Abstract
We have isolated and analyzed the DNA encoding the mu heavy chain constant region of a mutant IgM that is defective in initiating complement-dependent cytolysis. By assaying the expression of mu-chain genes that were constructed in vitro from mutant and wild-type gene segments, we have mapped the mutation into a 555-base-pair segment that spans part of the third and fourth constant region domains. In this segment there is one nucleotide change, such that the mutant mu-chain gene encodes asparagine rather than the normal serine at amino acid position 406 in the third constant domain. We have used site-directed mutagenesis to introduce a comparable mutation into the normal mu-chain gene and confirmed that this substitution causes the production of IgM with the original mutant phenotype. Evidence is also provided that the serine-406----asparagine substitution might cause the mutant mu chain to be abnormally glycosylated.
Full text
PDF
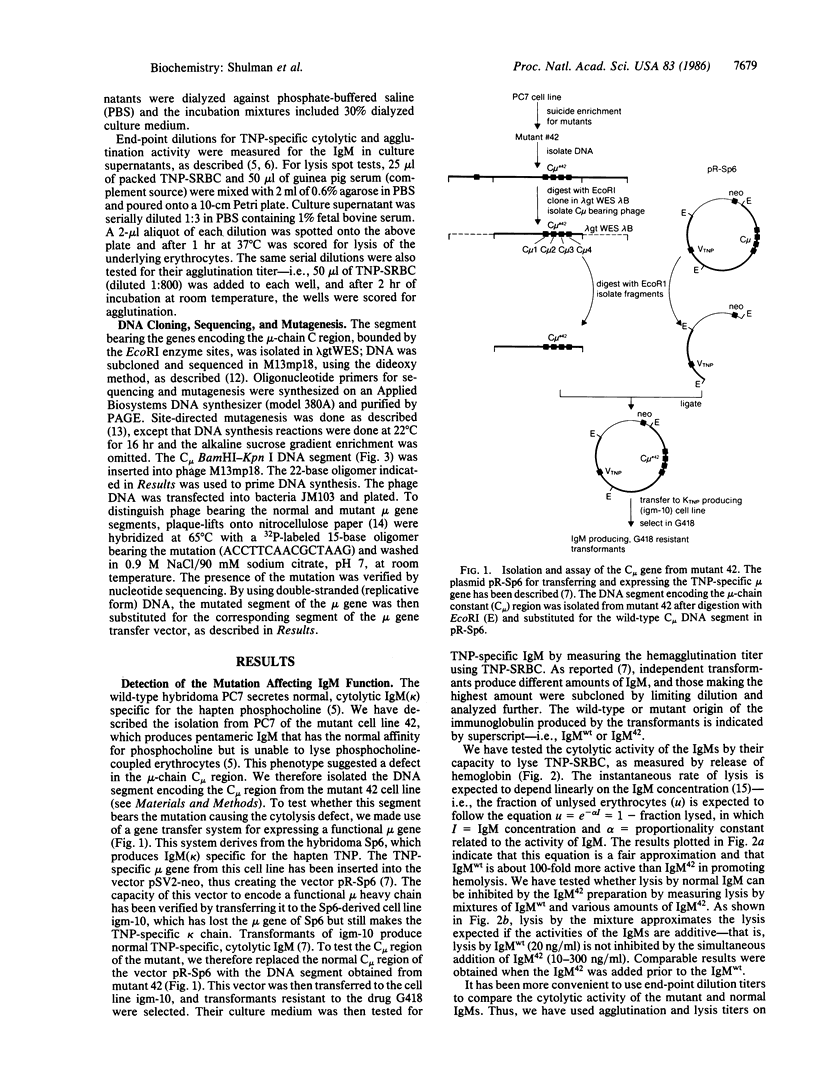
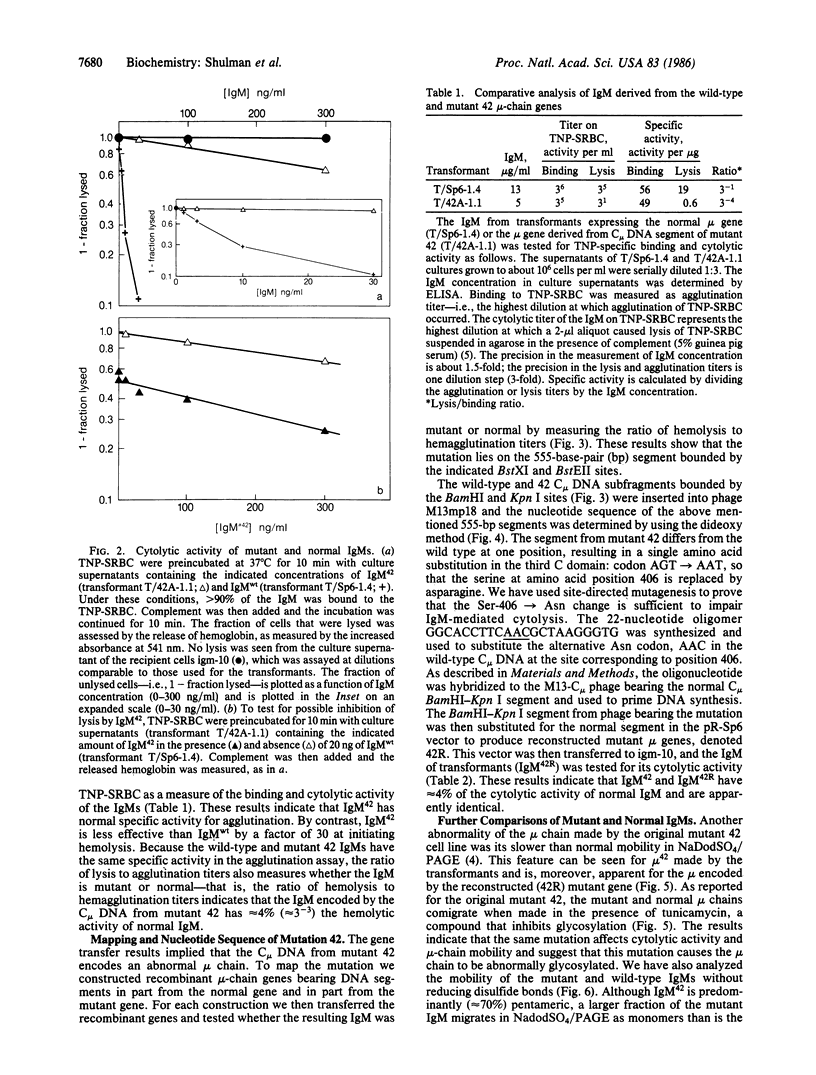


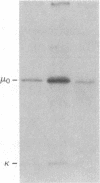
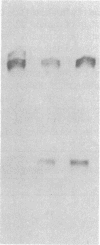
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baczynsky W. O., Sugii S., Murialdo H., Pennell N., Filkin C., Hozumi N., Shulman M. J. Nucleotide sequences of immunoglobulin mu heavy chain deletion mutants. Nucleic Acids Res. 1983 Nov 11;11(21):7471–7485. doi: 10.1093/nar/11.21.7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Borsos T., Rapp H. J. Hemolysin titration based on fixation of the activated first component of complement: evidence that one molecule of hemolysin suffices to sensitize an erythrocyte. J Immunol. 1965 Sep;95(3):559–566. [PubMed] [Google Scholar]
- Brown J. C., Koshland M. E. Activation of antibody Fc function by antigen-induced conformational changes. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5111–5115. doi: 10.1073/pnas.72.12.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubb M. O., Conradie J. D. Studies on the structural and biological functions of the Cmu4 domains of IgM. Immunology. 1978 Mar;34(3):449–458. [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Colomb M., Porter R. R. Characterization of a plasmin-digest fragment of rabbit immunoglobulin gamma that binds antigen and complement. Biochem J. 1975 Feb;145(2):177–183. doi: 10.1042/bj1450177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper N. R. The classical complement pathway: activation and regulation of the first complement component. Adv Immunol. 1985;37:151–216. doi: 10.1016/s0065-2776(08)60340-5. [DOI] [PubMed] [Google Scholar]
- Hurst M. M., Volanakis J. E., Stroud R. M., Bennett J. C. C1 fixation and classical complement pathway activation by a fragment of the Cmu4 domain of IgM. J Exp Med. 1975 Nov 1;142(5):1322–1326. doi: 10.1084/jem.142.5.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehry M., Sibley C., Fuhrman J., Schilling J., Hood L. E. Amino acid sequence of a mouse immunoglobulin mu chain. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2932–2936. doi: 10.1073/pnas.76.6.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide N., Nose M., Muramatsu T. Recognition of IgG by Fc receptor and complement: effects of glycosidase digestion. Biochem Biophys Res Commun. 1977 Apr 25;75(4):838–844. doi: 10.1016/0006-291x(77)91458-9. [DOI] [PubMed] [Google Scholar]
- Köhler G., Hengartner H., Shulman M. J. Immunoglobulin production by lymphocyte hybridomas. Eur J Immunol. 1978 Feb;8(2):82–88. doi: 10.1002/eji.1830080203. [DOI] [PubMed] [Google Scholar]
- Köhler G., Howe S. C., Milstein C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur J Immunol. 1976 Apr;6(4):292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leatherbarrow R. J., Rademacher T. W., Dwek R. A., Woof J. M., Clark A., Burton D. R., Richardson N., Feinstein A. Effector functions of a monoclonal aglycosylated mouse IgG2a: binding and activation of complement component C1 and interaction with human monocyte Fc receptor. Mol Immunol. 1985 Apr;22(4):407–415. doi: 10.1016/0161-5890(85)90125-7. [DOI] [PubMed] [Google Scholar]
- Leptin M., Melchers F. A monoclonal antibody with specificity for murine mu heavy chain which inhibits the formation of antigen-specific direct IgM plaques. J Immunol Methods. 1983 Apr 15;59(1):53–61. doi: 10.1016/0022-1759(83)90145-x. [DOI] [PubMed] [Google Scholar]
- Marshall R. D. The nature and metabolism of the carbohydrate-peptide linkages of glycoproteins. Biochem Soc Symp. 1974;(40):17–26. [PubMed] [Google Scholar]
- Nose M., Wigzell H. Biological significance of carbohydrate chains on monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6632–6636. doi: 10.1073/pnas.80.21.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi A., Hawley R. G., Hawley T., Shulman M. J., Traunecker A., Köhler G., Hozumi N. Functional immunoglobulin M production after transfection of cloned immunoglobulin heavy and light chain genes into lymphoid cells. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6351–6355. doi: 10.1073/pnas.80.20.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam F. W., Florent G., Paul C., Shinoda T., Shimizu A. Complete amino acid sequence of the Mu heavy chain of a human IgM immunoglobulin. Science. 1973 Oct 19;182(4109):287–291. doi: 10.1126/science.182.4109.287. [DOI] [PubMed] [Google Scholar]
- Sandri-Goldin R. M., Goldin A. L., Levine M., Glorioso J. C. High-frequency transfer of cloned herpes simplex virus type 1 sequences to mammalian cells by protoplast fusion. Mol Cell Biol. 1981 Aug;1(8):743–752. doi: 10.1128/mcb.1.8.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman M. J., Heusser C., Filkin C., Köhler G. Mutations affecting the structure and function of immunoglobulin M. Mol Cell Biol. 1982 Sep;2(9):1033–1043. doi: 10.1128/mcb.2.9.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. C., Cathou R. E. Effects of limited denaturation by heat on the dynamic conformation of equine immunoglobulin M antibody and on interaction with antigen and complement. Biochemistry. 1981 Jan 6;20(1):192–198. doi: 10.1021/bi00504a032. [DOI] [PubMed] [Google Scholar]
- Winkelhake J. L., Kunicki T. J., Elcombe B. M., Aster R. H. Effects of pH treatments and deglycosylation of rabbit immunoglobulin G on the binding of C1q. J Biol Chem. 1980 Apr 10;255(7):2822–2828. [PubMed] [Google Scholar]
- Ziccardi R. J., Cooper N. R. Activation of C1r by proteolytic cleavage. J Immunol. 1976 Feb;116(2):504–509. [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis using M13-derived vectors: an efficient and general procedure for the production of point mutations in any fragment of DNA. Nucleic Acids Res. 1982 Oct 25;10(20):6487–6500. doi: 10.1093/nar/10.20.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]