Abstract
Motor control circuitry of the central nervous system must be flexible so that motor behaviours can be adapted to suit the varying demands of different states, developmental stages, and environments. Flexibility in motor control is largely provided by neuromodulatory systems which can adjust the output of motor circuits by modulating the properties and connectivity of neurons within them. The spinal circuitry which controls locomotion is subject to a range of neuromodulatory influences, including some which are intrinsic to the spinal cord. One such intrinsic neuromodulatory system, for which a wealth of anatomical information has recently been combined with new physiological data, is the C bouton system. C boutons are large, cholinergic inputs to motor neurons which were first described over 40 years ago but whose source and function have until recently remained a mystery. In this review we discuss how the convergence of anatomical, molecular genetic and physiological data has recently led to significant advances in our understanding of this unique neuromodulatory system. We also highlight evidence that C boutons are involved in spinal cord injury and disease, revealing their potential as targets for novel therapeutic strategies.
Keywords: C terminal, motor control, neuromodulation, spinal cord
Introduction
The successful execution of movement typically involves the integration of numerous internally generated motor commands with an abundance of afferent sensory signals. The ‘final common pathway’ for motor output from the central nervous system is then mediated by motor neurons which innervate all skeletal muscles. However, motor neurons are far from static relay stations for motor commands. Due to the influence of a range of neuromodulatory systems, motor neurons can adjust the final motor output of the CNS to ensure that movement is smooth, accurate and matched to environmental and organismal demands. Detailed knowledge of the modulatory inputs which motor neurons receive is therefore important for both understanding how the healthy nervous system generates movement and in searching for potential therapeutic targets for conditions in which a lack of or inappropriate motor output is generated. Much work has focused on the anatomy and function of modulatory inputs which arise from brainstem nuclei (reviewed by Rekling et al. 2000; Heckman et al. 2009). In the present review we focus on discrete, spinally derived modulatory inputs to motor neurons, called C boutons, for which anatomical and physiological data have recently converged to provide compelling evidence of how neuromodulatory systems can fine-tune motor programs.
In this review we will discuss: early observations of C bouton structure; more recent analyses of proteins expressed at these abundant synapses; links between the structure of the C bouton system and its functional role in motor control; and, finally, the potential involvement of C boutons in disorders such as amyotrophic lateral sclerosis (ALS) and spinal cord injury.
Anatomy of the C bouton synapse
C bouton inputs to motor neurons were first reported in electron microscopy studies over 40 years ago (Conradi, 1969; Conradi & Skoglund, 1969). This work described large (2–6 μm in diameter) synaptic inputs on the soma and proximal dendrites of motor neurons which were associated with highly specialised postsynaptic structures. Perhaps the most distinctive of these postsynaptic structures were the sub-surface cisternae, which are flattened membrane disks positioned just below the narrow synaptic cleft, typically extending along the entire length of the synapse (Fig. 1A). These large, distinctive synapses were given the label C-type to distinguish them from synapses with spherical vesicles (S-type), flattened vesicles (F-type), dense postsynaptic “Taxi” bodies (T-type) and synapses contacting smaller presynaptic boutons (M-type). Following the first description of C boutons in cats, these specialised synapses have been reported in a wide range of mammals (Conradi & Skoglund, 1969; Bodian, 1975; Bernstein & Bernstein, 1976; Hamos & King, 1980; Pullen et al. 1992; Wilson et al. 2004).
Fig. 1.
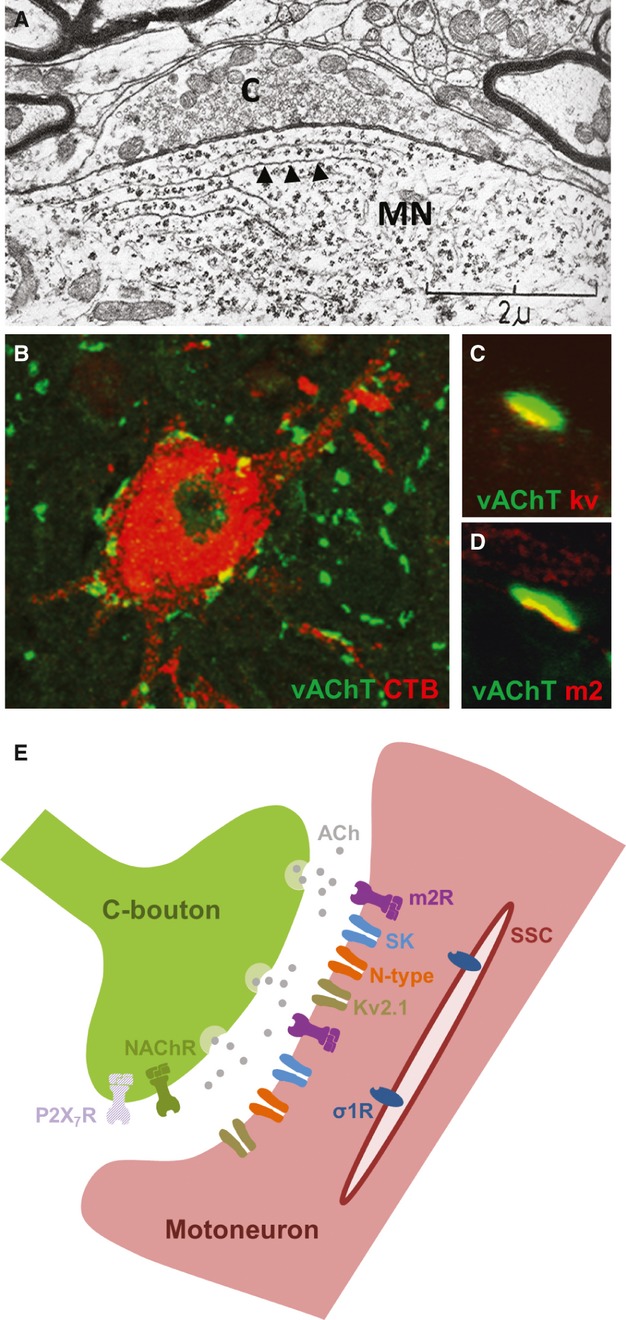
Anatomy of the C bouton synpase. (A) An electron micrograph (adapted from Conradi, 1969) showing a C bouton (C) contacting a motor neuron (MN). Note the elongated subsurface cisternae (arrowheads) located postsynaptically. (B) A single motor neuron, labelled via injection of CTB into the gastrocnemius muscle, with numerous C boutons (vAChT-immunolabelled terminals) contacting the soma and proximal dendrites. (C,D) Immunolabelling reveals postsynaptic clusters of Kv2.1 channels (C) and m2 (D) receptors aligned with vAChT-labelled C boutons. (E) Schematic representation of a C bouton synapse. Nicotonic acetylcholine (NACh) and ATP (P2X7) receptors are thought to be located presynaptically. Muscarinic acetylcholine receptors (m2), Ca2+-dependent K+ channels (SK), voltage-activated Ca2+ channels (N-type) and delayed rectifier K+ channels (Kv2.1) are clustered postsynaptically. Sigma-1 receptors (σ1R) appear to be located beneath the postsynaptic plasma membrane, on subsurface cisternae (SSC).
Although motor neurons were already known to receive cholinergic inputs (Lewis & Shute, 1966), C boutons were not definitively shown to be cholinergic until many decades after their first description. The cholinergic identity of C boutons was eventually confirmed using antibodies against choline acetyl transferase, acetylcholinesterase and vesicular acetylcholine transporter (Connaughton et al. 1986; Nagy et al. 1993; Li et al. 1995; Arvidsson et al. 1997) (Fig. 1B).
Following the identification of C boutons as cholinergic, a large number of studies using immunohistochemical techniques also demonstrated complex but specific sets of proteins clustered on motor neurons adjacent to C boutons which presumably mediate transmission at these synapses (Fig. 1E). Postsynaptic clusters of metabotropic m2-type muscarinic receptors (Hellstrom et al. 2003) suggest that these receptors are the target of ACh released from C boutons (Fig. 1D). Nicotinic acetylcholine receptors have not been found postsynaptically, suggesting an absence of ionotropic transmission at C boutons. Nicotinic acetylcholine receptors (subunits alpha3, alpha4, alpha5 and beta2; Khan et al. 2003) have, however, been reported presynaptically, as have ATP (P2X7) receptors (Deng & Fyffe, 2004), although it should be noted that there has been some debate regarding the specificity of P2X7 antibodies (Kaczmarek-Hajek et al. 2012). Together, such presynaptic receptors may contribute to feedback control of C bouton synapses.
There is also postsynaptic clustering of ion channels at C bouton synapses, which likely act as downstream targets of metabotropic muscarinic receptors. One of the most clearly clustered proteins is the Kv2.1 channel (Fig. 1C). In addition, Ca2+-dependent K+ channels are found clustered postsynaptically at C boutons (Deardorff et al. 2013). Interestingly, in mice, the SK2 subtype of Ca2+-dependent K+ channels is found on all alpha motor neurons,whereas the SK3 subtype is only found on a small subpopulation of alpha motor neurons (Deardorff et al. 2013). In comparison, SK3 channels are found on all cat motor neurons, whereas the exact distribution of SK2 channels is not clear (Deardorff et al. 2013). These data support, at least in rodents, some degree of motor neuron-subtype specificity in C bouton signalling. N-type calcium channels also show high levels of postsynaptic expression at C bouton synapses (Wilson et al. 2004), perhaps linked to the Ca2+ requirements of SK channels.
Finally, a recent study has reported clustering of sigma-1 receptors at C bouton synapses (Mavlyutov et al. 2012). In contrast to other proteins at the synapse, sigma-1 receptors appear to be located beneath the postsynaptic plasma membrane, on subsurface cisternae. In support of a functional role for these receptors, the enzyme which converts tryptamine to the sigma-1 receptor agonist dimethyltryptamine is also localised in close proximity to C bouton synapses.
Neuronal source of C boutons
Soon after the first description of C boutons (Conradi, 1969; Conradi & Skoglund, 1969), studies began investigating their neuronal source. Initial work found that C boutons remained intact following complete spinal transections in cats, supporting a propriospinal rather than supraspinal origin for C boutons (McLaughlin, 1972). This was further reinforced by a comprehensive description of bulbospinal connections in mice, showing that cholinergic neurons of the brainstem do not project to the spinal cord (VanderHorst & Ulfhake, 2006).
Research next focused on which class of cholinergic neurons within the spinal cord gives rise to C boutons. Motor neuron axon collaterals were considered as a potential source. However, given differences in synaptic proteins at C boutons vs. motor neuron terminals on Renshaw cells (Hellstrom et al. 1999), a motor neuronal source for C boutons appeared unlikely. The definitive exclusion of motor neurons as a C bouton source was later demonstrated using a ChAT-GFP mouse line in which GFP was expressed by all motor neurons, but surprisingly not all cholinergic interneurons. In this mouse line, C boutons did not express GFP (Miles et al. 2007), demonstrating that they must arise from cholinergic interneurons and not motor neurons.
With motor neurons excluded as the source of C boutons, attention shifted to cholinergic interneurons of the spinal cord. Several classes of cholinergic spinal interneurons have been described including: partition cells located between the border of the dorsal and ventral horns; central canal cluster cells closely associated with the central canal; and neurons scattered in the dorsal horn (Barber et al. 1984; Phelps et al. 1984; Arvidsson et al. 1997; Huang et al. 2000; VanderHorst & Ulfhake, 2006). Research utilising knowledge of the molecular identity of C boutons revealed that the most likely source of C boutons is a medially positioned population of partition cells (Miles et al. 2007). Both C boutons and these interneurons are fluorescently labelled in mice in which YFP expression is dependent on the transcription factor Dbx-1 (a marker of the developmentally defined V0 interneuron cohort; Jessell, 2000). In addition, both Dbx-1 expressing partition cells and C boutons lack expression of neuronal nitric oxide synthase (nNOS). Several years later, the paired-like homeodomain transcription factor Pitx2 was also found to label medially positioned partition cells (Zagoraiou et al. 2009). Pitx2 expression was found to define a small cluster of V0 (Dbx1+) interneurons which form a longitudinal column near the central canal of the spinal cord (Fig. 2A,B) and can be further subdivided into cholinergic (V0C) and glutamatergic (V0G) subtypes. Remarkably, molecular genetic techniques demonstrated that this small cluster of V0C interneurons, which is outnumbered by motor neurons by a factor of ∼10 : 1, represent the sole source of C bouton inputs to all spinal motor neurons (Fig. 2C).
Fig. 2.
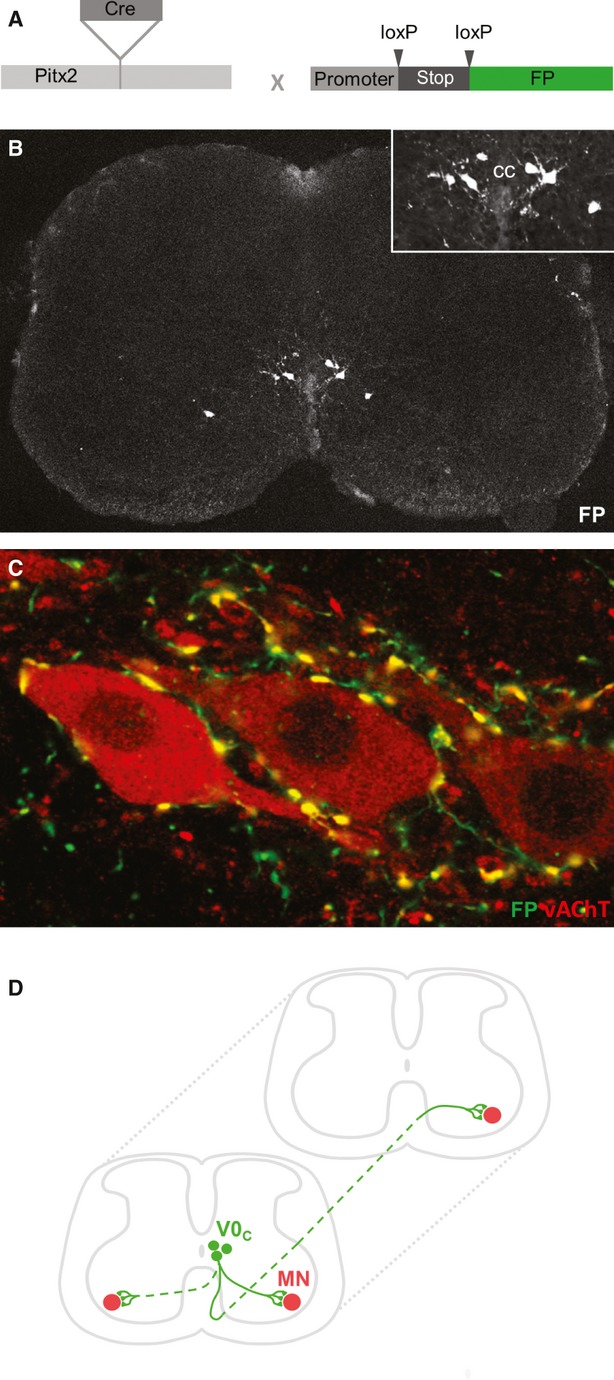
Source and distribution of C boutons. (A,B) A Pitx2-Cre mouse line, which directs expression of Cre recombinase selectively in Pitx2+ neurons, was crossed with conditional promoter-stop-FP reporter lines (Zagoraiou et al., 2009). Fluorescent protein (FP) is expressed in a small population of neurons located close to the central canal (CC). Inset shows a higher magnification view of fluorescent Pitx2+ interneurons. (C) Coexpression of vAChT and FP in a Pitx2-Cre; promotor-stop-FP mouse, confirming Pitx2+ interneurons as the sole source of C boutons. (D) Schematic representation of C bouton source neurons (V0C interneurons) and their axonal projections. The majority of V0C neurons (∼70%) project solely to ipsilateral motor neurons (MN), whereas the remaining cells project contralaterally, or possibly bilaterally. The axons of V0C interneurons may also project rostrocaudally to innervate motor neurons in different segments of the spinal cord.
The discovery of a molecular marker for C bouton source interneurons not only clarified the long-standing mystery of the source of C boutons (reviewed by Frank, 2009) but also became critical for the goal of understanding both the circuitry and the function of the C bouton system.
Circuitry of the C bouton system
In comparison with the anatomy of the C bouton synapse, much remains to be determined regarding the circuitry of their source interneurons, including the projection patterns of V0C interneurons and the inputs that they receive. This information, regarding the contribution of the C bouton system to central motor circuitry, is critical for a complete understanding of motor function.
C boutons selectively innervate the soma and proximal dendrites of motor neurons throughout the spinal cord (Hamos & King, 1980), showing selectivity for alpha over gamma motor neurons (Arvidsson et al. 1987). However, their innervation of brainstem motor neurons is more selective, with C boutons identified in the trigeminal, facial and hypoglossal motor nuclei but not the ocular motor nuclei (Hellstrom et al. 2003). Within the spinal cord C boutons appear to preferentially innervate certain classes of alpha motor neurons; greater numbers of C boutons are found on motor neurons innervating large proximal compared with smaller distal muscles, and fast twitch compared with slow twitch muscles (Hellstrom et al. 2003).
Beyond the profile of motor neuron subtypes preferentially innervated by C boutons, many things remain to be determined regarding the patterns by which V0C interneurons innervate motor neurons. It is clear that there is considerable divergence in the output of V0C interneurons, with individual cells likely to form ∼1000 synaptic contacts with motor neurons (Zagoraiou et al. 2009). Transynaptic viral tracing experiments have shown that the majority of V0C interneurons project to ipsilateral motor neurons, although up to a third of cells have contralateral projections (Zagoraiou et al. 2009). More recent viral work has suggested that a proportion of V0C interneurons may project bilaterally to functionally equivalent motor neurons on each side of the spinal cord (Fig. 2D). However, it should be noted that this work concentrated on premotor, cholinergic interneurons near the central canal and did not define these cells as Pitx2+, V0c interneurons (Stepien et al. 2010). As well as suggesting bilateral output from V0C interneurons, this work also raised the possibility that motor neurons receive C bouton inputs from a number of V0C interneurons distributed throughout the rostrocaudal axis of the spinal cord.
In addition to innervating motor neurons, the axons of V0C interneurons also appear to synapse onto other interneurons within the intermediate zone of the spinal cord. Although the full complement of interneurons contacted remains to be determined, there is evidence that V0C interneurons project to Ia inhibitory interneurons (Siembab et al. 2010).
To determine the circuitry of the C bouton system it is also important to consider the inputs that V0C interneurons receive. Although data are limited, there is anatomical evidence of descending serotonergic inputs and a lack of direct primary afferent input to V0C interneurons. Physiological experiments demonstrating oligosynaptic, but not monosynaptic, dorsal root-evoked input to V0C interneurons confirm a lack of direct primary afferent input (Zagoraiou et al. 2009). In addition, Zagoraiou et al. (2009) also demonstrated that V0C interneurons receive input from the spinal locomotor central pattern generator (CPG) but are themselves unlikely to be involved in generating the locomotor rhythm. These CPG inputs appear to be very closely related to those driving the locomotor activity of motor neurons. Taken together, data regarding inputs to V0C interneurons suggest that they may receive copies of the descending, sensory and local synaptic drive received by motor neurons, perhaps enabling the C bouton system to control motor neuron activity in a manner appropriate for ongoing motor activity (Zagoraiou et al. 2009) (Fig. 3A).
C bouton function
Advances in our knowledge of the anatomy of C bouton synapses and the circuitry of their source interneurons (V0C interneurons) have guided physiological research aiming to answer long-standing questions regarding the function of the C bouton system. Such research aims to address: the mechanisms of transmission at C bouton synapses; the consequences of C bouton activation for motor neuron function; the role of the C bouton system within spinal motor circuitry; and ultimately the contribution of the C bouton system to the control of motor behaviour.
Prior to the discovery of V0C interneurons, knowledge of the proteins clustered at C bouton synapses formed the basis of experiments investigating C bouton function. Given the clustering of postsynaptic m2 receptors at C bouton synapses, pharmacological activation of muscarinic receptors on motor neurons was used to indirectly investigate the effects of C bouton activation. Data obtained from whole-cell patch-clamp recordings of motor neurons in neonatal mouse spinal cord slice preparations revealed that activation of m2 receptors increases motor neuron excitability (Fig. 3C) by reducing the action potential after hyperpolarisation (AHP; Miles et al. 2007). Analysis of the mechanisms underlying reductions in AHP amplitude revealed a direct blockade of Ca2+-dependent K+ (SK) channels, with no effect on voltage-activated Ca+ channels (sources of Ca2+ for SK channel activation; Fig. 3B). Physiological experiments demonstrating the effects of m2 receptor activation on motor neurons thus provide an explanation for postsynaptic clustering of m2 receptors, SK channels and N-type Ca2+ channels at C bouton synapses. However, the roles of other protein clusters remain less clear.
Fig. 3.
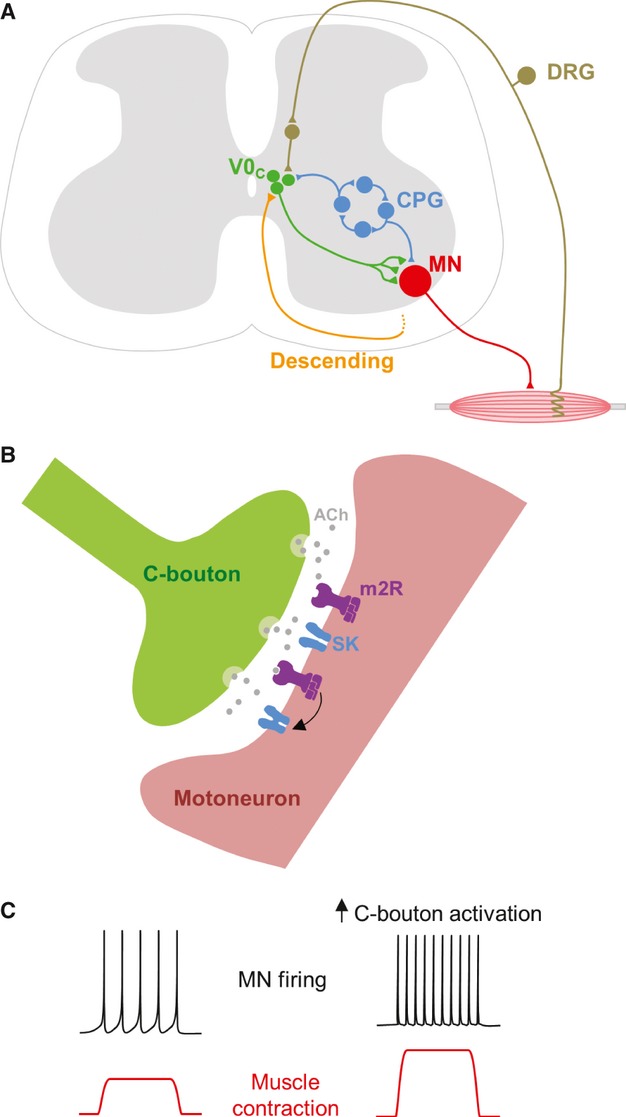
Function of the C bouton system. (A) C bouton source cells (V0C interneurons) are well positioned to control motor neuron (MN) output in a task-dependent manner. They receive a range of inputs including: oligosynaptic sensory input; descending input likely from the brainstem; and rhythmic input from the locomotor central pattern generator (CPG). DRG, dorsal root ganglion. (B) Signalling at C bouton synapses is thought to involve release of acetylcholine (ACh), activation of postsynaptic m2-type muscarinic acetylcholine receptors (m2R), and the subsequent blockade of Ca2+-dependent K+ channels (SK-type) which underlie the action potential after hyperpolarisation (AHP). (C) Increases in C bouton activation should lead to reductions in the motor neuron AHP, increased motor neuron firing rates, greater activation of muscles, and ultimately more intense muscle contractions.
Perhaps most strikingly, despite their specific localisation to C bouton synapses, the roles of Kv2.1 channels remain unknown. Evidence of K+ channel modulation by C boutons was also provided by experiments activating m2 receptors on motor neurons; a small hyperpolarising current likely reflecting facilitation of a resting K+ current was observed in some, but not all, motor neurons (Miles et al. 2007). However, given that Kv2.1 channels mediate delayed rectifier currents which are not normally active at rest, it is unlikely that their facilitation could underlie any m2-induced hyperpolarisation. Kv2.1 channels are nonetheless known to control neuronal excitability, perhaps fulfilling a homeostatic role to limit hyperexcitability (Misonou et al. 2005). Interestingly, the voltage-dependent activation properties and clustering of Kv2.1 channels depend on their phosphorylation state. Dephosphorylation of Kv2.1 channels, which can be induced by glutamatergic simulation, leads to the dispersion of Kv2.1 channel clusters and a large (∼30 mV) leftward shift in their activation curve (Misonou et al. 2005). Conversely, phosphorylation of Kv2.1 channels, which might be induced by m2 receptor activation (Zhou et al. 2003), leads to a rightward shift in their activation curve (Murakoshi et al. 1997). It is thus plausible that following activation of C bouton synapses, greater depolarisation is required to open Kv2.1 channels, providing another mechanism by which C bouton synapses might increase motor neuron excitability besides blocking the AHP. In addition to mediating delayed rectifier currents, Kv2.1 channels have the potential to fulfil non-conducting roles at C bouton synapses. Kv2.1 channels are predominantly non-conducting when clustered on the plasma membrane (Fox et al. 2013). In this clustered, non-conducting state they may instead act as cell surface insertion platforms for ion channel trafficking (Deutsch et al. 2012).
Another protein found at C bouton synapses for which a function remains to be ascribed is the sigma-1 receptor (Mavlyutov et al. 2012). One potential role for these receptors is the regulation of Ca2+ availability at C bouton synapses. This possibility is supported by their localisation to subsurface cisternae, which form intracellular Ca2+ stores (Berridge, 1998), and their known association with IP3 receptors (Kourrich et al. 2012). Sigma-1 receptors might therefore contribute to the control of Ca2+ release from subsurface cisternae, which could in turn contribute to the activation of SK channels and AHP generation (Berridge, 1998). Alternatively, sigma-1 receptors could modulate the function of ion channels at C bouton synapses to regulate motor neuron excitability (Kourrich et al. 2012). For example, given their direct interactions with Kv channels, sigma-1 receptors could be involved in the redistribution of Kv channels from intracellular compartments to the plasma membrane (Kourrich et al. 2013).
In addition to modulating the intrinsic properties of motor neurons, C boutons might also modulate synaptic input to motor neurons. Two recent studies in rodents have provided indirect evidence of this. The first showed that the activation of projections from cholinergic neurons located near the central canal potentiates commissural glutamatergic inputs to motor neurons via muscarinic receptor-dependent mechanisms (Bertrand & Cazalets, 2011). Conversely, the second study showed that muscarinic receptor activation can inhibit synaptic currents mediated by postsynaptic AMPA receptors on motor neurons (Mejia-Gervacio, 2012). Further work will be required to determine whether either of these cholinergic modulatory effects on synaptic transmission involves the C bouton system.
Advances in our understanding of C bouton function at the cellular level have been paralleled by recent insight into their roles in spinal motor circuits and motor behaviour. Research regarding the function of the C bouton system has primarily focused on their contribution to the control of locomotion.
Early evidence that the C bouton system contributes to locomotor control came from work in cats which demonstrated that fictive locomotor activity induces c-fos expression in cholinergic interneurons near the central canal (Huang et al. 2000). It was later demonstrated that the activity of V0C interneurons is indeed tightly phase-locked to motor neuron activity during fictive locomotion in isolated neonatal mouse spinal cord preparations (Zagoraiou et al. 2009). Initial work investigating the role of C boutons in the control of locomotor-related motor neuron output was directed by knowledge of postsynaptic m2 receptor clustering at C bouton synapses. This work utilised an m2 receptor antagonist to block C bouton signalling and a cholinesterase inhibitor to turn up C bouton signalling during on-going, fictive locomotor activity recorded from isolated neonatal mouse spinal cord preparations (Miles et al. 2007). Blockade of m2 receptors reduced the amplitude of locomotor-related bursts of motor neuron output. Conversely, cholinesterase inhibition increased tonic and phasic locomotor-related motor neuron output. Neither treatment affected the frequency or pattern of locomotor-related output, indicating a specific role for C boutons in the modulation of motor neuron activity, but no effect on spinal interneurons within locomotor CPG circuitry.
In addition to providing functional knowledge at the cellular and circuit levels, recent research has importantly demonstrated a functional role for the C bouton system in whole animal behaviour (Zagoraiou et al. 2009). This work utilised mice in which the cholinergic output of V0c interneurons is inactivated due to the conditional knockout of the enzyme responsible for the biosynthesis of acetylcholine (choline acetyltransferase). The motor performance of mutant and control animals was assessed during locomotor behavioural assays designed to uncover task-dependent modulation in the activation of hind limb muscles. The degree of muscle activation was monitored via recordings of electromyographic (EMG) activity while animals were subjected to sequential walking and swimming tasks. In rodents, swimming elicits greater activation of some hind limb muscles compared with walking. However, in mutant animals in which C boutons were inactivated, the enhancement of muscle activation during swimming was significantly diminished compared with controls (Zagoraiou et al. 2009). This suggests that C boutons modulate motor neuron activity during locomotion to match the intensity of muscle activation to the biomechanical demands of different motor tasks. It remains unclear how V0c interneuron activity is adjusted to suit different locomotor tasks; possible mechanisms include feedback from sensory systems, or feed-forward control originating from higher motor control centres (Fig. 3A).
Many of the questions that remain regarding the function of the C bouton system relate to our incomplete knowledge of the inputs received by V0C interneurons and the pattern of their innervation of motor neurons. As discussed above, there are varying levels of specificity with respect to C bouton connectivity to motor neurons, including preferences based on the muscle types that motor neurons innervate (Hellstrom et al. 2003). In addition, it has recently become apparent that although ubiquitous, C bouton synapses are not completely uniform. Some degree of specialisation in C bouton signalling is, for example, indicated by variability in the complement of SK channel subtypes clustered at C bouton synapses on different motor neurons (Deardorff et al. 2013). Although the functional significance of such motor neuron subtype-specific features remains to be elucidated, it seems likely that C bouton-mediated modulation does more than simply set a global tone of motor neuron excitability, but rather fine-tunes the excitability of functionally distinct groups of motor neurons to help orchestrate complex motor behaviours.
Clinical significance of the C bouton system
Given the importance of C boutons in spinal motor circuitry and the control of motor behaviour, it is perhaps not surprising that their dysfunction is also implicated in injury and disease affecting the spinal cord.
Changes in the C bouton system have been described in a number of studies involving animal models of spinal cord injury. Rodent studies utilising either contusion injuries (Apostolova et al. 2006; Jakovcevski et al. 2007; Mehanna et al. 2010) or complete spinal transections (Kitzman, 2006; Skup et al. 2012) have shown that the number of C boutons contacting spinal motor neurons decreases soon after injury (Fig. 4B), although C bouton loss has not been observed in all motoneuron types (Skup et al. 2012; Ichiyama et al. 2011). In addition to loss of C boutons, levels of Kv2.1 protein are reduced and Kv2.1 channel clusters on motor neurons dispersed, following contusion injuries in mice (Song et al. 2012). Interestingly, where post-injury training has been used to aid in the recovery of motor function there appears to be a concomitant recovery of C boutons (Skup et al. 2012). In contrast to rodent studies, work involving double spinal hemisections in cats failed to show reductions in C bouton number post-injury (Pullen & Sears, 1983). These animals gradually recovered their locomotor function and C boutons were eventually found to increase in number (72 days post-injury) and size (200 days post injury) compared with pre-injury values. Overall, these studies demonstrate a link between the fate of C bouton synapses and the loss, and perhaps recovery, of motor function following spinal cord injury (Fig. 4A,B). Thus, C boutons may represent useful targets for novel treatments of spinal cord injury.
Fig. 4.
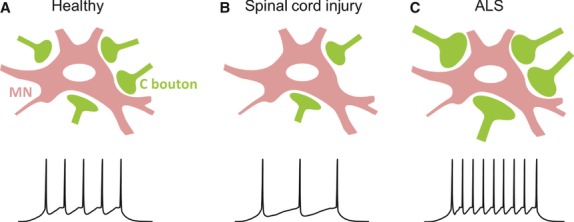
Involvement of C boutons in spinal cord injury and disease. Compared to ‘healthy’ control conditions (A), C boutons appear to be reduced in number in spinal cord injury (B) and enlarged in amyotrophic lateral sclerosis (ALS; C). Decreased C bouton numbers in spinal cord injury should reduce motor neuron excitability and the frequency of motor neuron output (B, bottom trace), perhaps contributing to overall motor dysfunction. Conversely, enlarged C boutons in ALS should lead to greater motor neuron excitability, which might contribute to excitotoxic disease mechanisms.
There is also evidence of changes in the C bouton system in the motor neuron disease Amyotrophic Lateral Sclerosis (ALS). ALS is a fatal neurodegenerative disease characterised by loss of motor neurons in the spinal cord and brainstem and corticospinal neurons of the motor cortex. However, it is now clear that interneurons, and their inputs to motor neurons, are also affected in the disease (Matsumoto et al. 1994; Sasaki & Maruyama, 1994; Ince et al. 1995; Stephens et al. 2006). C boutons represent one of the classes of synaptic inputs to motor neurons which are perturbed in human ALS (Nagao et al. 1998) and rodent models of the disease (Chang & Martin, 2009; Pullen & Athanasiou, 2009; Herron & Miles, 2012). Work on mouse models of ALS have shown enlargement of C boutons in mutant animals (Pullen & Athanasiou, 2009; Herron & Miles, 2012). To date, equivalent analyses are lacking in human tissue, although one study performed on post-mortem tissue from human ALS patients has shown a reduction in C bouton number (Nagao et al. 1998). This has also been reported in ALS model mice (Chang & Martin, 2009) and is likely to reflect global degenerative processes occurring towards the end-stage of the disease. It has been hypothesised that enlarged C boutons represent a compensatory effort to increase motor neuron output. However, recent work showing that C boutons are enlarged early in development (between 8 and 30 days of age), long before motor symptoms arise (∼90–100 days), may support involvement of C bouton pathology in the disease mechanisms underlying ALS (Herron & Miles, 2012). Larger C boutons should lead to greater motor neuron excitability (higher firing frequencies; Fig. 4C) which might over time contribute to motor neuron degeneration via excitotoxic mechanisms. One caveat to this idea is the finding that C boutons are only altered in male ALS model mice (Herron & Miles, 2012). Thus, C bouton pathology may not be central to motor neuron loss in ALS but rather involved in the greater susceptibility of males to the disease; ALS affects males more often than females with male : female ratios as high as 3 : 1 in some populations (McCombe & Henderson, 2010).
Another link between C boutons and ALS has recently come from the demonstration that sigma-1 receptors (enriched at C boutons) are mutated in some forms of familial ALS (Luty et al. 2010; Al-Saif et al. 2012). In addition, research investigating the fate of sigma-1 receptors in ALS has revealed an abnormal distribution of sigma-1 receptors in motor neurons and an overall reduction in sigma-1 receptor protein levels in spinal cord tissue from ALS patients and ALS model mice (Prause et al. 2013). Finally, treatment of ALS model mice with a sigma-1 receptor agonist has been shown to reduce both motor neuron loss and deficits in motor unit function, improve locomotor performance, and prolong survival (Mancuso et al. 2012). While providing support for further development of ALS therapies targeting sigma-1 receptors, this work also emphasises the potential for therapeutic strategies focused on C boutons.
Summary
Our understanding of C bouton synapses has advanced considerably since their first detailed description over 40 years ago. Steady progress regarding separate components of the C bouton system has paved the way for the recent synthesis of anatomical, molecular genetic and physiological findings, which has led to significant advances in our understanding of the C bouton system. It is now clear that the C bouton system provides neuromodulatory control of motor neuron output, in a task-dependent fashion, from which appropriate motor behaviour ensues. Furthermore, considerable evidence is accumulating in support of a role for C boutons in injury and disease, highlighting their potential as targets for novel therapeutic strategies. Our recent progress should facilitate the translation of basic research to clinical applications, but there still remains much to be determined regarding the finer details and nuances of the C bouton system.
References
- Al-Saif A, Bohlega S, Al-Mohanna F. Loss of ERLIN2 function leads to juvenile primary lateral sclerosis. Ann Neurol. 2012;72:510–516. doi: 10.1002/ana.23641. [DOI] [PubMed] [Google Scholar]
- Apostolova I, Irintchev A, Schachner M. Tenascin-R restricts posttraumatic remodeling of motoneuron innervation and functional recovery after spinal cord injury in adult mice. J Neurosci. 2006;26:7849–7859. doi: 10.1523/JNEUROSCI.1526-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson U, Svedlund J, Lagerback PA, et al. An ultrastructural study of the synaptology of gamma-motoneurones during the postnatal development in the cat. Brain Res. 1987;465:303–312. doi: 10.1016/0165-3806(87)90251-3. [DOI] [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Elde R, et al. Vesicular acetylcholine transporter (VAChT) protein: a novel and unique marker for cholinergic neurons in the central and peripheral nervous systems. J Comp Neurol. 1997;378:454–467. [PubMed] [Google Scholar]
- Barber RP, Phelps PE, Houser CR, et al. The morphology and distribution of neurons containing choline acetyltransferase in the adult rat spinal cord: an immunocytochemical study. J Comp Neurol. 1984;229:329–346. doi: 10.1002/cne.902290305. [DOI] [PubMed] [Google Scholar]
- Bernstein JJ, Bernstein ME. Ventral horn synaptology in the rat. J Neurocytol. 1976;5:109–123. doi: 10.1007/BF01176185. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Bertrand SS, Cazalets JR. Cholinergic partition cells and lamina x neurons induce a muscarinic-dependent short-term potentiation of commissural glutamatergic inputs in lumbar motoneurons. Front Neural Circuits. 2011;5:15. doi: 10.3389/fncir.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodian D. Origin of specific synaptic types in the motoneuron neuropil of the monkey. J Comp Neurol. 1975;159:225–243. doi: 10.1002/cne.901590205. [DOI] [PubMed] [Google Scholar]
- Chang Q, Martin LJ. Glycinergic innervation of motoneurons is deficient in amyotrophic lateral sclerosis mice: a quantitative confocal analysis. Am J Pathol. 2009;174:574–585. doi: 10.2353/ajpath.2009.080557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connaughton M, Priestley JV, Sofroniew MV, et al. Inputs to motoneurones in the hypoglossal nucleus of the rat: light and electron microscopic immunocytochemistry for choline acetyltransferase, substance P and enkephalins using monoclonal antibodies. Neuroscience. 1986;17:205–224. doi: 10.1016/0306-4522(86)90237-x. [DOI] [PubMed] [Google Scholar]
- Conradi S. Ultrastructure and distribution of neuronal and glial elements on the motoneuron surface in the lumbosacral spinal cord of the adult cat. Acta Physiol Scand Suppl. 1969;332:5–48. [PubMed] [Google Scholar]
- Conradi S, Skoglund S. Observations on the ultrastruture and distribution of neuronal and glial elements on the motoneuron surface in the lumbosacral spinal cord of the cat during postnatal development. Acta Physiol Scand Suppl. 1969;333:5–52. [PubMed] [Google Scholar]
- Deardorff AS, Romer SH, Deng Z, et al. Expression of postsynaptic Ca2+-activated K+ (SK) channels at C-bouton synapses in mammalian lumbar alpha-motoneurons. J Physiol. 2013;591(Pt4):875–897. doi: 10.1113/jphysiol.2012.240879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z, Fyffe RE. Expression of P2X7 receptor immunoreactivity in distinct subsets of synaptic terminals in the ventral horn of rat lumbar spinal cord. Brain Res. 2004;1020:53–61. doi: 10.1016/j.brainres.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Deutsch E, Weigel AV, Akin EJ, et al. Kv2.1 cell surface clusters are insertion platforms for ion channel delivery to the plasma membrane. Mol Biol Cell. 2012;23:2917–2929. doi: 10.1091/mbc.E12-01-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PD, Loftus RJ, Tamkun MM. Regulation of kv2.1 k+ conductance by cell surface channel density. J Neurosci. 2013;33:1259–1270. doi: 10.1523/JNEUROSCI.3008-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E. A new class of spinal interneurons: the origin and function of C boutons is solved. Neuron. 2009;64:593–595. doi: 10.1016/j.neuron.2009.11.030. [DOI] [PubMed] [Google Scholar]
- Hamos JE, King JS. The synaptic organization of the motor nucleus of the trigeminal nerve in the opossum. J Comp Neurol. 1980;194:441–463. doi: 10.1002/cne.901940210. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Mottram C, Quinlan K, et al. Motoneuron excitability: the importance of neuromodulatory inputs. Clin Neurophysiol. 2009;120:2040–2054. doi: 10.1016/j.clinph.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom J, Arvidsson U, Elde R, et al. Differential expression of nerve terminal protein isoforms in VAChT-containing varicosities of the spinal cord ventral horn. J Comp Neurol. 1999;411:578–590. [PubMed] [Google Scholar]
- Hellstrom J, Oliveira AL, Meister B, et al. Large cholinergic nerve terminals on subsets of motoneurons and their relation to muscarinic receptor type 2. J Comp Neurol. 2003;460:476–486. doi: 10.1002/cne.10648. [DOI] [PubMed] [Google Scholar]
- Herron LR, Miles GB. Gender-specific perturbations in modulatory inputs to motoneurons in a mouse model of amyotrophic lateral sclerosis. Neuroscience. 2012;226:313–323. doi: 10.1016/j.neuroscience.2012.09.031. [DOI] [PubMed] [Google Scholar]
- Huang A, Noga BR, Carr PA, et al. Spinal cholinergic neurons activated during locomotion: localization and electrophysiological characterization. J Neurophysiol. 2000;83:3537–3547. doi: 10.1152/jn.2000.83.6.3537. [DOI] [PubMed] [Google Scholar]
- Ichiyama RM, Broman J, Roy RR, et al. Locomotor training maintains normal inhibitory influence on both alpha- and gamma-motoneurons after neonatal spinal cord transection. J Neurosci. 2011;31:26–33. doi: 10.1523/JNEUROSCI.6433-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince PG, Slade J, Chinnery RM, et al. Quantitative study of synaptophysin immunoreactivity of cerebral cortex and spinal cord in motor neuron disease. J Neuropathol Exp Neurol. 1995;54:673–679. doi: 10.1097/00005072-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Jakovcevski I, Wu J, Karl N, et al. Glial scar expression of CHL1, the close homolog of the adhesion molecule L1, limits recovery after spinal cord injury. J Neurosci. 2007;27:7222–7233. doi: 10.1523/JNEUROSCI.0739-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Kaczmarek-Hajek K, Lorinczi E, Hausmann R, et al. Molecular and functional properties of P2X receptors – recent progress and persisting challenges. Purinergic Signal. 2012;8:375–417. doi: 10.1007/s11302-012-9314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I, Osaka H, Stanislaus S, et al. Nicotinic acetylcholine receptor distribution in relation to spinal neurotransmission pathways. J Comp Neurol. 2003;467:44–59. doi: 10.1002/cne.10913. [DOI] [PubMed] [Google Scholar]
- Kitzman P. Changes in vesicular glutamate transporter 2, vesicular GABA transporter and vesicular acetylcholine transporter labeling of sacrocaudal motoneurons in the spastic rat. Exp Neurol. 2006;197:407–419. doi: 10.1016/j.expneurol.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Su TP, Fujimoto M, et al. The sigma-1 receptor: roles in neuronal plasticity and disease. Trends Neurosci. 2012;35:762–771. doi: 10.1016/j.tins.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Hayashi T, Chuang JY, et al. Dynamic interaction between sigma-1 receptor and Kv1.2 shapes neuronal and behavioral responses to cocaine. Cell. 2013;152:236–247. doi: 10.1016/j.cell.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PR, Shute CC. The distribution of cholinesterase in cholinergic neurons demonstrated with the electron microscope. J Cell Sci. 1966;1:381–390. doi: 10.1242/jcs.1.3.381. [DOI] [PubMed] [Google Scholar]
- Li W, Ochalski PAY, Brimijoin S, et al. C-terminals on motoneurons: Electron microscope localization of cholinergic markers in adult rats and antibody-induced depletion in neonates. Neuroscience. 1995;65:879–891. doi: 10.1016/0306-4522(94)00511-3. [DOI] [PubMed] [Google Scholar]
- Luty AA, Kwok JB, Dobson-Stone C, et al. Sigma nonopioid intracellular receptor 1 mutations cause frontotemporal lobar degeneration-motor neuron disease. Ann Neurol. 2010;68:639–649. doi: 10.1002/ana.22274. [DOI] [PubMed] [Google Scholar]
- Mancuso R, Oliván S, Rando A, et al. Sigma-1R agonist improves motor function and motoneuron survival in ALS mice. Neurotherapeutics. 2012;9:814–826. doi: 10.1007/s13311-012-0140-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S, Goto S, Kusaka H, et al. Synaptic pathology of spinal anterior horn cells in amyotrophic lateral sclerosis: an immunohistochemical study. J Neurol Sci. 1994;125:180–185. doi: 10.1016/0022-510x(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Mavlyutov TA, Epstein ML, Liu P, et al. Development of the sigma-1 receptor in C-terminals of motoneurons and colocalization with the N, N'-dimethyltryptamine forming enzyme, indole-N-methyl transferase. Neuroscience. 2012;206:60–68. doi: 10.1016/j.neuroscience.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCombe PA, Henderson RD. Effects of gender in amyotrophic lateral sclerosis. Gend Med. 2010;7:557–570. doi: 10.1016/j.genm.2010.11.010. [DOI] [PubMed] [Google Scholar]
- McLaughlin BJ. Propriospinal and supraspinal projections to the motor nuclei in the cat spinal cord. J Comp Neurol. 1972;144:475–500. doi: 10.1002/cne.901440406. [DOI] [PubMed] [Google Scholar]
- Mehanna A, Jakovcevski I, Acar A, et al. Polysialic acid glycomimetic promotes functional recovery and plasticity after spinal cord injury in mice. Mol Ther. 2010;18:34–43. doi: 10.1038/mt.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia-Gervacio S. Muscarinic control of AMPA receptor responsiveness in mouse spinal cord motoneurons. J Physiol. 2012;590:4663–4671. doi: 10.1113/jphysiol.2012.238444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Hartley R, Todd AJ, et al. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci U S A. 2007;104:2448–2453. doi: 10.1073/pnas.0611134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H, Mohapatra DP, Trimmer JS. Kv2.1: a voltage-gated k+ channel critical to dynamic control of neuronal excitability. Neurotoxicology. 2005;26:743–752. doi: 10.1016/j.neuro.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Murakoshi H, Shi G, Scannevin RH, et al. Phosphorylation of the Kv2.1 K+ channel alters voltage-dependent activation. Mol Pharmacol. 1997;52:821–828. doi: 10.1124/mol.52.5.821. [DOI] [PubMed] [Google Scholar]
- Nagao M, Misawa H, Kato S, et al. Loss of cholinergic synapses on the spinal motor neurons of amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 1998;57:329–333. doi: 10.1097/00005072-199804000-00004. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Yamamoto T, Jordan LM. Evidence for the cholinergic nature of C-terminals associated with subsurface cisterns in alpha-motoneurons of rat. Synapse. 1993;15:17–32. doi: 10.1002/syn.890150103. [DOI] [PubMed] [Google Scholar]
- Phelps PE, Barber RP, Houser CR, et al. Postnatal development of neurons containing choline acetyltransferase in rat spinal cord: an immunocytochemical study. J Comp Neurol. 1984;229:347–361. doi: 10.1002/cne.902290306. [DOI] [PubMed] [Google Scholar]
- Prause J, Goswami A, Katona I, et al. Altered localization, abnormal modification and loss of function of Sigma receptor-1 in amyotrophic lateral sclerosis. Hum Mol Genet. 2013;22:1581–1600. doi: 10.1093/hmg/ddt008. [DOI] [PubMed] [Google Scholar]
- Pullen AH, Athanasiou D. Increase in presynaptic territory of C-terminals on lumbar motoneurons of G93A SOD1 mice during disease progression. Eur J Neurosci. 2009;29:551–561. doi: 10.1111/j.1460-9568.2008.06602.x. [DOI] [PubMed] [Google Scholar]
- Pullen AH, Sears TA. Trophism between C-type axon terminals and thoracic motoneurones in the cat. J Physiol. 1983;337:373–388. doi: 10.1113/jphysiol.1983.sp014629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen AH, Martin JE, Swash M. Ultrastructure of pre-synaptic input to motor neurons in Onuf's nucleus: controls and motor neuron disease. Neuropathol Appl Neurobiol. 1992;18:213–231. doi: 10.1111/j.1365-2990.1992.tb00784.x. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, et al. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S, Maruyama S. Decreased synaptophysin immunoreactivity of the anterior horns in motor neuron disease. Acta Neuropathol. 1994;87:125–128. doi: 10.1007/BF00296180. [DOI] [PubMed] [Google Scholar]
- Siembab VC, Smith CA, Zagoraiou L, et al. Target selection of proprioceptive and motor axon synapses on neonatal V1-derived Ia inhibitory interneurons and Renshaw cells. J Comp Neurol. 2010;518:4675–4701. doi: 10.1002/cne.22441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skup M, Gajewska-Wozniak O, Grygielewicz P, et al. Different effects of spinalization and locomotor training of spinal animals on cholinergic innervation of the soleus and tibialis anterior motoneurons. Eur J Neurosci. 2012;36:2679–2688. doi: 10.1111/j.1460-9568.2012.08182.x. [DOI] [PubMed] [Google Scholar]
- Song MY, Hong C, Bae SH, et al. Dynamic modulation of the kv2.1 channel by SRC-dependent tyrosine phosphorylation. J Proteome Res. 2012;11:1018–1026. doi: 10.1021/pr200770v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens B, Guiloff RJ, Navarrete R, et al. Widespread loss of neuronal populations in the spinal ventral horn in sporadic motor neuron disease. A morphometric study. J Neurol Sci. 2006;244:41–58. doi: 10.1016/j.jns.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Stepien AE, Tripodi M, Arber S. Monosynaptic rabies virus reveals premotor network organization and synaptic specificity of cholinergic partition cells. Neuron. 2010;68:456–472. doi: 10.1016/j.neuron.2010.10.019. [DOI] [PubMed] [Google Scholar]
- VanderHorst VG, Ulfhake B. The organization of the brainstem and spinal cord of the mouse: relationships between monoaminergic, cholinergic, and spinal projection systems. J Chem Neuroanat. 2006;31:2–36. doi: 10.1016/j.jchemneu.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Rempel J, Brownstone RM. Postnatal development of cholinergic synapses on mouse spinal motoneurons. J Comp Neurol. 2004;474:13–23. doi: 10.1002/cne.20089. [DOI] [PubMed] [Google Scholar]
- Zagoraiou L, Akay T, Martin JF, et al. A cluster of cholinergic premotor interneurons modulates mouse locomotor activity. Neuron. 2009;64:645–662. doi: 10.1016/j.neuron.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Das S, Murthy KS. Erk1/2- and p38 MAP kinase-dependent phosphorylation and activation of cPLA2 by m3 and m2 receptors. Am J Physiol Gastrointest Liver Physiol. 2003;284:G472–G480. doi: 10.1152/ajpgi.00345.2002. [DOI] [PubMed] [Google Scholar]


