced early breakdown of inositol phospholipids and Ca2+ mobilization in guinea pig neutrophils. J Biol Chem. 1985 Dec 15;260(29):15771–15780. [PubMed] [Google Scholar]
- Oreffo R. O., Francis J. A., Triffitt J. T. Vitamin A effects on UMR 106 osteosarcoma cells are not mediated by specific cytosolic receptors. Biochem J. 1985 Dec 1;232(2):599–603. doi: 10.1042/bj2320599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer F. B. Chromatography of acidic phospholipids on immobilized neomycin. J Lipid Res. 1981 Nov;22(8):1296–1300. [PubMed] [Google Scholar]
- Repine J. E., White J. G., Clawson C. C., Holmes B. M. Effects of phorbol myristate acetate on the metabolism and ultrastructure of neutrophils in chronic granulomatous disease. J Clin Invest. 1974 Jul;54(1):83–90. doi: 10.1172/JCI107752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse S. E., Allen C. L. Synergistic activation by collagen and 15-hydroxy-9 alpha,11 alpha-peroxidoprosta-5,13-dienoic acid (PGH2) of phosphatidylinositol metabolism and arachidonic acid release in human platelets. J Clin Invest. 1982 Dec;70(6):1216–1224. doi: 10.1172/JCI110720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M., Badwey J. A., Karnovsky M. L., Karnovsky M. J. Release of superoxide and change in morphology by neutrophils in response to phorbol esters: antagonism by inhibitors of calcium-binding proteins. J Cell Biol. 1985 Sep;101(3):1052–1058. doi: 10.1083/jcb.101.3.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht J. Extraction and purification of polyphosphoinositides. Methods Enzymol. 1981;72:626–631. doi: 10.1016/s0076-6879(81)72054-8. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Role of retinoids in differentiation and carcinogenesis. J Natl Cancer Inst. 1984 Dec;73(6):1381–1387. [PubMed] [Google Scholar]
- Stillwell W., Ricketts M., Hudson H., Nahmias S. Effect of retinol and retinoic acid on permeability, electrical resistance and phase transition of lipid bilayers. Biochim Biophys Acta. 1982 Jun 14;688(2):653–659. doi: 10.1016/0005-2736(82)90376-5. [DOI] [PubMed] [Google Scholar]
- Taffet S. M., Greenfield A. R., Haddox M. K. Retinal inhibits TPA activated, calcium-dependent, phospholipid-dependent protein kinase ("C" kinase). Biochem Biophys Res Commun. 1983 Aug 12;114(3):1194–1199. doi: 10.1016/0006-291x(83)90689-7. [DOI] [PubMed] [Google Scholar]
- Takenawa T., Nagai Y. Purification of phosphatidylinositol-specific phospholipase C from rat liver. J Biol Chem. 1981 Jul 10;256(13):6769–6775. [PubMed] [Google Scholar]
- Usher J. R., Epand R. M., Papahadjopoulos D. The effect of free fatty acids on the thermotropic phase transition of dimyristoyl glycerophosphocholine. Chem Phys Lipids. 1978 Oct;22(3):245–253. doi: 10.1016/0009-3084(78)90031-2. [DOI] [PubMed] [Google Scholar]
- Valtersson C., van Duÿn G., Verkleij A. J., Chojnacki T., de Kruijff B., Dallner G. The influence of dolichol, dolichol esters, and dolichyl phosphate on phospholipid polymorphism and fluidity in model membranes. J Biol Chem. 1985 Mar 10;260(5):2742–2751. [PubMed] [Google Scholar]
- Vandenbark G. R., Kuhn L. J., Niedel J. E. Possible mechanism of phorbol diester-induced maturation of human promyelocytic leukemia cells. J Clin Invest. 1984 Feb;73(2):448–457. doi: 10.1172/JCI111231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A. K., Shapas B. G., Rice H. M., Boutwell R. K. Correlation of the inhibition by retinoids of tumor promoter-induced mouse epidermal ornithine decarboxylase activity and of skin tumor promotion. Cancer Res. 1979 Feb;39(2 Pt 1):419–425. [PubMed] [Google Scholar]
- Wolfson M., McPhail L. C., Nasrallah V. N., Snyderman R. Phorbol myristate acetate mediates redistribution of protein kinase C in human neutrophils: potential role in the activation of the respiratory burst enzyme. J Immunol. 1985 Sep;135(3):2057–2062. [PubMed] [Google Scholar]



