Abstract
We report here that when untreated mice underwent heat stress, they displayed thermoregulatory deficit (e.g., animals display hypothermia during room temperature exposure), brain (or hypothalamic) inflammation, ischemia, oxidative damage, hypothalamic-pituitary-adrenal axis impairment (e.g., decreased plasma levels of both adrenocorticotrophic hormone and corticosterone during heat stress), multiple organ dysfunction or failure, and lethality. Melatonin therapy significantly reduced the thermoregulatory deficit, brain inflammation, ischemia, oxidative damage, hypothalamic-pituitary-adrenal axis impairment, multiple organ dysfunction, and lethality caused by heat stroke. Our data indicate that melatonin may improve outcomes of heat stroke by reducing brain inflammation, oxidative damage, and multiple organ dysfunction.
1. Introduction
Melatonin, the main product of the pineal gland, is found in high concentrations in other body fluids and tissues [1, 2] and possesses anti-inflammatory and antioxidant actions [3–6]. We have evaluated the prophylactic [7] as well as the therapeutic [8] effect of melatonin in heatstroke rats under general anesthesia and showed the therapeutic effects of melatonin on heatstroke-induced multiple organ dysfunction syndrome. According to a more recent review [9], the ischemic and oxidative damage to the hypothalamus during heatstroke may cause multiple organ dysfunction or failure through hypothalamic-pituitary-adrenal (HPA) axis mechanisms. Studies are warranted to know whether the heatstroke-induced brain (or hypothalamic) inflammation and damage, thermoregulatory deficits, and multiple organ dysfunction can be affected by melatonin therapy in an unanesthetized and unrestrained mouse model [10–12].
To deal with the hypothesis, we assessed the temporal profiles of cellular markers of ischemia (e.g., glutamate and lactate/pyruvate ratio), damage (e.g., glycerol), inflammation (e.g., tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1β), IL-10, and myeloperoxidase (MPO) activity), and oxidative damage (e.g., prooxidant enzymes (e.g., lipid peroxidation and glutathione oxidation), anti-oxidant defenses (e.g., glutathione peroxidase (GPx), and glutathione reductase (GR), oxidant radicals, nitric oxide metabolites (NOx), and dihydroxybenzoic acid (DHBA)) in the hypothalamus that occurred after heat regimen in mice treated with or without melatonin therapy. In addition, the influence of melatonin therapy on the heatstroke-induced thermoregulatory deficits as well as increased plasma levels of multiple organ dysfunction or failure [10–12] was assessed.
2. Materials and Methods
2.1. Mice
Present studies were performed in male ICR mice (29–37 g), whose stock originated from the Institute of Cancer Research of the National Institutes of Health in the USA. They were purchased from the National Animal Center (Taipei, Taiwan) and kept under a 12-hour light-dark cycle at controlled temperature (21 ± 2°C) with free access to food and tap water.
2.2. Murine Model of Heatstroke
Institute of Cancer Research male mice 8 to 10 weeks old were exposed to whole body heating (WBH; 41.2°C; relative humidity 50%–55%; 1 h) in an environment-controlled chamber [10–12]. The heat-stressed mice were returned to the normal room temperature (26°C) after the end of the heat exposure. Mice that survived to day 4 of heat treatment were considered survivors, and the data were used for analysis of the results. Core temperatures were measured every 1 min with a copper constant thermocouple inserted into the rectum and connected to a thermometer (HR1300; Yokogawa, Tokyo, Japan). Before the start of thermal experiments, mice were housed at an ambient temperature (26°C) below the neutral zone for this species. After 1-hour heating period, animals were properly fed and hydrated. In separate experiments, 4 h post-WBH, animals were sacrificed and their brains and blood were obtained for biochemical verification [10–12].
2.3. Experimental Groups
Three hundred mice were randomly divided into 3 major groups: (a) nonheated mice treated with vehicle solution (n = 60): these groups of animals were s.c. injected with one dose of 0.2 mL of 0.25% ethanol-saline [13] immediately post-WBH; (b) heated mice treated with vehicle solution (n = 60); and (c) heated mice treated with melatonin (N-acetyl-5-methoxytryptamine) (0.2 mg/kg, 1 mg/kg, or 5 mg/kg) [14] immediately post-WBH (n = 180).
In Experiment 1, effects of heat exposure on body core temperature and % survival of different groups of mice were assessed (n = 60).
In Experiment 2, effects of heat exposure on cellular ischemia and damage markers in brain (or hypothalamus) of different groups of mice were measured (n = 60).
In Experiment 3, effects of heat exposure on inflammatory mediators in brain (or hypothalamus) of different groups of mice were measured (n = 60).
In Experiment 4, effects of heat exposure on oxidative stress markers in brain (or hypothalamus) of different groups of mice were measured (n = 60).
In Experiment 5, effects of heat exposure on serum levels of multiple organ dysfunction indicators, ACTH, and corticosterone of different groups were measured (n = 60).
2.4. Extracellular Levels of Glutamate, Lactate-to-Pyruvate Ratio, Glycerol, Nitric Oxide, and Hydroxyl Radicals in the Hypothalamus
Hypothalamic samples were homogenized in 0.05 M phosphate buffer at pH7.0 and then centrifuged at 4000 ×g for 20 min at 4°C. The supernatants were used for the determination of cellular levels of glutamate, lactate-to-pyruvate ratio, glycerol, nitric oxide, and hydroxyl radicals. The dialysis probe (4 nm in length c CMA/12; Carnegie Medicine, Stockholm, Sweden) was put into the supernatants to obtain the dialysates.
The nitric oxide (NOx −) concentration in the dialysates of hypothalamus was measured with the Eicom ENO-20 NOx − analysis system (Eicom, Kyoto) [15]. In the Eicom ENO-20 NOx − analysis system, after the NO2 − and NO3 − in the sample have been separated by the column, the NO2 − reacts in the acidic solution with the primary aromatic amine to produce an azo compound. Following this, the addition of aromatic amines to the azo compound results in a coupling that produces a diazo compound, and the absorbance rate of the red color in this compound is then measured. For measurement of glutamate, lactate-to-pyruvate ratio, and glycerol, the dialysates were injected onto a CMA600 microdialysis analyzer (Carnegie Medicine, Stockholm, Sweden). The concentrations of hydroxyl radicals were measured by a modified procedure based on the hydroxylation of sodium salicylates by hydroxyl radicals, leading to the production of 2,3-dihydroxybenzoic acid and 2,5-dihydroxybenzoic acid [16].
2.5. Determination of Lipid Peroxidation
Lipid peroxidation was assessed by measuring the levels of malondialdehyde (MDA) with 2-thiobarbituric acid (TBA) to form a chromophore absorbing at 532 nm [17]. About 0.1 g of tissue was homogenized with 1.5 mL of 0.1 M phosphate buffer at pH3.5. The reaction mixture (0.2 mL of sample, 1.5 mL of 20% acetic acid, 0.2 mL of 8.1% sodium dodecyl sulfate, and 1.5 mL of aqueous solution of 0.8% TBA, up to 4 mL with distilled water) was heated to 95°C for 1 h, and then 5 mL of N-butanol and pyridine (15 : 1 vol/vol) was added. The mixture was vortexed vigorously and centrifuged at 1500 g for 10 min, and the absorbance of the organic phase was measured at 532 nm. The values were expressed as nanomoles of TBA-reactive substances (MDA equivalent) per milligram of protein.
2.6. Quantification of Total and Oxidized Glutathione
Tissues were homogenized in 5% 5-sulfosalicylic acid (1 : 10 wt/vol) at 0°C, and the supernatants were used for analysis of total and oxidized glutathione. Total glutathione (reduced-form glutathione (GSH) + oxidized-form glutathione (GSSG)) was analyzed according to the Tietze method [18], and GSSG was determined as described by Griffith [19]. The recycling assay for total glutathione is oxidized by 5,5-Dithiobis (2 acid) (DTNB) to give GSSG with stoichiometric formation of 5-thio-2-nitrobenzoic acid. GSSG is reduced to GSH by the action of the highly specific glutathione reductase (GR) and nicotinamide adenine dinucleotide phosphate (reduced form; NADPH). The rate of 5-thio-2-nitrobenzoic acid formation is followed at 412 nm and is proportional to the sum of GSH and GSSG present.
2.7. Determination of Glutathione Peroxidase (GPx) and Glutathione Reductase (GR) Activity
Tissues were homogenized in 0.05 M phosphate buffer, pH7.0 and then centrifuged at 4000 ×g for 20 min at 4°C. The supernatants were used for GPx and GR activity assay. The GPx and GR activities were performed with a commercial GPx assay kit (Sigma, USA) and a GP assay kit (Sigma, USA), respectively. One unit of GPx and GR activity was defined as the amount of sample required to oxidize 1 mmol of NADPH per minute based on the molecular absorbance of 6.22 × 106 for NADPH.
2.8. Myeloperoxidase Activity
MPO activity, an indicator of polymorphonuclear leukocyte accumulation, was determined in the hypothalamus as described previously [20] at 4 hours after heat stress. MPO activity was defined as the quantity of enzyme degrading 1 μmol of peroxide/min at 37°C and was expressed in milliunits/gram of wet tissue.
2.9. Determination of Cytokines in the Hypothalamus
The hypothalamic samples were prepared according to previous reports [21]. The tissues were homogenized in five volumes of ice-cold Ripa buffer. The homogenates were incubated on ice for 30 min and then centrifuged (15,000 ×g, 30 min, 4°C) twice. The concentrations of these cytokines in the supernatants were determined by commercially available ELISA kits (R & D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. Optical densities were read on a plate reader set at 450 nm for these cytokines. The concentrations of these cytokines in the samples were calculated from the standard curve multiplied by the dilution factor and were expressed as pg/g.
2.10. Quantification of Multiple Organ Dysfunction and Injury
Creatinine, blood urea nitrogen (BUN), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) were estimated in blood samples collected 4 hours after the start of heat stress or the equivalent time point for the nonheated animals. The serum levels of creatinine, BUN, ALT, AST, and ALP were determined by spectrophotometry (HITACHI 7600, Tokyo, Japan).
2.11. Plasma Assessment of Corticosterone and Adrenocorticotrophic Hormone (ACTH)
Plasma corticosterone and ACTH were assessed using corticosterone Double Antibody RIA kit (MP Biomedicals, Solon, Oh, USA) and ACTH (Rat, Mouse)-RIA kit (Phoenix Pharmaceuticals, Burlingame, CA, USA), respectively. All analyses were performed according to manufacturer's instructions.
2.12. Statistical Analysis
All values in the tables and text are expressed as mean ± S.E.M. of n observations, where n represents the number of animals studied. Statistical evaluation was performed by using analysis of variance (ANOVA) followed by a multiple-comparison test (Scheffe's test). A P value of less than 0.05 was considered to be statistically significant.
3. Results
3.1. Melatonin Prevents Heat-Induced Hypothermia and Lethality
As summarized in Table 1, the body core temperature values of heated mice were significantly lower than those of nonheated mice kept at a normal ambient temperature (26°C) (33.2 ± 0.2°C and 37.2 ± 0.3°C, resp.) (P < 0.001). Additionally, the fraction survival of heated mice was significantly lower than those of nonheated mice (1/12 and 12/12, resp.) (P < 0.001) (Figure 1). The heat-induced hypothermia and lethality were significantly reduced by melatonin therapy (Table 1 and Figure 1).
Table 1.
Effects of heat exposure on body core temperatures of different groups of mice.
| Treatment groups | Core temperature (°C) | P values |
|---|---|---|
| (1) Nonheated mice untreated | 37.2 ± 0.3 | |
| (2) Heated mice treated with vehicle saline | 33.2 ± 0.2 | a P < 0.01 |
| (3) Heated mice treated with melatonin 0.2 mg/kg | 33.5 ± 0.3 | a P < 0.01 |
| (4) Heated mice treated with melatonin 1 mg/kg | 36.3 ± 0.3 | b P < 0.01 |
| (5) Heated mice treated with melatonin 5 mg/kg | 37.4 ± 0.4 | b P < 0.01 |
Core temperatures were measured 4 hours after whole body heating for heated groups or the equivalent time period for non-heated groups. aCompared with non-heated groups; bCompared with group 2. Data are means ± S.E.M. of 12 mice per group.
Figure 1.
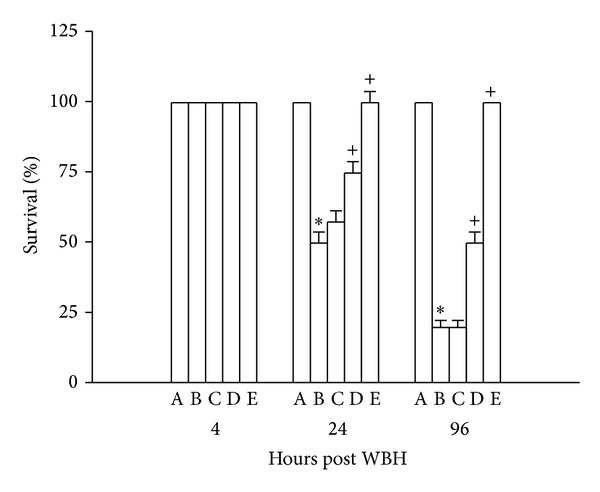
Percent survival values for nonheated mice untreated (A), heated mice treated with vehicle solution (B), heated mice treated with melatonin (0.2 mg/kg) (C), heated mice treated with melatonin (1 mg/kg) (D), and heated mice treated with melatonin (5 mg/kg) (E) 4–96 hours after whole body heating (WBH). Data are expressed as means ± S.E.M. for 12 mice per group. *P < 0.05 compared with nonheated mice untreated mice; + P < 0.05 compared with heated mice treated with vehicle solution.
3.2. Melatonin Reduces Heat-Induced Increased Levels of Glutamate, Lactate-to-Pyruvate Ratio, Glycerol, Nitrite (NO), and Dihydroxybenzoic Acid (DHBAs) in the Hypothalamus
Table 2 summarizes the effects of heat exposure on cellular levels of glutamate, lactate-to-pyruvate ratio, glycerol, nitrite, and DHBA in the hypothalamus in different groups of mice. As compared with nonheated mice, the heated mice had higher levels of glutamate, lactate-to-pyruvate ratio, glycerol, nitrite, and DHBA in the hypothalamus (P < 0.05). The heat-induced increased levels of glutamate, lactate-to-pyruvate ratio, glycerol, nitrite, and DHBA in the hypothalamus were all significantly and dose-dependently reduced by melatonin (0.2–5.0 mg/kg).
Table 2.
Effect of heat exposure on hypothalamic levels of various parameters in different groups of mice.
| Treatment groups | Glutamate (% of baseline) |
Lactate/pyruvate ratio | Glycerol (% of baseline) |
Nitric oxide (µM) |
2,3-DHBA (% of baseline) |
|---|---|---|---|---|---|
| (1) Non-heated mice | 100 ± 6 | 10 ± 3 | 100 ± 5 | 16 ± 2 | 100 ± 5 |
| (2) Heated mice treated with vehicle solution | 207 ± 11a | 219 ± 15a | 173 ± 7a | 99 ± 11a | 151 ± 8a |
| (3) Heated mice treated with melatonin 0.2 mg/kg | 214 ± 12 | 206 ± 14 | 214 ± 11 | 106 ± 10 | 165 ± 7 |
| (4) Heated mice treated with melatonin 1 mg/kg | 141 ± 8b | 87 ± 5b | 135 ± 5b | 61 ± 4b | 118 ± 5b |
| (5) Heated mice treated with melatonin 5 mg/kg | 106 ± 5b | 10 ± 2b | 102 ± 4b | 15 ± 2b | 99 ± 6b |
Samples were measured 4 hours after whole body heating or the equivalent time period for non-heated groups. aCompared with non-heated groups (P < 0.05); bCompared with group 2 (P < 0.05). Data are means ± S.E.M. of 12 mice per group.
3.3. Melatonin Attenuates Heat-Induced Increased Levels of TNF-α, IL-1β, and MPO but Increases Production of IL-10 in the Hypothalamus
As shown in Table 3, hypothalamic levels of TNF-α, IL-1β, and MPO were all increased in heated mice. Again, heat-induced overproduction of TNF-α, IL-1β, and MPO was significantly and dose-dependently reduced by melatonin therapy (0.2–5.0 mg/kg) (P < 0.05). In contrast, hypothalamic levels of IL-10 were significantly and dose-dependently increased by melatonin treatment in heated mice.
Table 3.
Effects of heat exposure on hypothalamic levels of various parameters in different groups of mice.
| Treatment groups | TNF-α (pg/g) | IL-1β (pg/g) | IL-10 (pg/g) | MPO activity (pg/wet tissue) |
|---|---|---|---|---|
| (1) Non-heated mice | 10 ± 3 | 8 ± 2 | 5 ± 2 | 51 ± 8 |
| (2) Heated mice treated with vehicle solution | 208 ± 18a | 426 ± 51a | 6 ± 3 | 448 ± 14 |
| (3) Heated mice treated with melatonin 0.2 mg/kg | 224 ± 21 | 453 ± 56 | 4 ± 2 | 507 ± 17 |
| (4) Heated mice treated with melatonin 1 mg/kg | 88 ± 8b | 190 ± 18b | 212 ± 22b | 144 ± 9b |
| (5) Heated mice treated with melatonin 5 mg/kg | 9 ± 3b | 8 ± 3b | 256 ± 30b | 51 ± 11b |
Samples were measured 4 hours after whole body heating for non-heated groups. aCompared with non-heated groups (P < 0.05); bCompared with group 2 (P < 0.05). Data are means ± S.E.M. of 12 mice per group.
3.4. Melatonin Decreases Heat-Induced Hypothalamic Oxidative Stress
As shown in Table 4, the hypothalamic levels of both MDA and GSSG/GSH in heated mice were significantly higher than those of nonheated mice. In contrast, the hypothalamic levels of GP, GR of heated mice were significantly higher than those of nonheated mice (P < 0.05). The heat-induced increased levels of MDA and GSSG/GSH as well as the decreased levels of GP, GR in the hypothalamus were significantly and dose-dependently reduced by melatonin therapy (0.2–5.0 mg/kg) (P < 0.05).
Table 4.
Effects of heat exposure on hypothalamic levels of various parameters in different groups of mice.
| Treatment groups | MDA (n mol/mg protein) | GSSG/GSH | GP (m U/mg protein) | GR (m U/mg protein) |
|---|---|---|---|---|
| (1) Non-heated mice | 6.9 ± 0.6 | 0.55 ± 0.16 | 318 ± 39 | 182 ± 18 |
| (2) Heated mice treated with vehicle solution | 12 ± 2a | 2.24 ± 0.38a | 87 ± 18a | 76 ± 12a |
| (3) Heated mice treated with melatonin 0.2 mg/kg | 11 ± 1a | 2.42 ± 0.3b | 76 ± 15 | 72 ± 11 |
| (4) Heated mice treated with melatonin 1 mg/kg | 6 ± 1b | 0.98 ± 0.21b | 185 ± 25b | 144 ± 16b |
| (5) Heated mice treated with melatonin 5 mg/kg | 4 ± 1b | 0.42 ± 0.15b | 369 ± 41b | 192 ± 19b |
Samples were measured 4 hours after whole body heating for non-heated groups. aCompared with non-heated groups (P < 0.05); bCompared with group 2 (P < 0.05). Data are means ± S.E.M. of 12 mice per group.
3.5. Melatonin Attenuates Heat-Induced Increased Plasma Levels of Multiple Organ Injury Markers
The plasma levels of BUN, creatinine, ALT, AST, and AP in heated mice were all significantly higher than those of nonheated mice (Table 5). The heat-induced increased plasma levels of these parameters were all significantly and dose-dependently reduced by melatonin treatment (0.2–5.0 mg/kg) (P < 0.05).
Table 5.
Effect of heat exposure on serum levels of blood urea nitrogen (BUN), creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphate (AP) in different groups of mice.
| Treatment groups | BUN (mmol/L) | Creatinine (mmol/L) | ALT (U/L) | AST (U/L) | AP (U/L) |
|---|---|---|---|---|---|
| (1) Non-heated mice | 8 ± 1 | 27 ± 2 | 35 ± 4 | 111 ± 8 | 299 ± 23 |
| (2) Heated mice treated with vehicle solution | 23 ± 1a | 75 ± 3a | 129 ± 5a | 508 ± 22a | 533 ± 31a |
| (3) Heated mice treated with melatonin 0.2 mg/kg | 21 ± 2 | 69 ± 4 | 130 ± 6 | 492 ± 21 | 505 ± 28 |
| (4) Heated mice treated with melatonin 1 mg/kg | 15 ± 2b | 52 ± 2b | 85 ± 3b | 274 ± 16b | 395 ± 19b |
| (5) Heated mice treated 5 mg/kg | 10 ± 1b | 43 ± 3b | 67 ± 4b | 188 ± 12b | 364 ± 17b |
Samples were measured 4 hours after whole body heating or the equivalent time period for non-heated groups. aCompared with non-heated group (P < 0.05); bCompared with group 2 (P < 0.05). Data are means ± S.E.M. of 12 mice per group.
3.6. Melatonin Enhances Heat-Induced Increased Plasma Levels of Both ACTH and Corticosterone
As shown in Table 6, the plasma levels of both ACTH and corticosterone in heated mice were significantly higher than those of nonheated controls (P < 0.05). The heat-induced increased plasma levels of both ACTH and corticosterone were all significantly enhanced by melatonin therapy (P < 0.05; Table 6).
Table 6.
Effect of heat exposure on serum levels of adrenocorticotrophic-hormone (ACTH) and corticosterone in different groups of mice.
| Treatment groups | ACTH (pg·mL−1) | Corticosterone (ng·mL−1) |
|---|---|---|
| (1) Non-heated mice | 392 ± 98 | 142 ± 24 |
| (2) Heated mice treated with vehicle solution | 984 ± 127a | 505 ± 26a |
| (3) Heated mice treated with melatonin 0.2 mg/kg | 1001 ± 145 | 518 ± 28 |
| (4) Heated mice treated with melatonin 1 mg/kg | 1668 ± 162b | 719 ± 31b |
| (5) Heated mice treated with melatonin 5 mg/kg | 2167 ± 176b | 854 ± 35b |
Samples were measured 4 hours after whole body heating or the equivalent time period for non-heated groups. aCompared with non-heated group (P < 0.05); bCompared with group 2 (P < 0.05). Data are means ± S.E.M. of 12 mice per group.
4. Discussion
According to Chatterjee et al. [10, 11], heat-treated mice display body core temperatures of >40°C immediately after the termination of WBH and profound hypothermia at +4, +6, and +20 h after. This is confirmed by the present results. We further demonstrate that heat-induced hypothermia in mice can be significantly and dose-dependently prevented by melatonin therapy. However, the contention is not consistent with the findings of Leon et al. [22], who reported on mice exposed to an ambient temperature of ~39.5°C until a maximal core temperature of 42.7°C was attained. During their recovery, the mice had hypothermia (29.3°C) and, after 24 h of recovery, a fever-like elevation (37.8°C).
The hypothalamus is believed to be involved in regulating homeostasis, motivation, and emotional behavior; these functions are mediated through hypothalamic control of autonomic and endocrine activity [23]. The hypothalamus allows the output of pituitary hormones to response to changes in the autonomic nervous system activity and to the needs of temperature regulation, water balance, and energy requirements. Heat exposure is a stimulus that triggers biological stress reactions [24]. The hypothalamo-pituitary-adrenocortical (HPA) axis is also mobilized, as suggested by the increase in c-fos-positive cells [25] and c-fos mRNA content [26] in the hypothalamic paraventricular nucleus, as well as the increase in blood adrenocorticotrophic-hormone (ACTH) and corticosterone concentrations [27, 28]. Decreased heat tolerance has been associated with HPA axis impairment [29]. More than half a century ago, thermal injury to the thermoregulatory centers of the hypothalamus was hypothesized to be the primary mechanism of mortality [30]. Indeed, according to a more recent review [9], ischemic and oxidative damage to the hypothalamus may be responsible for heatstroke. Severe heat stress increases cutaneous blood flow and metabolism and decreases splanchnic blood flow. Severe heat stress also decreases mean arterial pressure, increases intracranial pressure, and decreases cerebral perfusion pressure, all of which lead to cerebral ischemia and hypoxia. Compared with normothermic controls, rodents with heatstroke have higher values of cellular ischemia (e.g., glutamate and lactate-to-pyruvate ratio) and damage (e.g., glycerol) markers, prooxidant enzymes (e.g., lipid peroxidation and glutathione oxidation markers), proinflammatory cytokines (e.g., interleukin-1β and tumor necrosis factor-α), inducible nitric oxide-synthase-dependent nitric oxide and an indicator for the accumulation of polymorphonuclear leukocytes (e.g., myeloperoxidase activity) as well as neuronal damage (e.g., apoptosis and necrosis) in the hypothalamus after heat stroke. Hypothalamic values of antioxidant defenses (e.g., glutathione peroxidase and glutathione reductase), however, are lower. Melatonin therapy, in addition to attenuating heat-induced hypothermia (thermoregulatory deficits), significantly attenuates heat-induced inflammatory, ischemic and oxidative damage to the hypothalamus.
Furthermore, our present results demonstrated that melatonin therapy significantly enhanced HPA axis mechanisms (as reflected by increased plasma levels of both ACTH and corticosterone in response to heat stress), led to reduction of multiple organ dysfunction or failure (as reflected by decreased plasma levels of BUN, creatinine, ALT, AST, and ALP), and resulted in attenuation of lethality in heatstroke mice.
Our present results are consistent with many previous investigations. For example, melatonin improved the clinical outcome of the septic newborns as judged by measurement of sepsis-related serum parameters via reducing the serum levels of lipid peroxidation products [31]. A major brain metabolite of melatonin acts as a potent nitric oxide scavenger, inhibitor, and/or downregulator of neuronal and inducible nitric oxide synthase and as a mitochondrial metabolism modulator [32]. Melatonin protected against mitochondrial reactive oxygen species-mediated apoptosis in astrocytes [33] and isoproterenol-induced myocardial injury in the rat via its antioxidative mechanism [34]. In addition, melatonin protected the mitochondria from oxidative damage by preventing cardiolipin [35]. Melatonin also may attenuate peritonitis-induced lethality in conscious rats by exerting its antioxidant effect [36]. These observations prompted us to think that these multiple mitochondrial layers of protection provided by melatonin may be crucial for future therapeutic prevention and treatment of heatstroke.
Systemic inflammatory response syndrome is characterized by increased serum levels of TNF-α, ICAM-1, E-selectin, IL-1β, and IL-6 [37–40]. Increased levels of these systemic inflammatory response syndrome molecules have also been shown in patients [41] or rats [42]. In addition, the increased serum levels of these molecules during heatstroke in an anesthetized rat model could be reduced by melatonin treatment [7]. The present results further showed that the increased levels of some of these molecules in the hypothalamus in a mice could also be reduced by melatonin. Additionally, melatonin therapy increased the hypothalamic levels of IL-10 in heatstroke, which was believed to be anti-inflammatory cytokine [43]. These results indicate that melatonin may reduce heat-induced activated inflammation by reducing the levels of these systemic inflammatory response molecules in the hypothalamus. It is known that melatonin intensifies the expression of protective heat shock proteins [44] and that it enhances heat shock protein 27 expression [45] as well as having anti-inflammatory properties and antioxidant properties.
5. Conclusion
In summary, we report here that when untreated mice underwent heat stress, they displayed thermoregulatory deficit (e.g., animals display hypothermia during room temperature exposure), brain (or hypothalamic) inflammation, ischemia, and oxidative damage, HPA axis impairment, multiple organ dysfunction or failure, and lethality. Melatonin therapy may improve outcomes of heatstroke in mice by reducing brain inflammation and oxidative damage, and multiple organ dysfunction.
Conflict of Interests
The authors report no conflict of interests related to this study or the findings specified in this paper.
Authors' Contribution
Yu-Fong Tian and Cheng-Hsien Lin equally contributed to the work.
Acknowledgments
This study was supported in part by the National Science Council (Grant nos. NSC90-2314-B-384-006-MY2, NSC99-2314-B-384-004-MY3, and NSC101-2314-B-218-001-MY3) and the Department of Health and Blessing, the Center of Excellence for Clinical Trial and Research in Neuroscience of the Republic of China (Grant no. DOH99-TD-B-111-003).
References
- 1.Menendez-Pelaez A, Reiter RJ. Distribution of melatonin in mammalian tissues: the relative importance of nuclear versus cytosolic localization. Journal of Pineal Research. 1993;15(2):59–69. doi: 10.1111/j.1600-079x.1993.tb00511.x. [DOI] [PubMed] [Google Scholar]
- 2.Reiter RJ, Tan D. What constitutes a physiological concentration of melatonin? Journal of Pineal Research. 2003;34(1):79–80. doi: 10.1034/j.1600-079x.2003.2e114.x. [DOI] [PubMed] [Google Scholar]
- 3.Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. Journal of Pineal Research. 2011;51(1):1–16. doi: 10.1111/j.1600-079X.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen CF, Wang D, Reiter RJ, Yeh DY. Oral melatonin attenuates lung inflammation and airway hyperreactivity induced by inhalation of aerosolized pancreatic fluid in rats. Journal of Pineal Research. 2011;50(1):46–53. doi: 10.1111/j.1600-079X.2010.00808.x. [DOI] [PubMed] [Google Scholar]
- 5.Manda K, Ueno M, Anzai K. AFMK, a melatonin metabolite, attenuates X-ray-induced oxidative damage to DNA, proteins and lipids in mice. Journal of Pineal Research. 2007;42(4):386–393. doi: 10.1111/j.1600-079X.2007.00432.x. [DOI] [PubMed] [Google Scholar]
- 6.Hardeland R, Tan D, Reiter RJ. Kynuramines, metabolites of melatonin and other indoles: the resurrection of an almost forgotten class of biogenic amines. Journal of Pineal Research. 2009;47(2):109–126. doi: 10.1111/j.1600-079X.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- 7.Lin XJ, Mei GP, Liu J, et al. Therapeutic effects of melatonin on heatstroke-induced multiple organ dysfunction syndrome in rats. Journal of Pineal Research. 2011;50(4):436–444. doi: 10.1111/j.1600-079X.2011.00863.x. [DOI] [PubMed] [Google Scholar]
- 8.Wu WS, Chou MT, Chao CM, et al. Melatonin reduces acute lung inflammation, edema, and hemorrhage in heatstroke rats. Acta Pharmacologica Sinica. 2012;33(6):775–782. doi: 10.1038/aps.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen SH, Lin MT, Chang CP. Ischemic and oxidative damage to the hypothalamus may be responsible for heat shock. Current Neupharmacology. 2013;11(2):129–140. doi: 10.2174/1570159X11311020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee S, Premachandran S, Bagewadikar RS, Bhattacharya S, Chattopadhyay S, Poduval TB. Arginine metabolic pathways determine its therapeutic benefit in experimental heatstroke: role of Th1/Th2 cytokine balance. Nitric Oxide. 2006;15(4):408–416. doi: 10.1016/j.niox.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee S, Premachandran S, Sharma D, Bagewadikar RS, Poduval TB. Therapeutic treatment with L-arginine rescues mice from heat stroke-induced death: physiological and molecular mechanisms. Shock. 2005;24(4):341–347. doi: 10.1097/01.shk.0000180983.55623.2b. [DOI] [PubMed] [Google Scholar]
- 12.Chen ZC, Wu WS, Lin MT, Hsu CC. Protective effect of transgenic expression of porcine heat shock protein 70 on hypothalamic ischemic and oxidative damage in a mouse model of heatstroke. BMC Neuroscience. 2009;10:p. 111. doi: 10.1186/1471-2202-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crespo E, Macías M, Pozo D, et al. Melatonin inhibits expression of the inducible NO synthase II in liver and lung and prevents endotoxemia in lipopolysaccharide-induced multiple organ dysfunction syndrome in rats. FASEB Journal. 1999;13(12):1537–1546. [PubMed] [Google Scholar]
- 14.Wu CC, Chiao CW, Hsiao G, Chen A, Yen MH. Melatonin prevents endotoxin-induced circulatory failure in rats. Journal of Pineal Research. 2001;30(3):147–156. doi: 10.1034/j.1600-079x.2001.300303.x. [DOI] [PubMed] [Google Scholar]
- 15.Togashi H, Mori K, Ueno K, et al. Consecutive evaluation of nitric oxide production after transient cerebral ischemia in the rat hippocampus using in vivo brain microdialysis. Neuroscience Letters. 1998;240(1):53–57. doi: 10.1016/s0304-3940(97)00918-x. [DOI] [PubMed] [Google Scholar]
- 16.Hassoun HT, Kozar RA, Kone BC, Safi HJ, Moore FA. Intraischemic hypothermia differentially modulates oxidative stress proteins during mesenteric ischemia/reperfusion. Surgery. 2002;132(2):369–376. doi: 10.1067/msy.2002.125722. [DOI] [PubMed] [Google Scholar]
- 17.Wang JL, Ke DS, Lin MT. Heat shock pretreatment may protect against heatstroke-induced circulatory shock and cerebral ischemia by reducing oxidative stress and energy depletion. Shock. 2005;23(2):161–167. doi: 10.1097/01.shk.0000150779.47107.d5. [DOI] [PubMed] [Google Scholar]
- 18.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Analytical Biochemistry. 1969;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 19.Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Analytical Biochemistry. 1980;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 20.Mullane KM, Kraemer R, Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. Journal of Pharmacological Methods. 1985;14(3):157–167. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- 21.Wolf D, Schümann J, Koerber K, et al. Low-molecular-weight hyaluronic acid induces nuclear factorκB-dependent resistance against tumor necrosis factor α-mediated liver injury in mice. Hepatology. 2001;34(3):535–547. doi: 10.1053/jhep.2001.27218. [DOI] [PubMed] [Google Scholar]
- 22.Leon LR, Blaha MD, DuBose DA. Time course of cytokine, corticosterone, and tissue injury responses in mice during heat strain recovery. Journal of Applied Physiology. 2006;100(4):1400–1409. doi: 10.1152/japplphysiol.01040.2005. [DOI] [PubMed] [Google Scholar]
- 23.Nunn N, Womack M, Dart C, Barrett-Jolley R. Function and pharmacology of spinally-projecting sympathetic pre-autonomic neurones in the paraventricular nucleus of the hypothalamus. Current Neuropharmacology. 2011;9(2):262–277. doi: 10.2174/157015911795596531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McEwen BS. Stressed or stressed out: what is the difference? Journal of Psychiatry and Neuroscience. 2005;30(5):315–318. [PMC free article] [PubMed] [Google Scholar]
- 25.Joo LC, Klein R, Owens NC, Mathai M, McKinley M, Badoer E. Activation of spinally projecting and nitrergic neurons in the PVN following heat exposure. American Journal of Physiology. 2006;291(1):R91–R101. doi: 10.1152/ajpregu.00675.2005. [DOI] [PubMed] [Google Scholar]
- 26.Tsay HJ, Li HY, Lin CH, Yang Y, Yeh J, Lin M. Heatstroke induces c-fos expression in the rat hypothalamus. Neuroscience Letters. 1999;262(1):41–44. doi: 10.1016/s0304-3940(99)00030-0. [DOI] [PubMed] [Google Scholar]
- 27.Cure M. Plasma corticosterone response in continuous versus discontinuous chronic heat exposure in rat. Physiology and Behavior. 1989;45(6):1117–1122. doi: 10.1016/0031-9384(89)90097-8. [DOI] [PubMed] [Google Scholar]
- 28.Djordjević J, Cvijić G, Davidović V. Different activation of ACTH and corticosterone release in response to various stressors in rats. Physiological Research. 2003;52(1):67–72. [PubMed] [Google Scholar]
- 29.Michel V, Peinnequin A, Alonso A, Buguet A, Cespuglio R, Canini F. Decreased heat tolerance is associated with hypothalamo-pituitary-adrenocortical axis impairment. Neuroscience. 2007;147(2):522–531. doi: 10.1016/j.neuroscience.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 30.Malamud N, Haymaker W, Custer RP. Heat stroke. Military Surgery. 1946;99(5):397–449. [PubMed] [Google Scholar]
- 31.Gitto E, Karbownik M, Reiter RJ, et al. Effects of melatonin treatment in septic newborns. Pediatric Research. 2001;50(6):756–760. doi: 10.1203/00006450-200112000-00021. [DOI] [PubMed] [Google Scholar]
- 32.Hardeland R, Backhaus C, Fadavi A. Reactions of the NO redox forms NO+, •NO and HNO (protonated NO-) with the melatonin metabolite N1-acetyl-5-methoxykynuramine. Journal of Pineal Research. 2007;43(4):382–388. doi: 10.1111/j.1600-079X.2007.00489.x. [DOI] [PubMed] [Google Scholar]
- 33.Jou M, Peng T, Hsu L, et al. Visualization of melatonin’s multiple mitochondrial levels of protection against mitochondrial Ca2+-mediated permeability transition and beyond in rat brain astrocytes. Journal of Pineal Research. 2010;48(1):20–38. doi: 10.1111/j.1600-079X.2009.00721.x. [DOI] [PubMed] [Google Scholar]
- 34.Mukherjee D, Roy SG, Bandyopadhyay A, et al. Melatonin protects against isoproterenol-induced myocardial injury in the rat: antioxidative mechanisms. Journal of Pineal Research. 2010;48(3):251–262. doi: 10.1111/j.1600-079X.2010.00749.x. [DOI] [PubMed] [Google Scholar]
- 35.Paradies G, Petrosillo G, Paradies V, Reiter RJ, Ruggiero FM. Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. Journal of Pineal Research. 2010;48(4):297–310. doi: 10.1111/j.1600-079X.2010.00759.x. [DOI] [PubMed] [Google Scholar]
- 36.Wu JY, Tsou MY, Chen TH, Chen SJ, Tsao CM, Wu CC. Therapeutic effects of melatonin on peritonitis-induced septic shock with multiple organ dysfunction syndrome in rats. Journal of Pineal Research. 2008;45(1):106–116. doi: 10.1111/j.1600-079X.2008.00567.x. [DOI] [PubMed] [Google Scholar]
- 37.Morecroft JA, Spitz L. The role of inflammatory mediators in necrotizing enterocolitis. Seminars in Neonatology. 1997;2(4):273–280. [Google Scholar]
- 38.Boldt J, Wollbruck M, Kuhn D, Linke LC, Hempelmann G. Do plasma levels of circulating soluble adhesion molecules differ between surviving and nonsurviving critically ill patients? Chest. 1995;107(3):787–792. doi: 10.1378/chest.107.3.787. [DOI] [PubMed] [Google Scholar]
- 39.Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88(9):3259–3287. [PubMed] [Google Scholar]
- 40.Goda K, Tanaka T, Monden M, Miyasaka M. Characterization of an apparently conserved epitope in E- and P-selectin identified by dual-specific monoclonal antibodies. European Journal of Immunology. 1999;29(5):1551–1560. doi: 10.1002/(SICI)1521-4141(199905)29:05<1551::AID-IMMU1551>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 41.Bouchama A, Knochel JP. Medical progress: heat stroke. New England Journal of Medicine. 2002;346(25):1978–1988. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- 42.Liu WS, Chen CT, Foo NH, et al. Human umbilical cord blood cells protect against hypothalamic apoptosis and systemic inflammation response during heatstroke in rats. Pediatrics and Neonatology. 2009;50(5):208–216. doi: 10.1016/S1875-9572(09)60065-6. [DOI] [PubMed] [Google Scholar]
- 43.Standiford TJ, Strieter RM, Lukacs NW, Kunkel SL. Neutralization of IL-10 increases lethality in endotoxemia: cooperative effects of macrophage inflammatory protein-2 and tumor necrosis factor. Journal of Immunology. 1995;155(4):2222–2229. [PubMed] [Google Scholar]
- 44.Bonior J, Jaworek J, Konturek SJ, Pawlik WW. Increase of heat shock protein gene expression by melatonin in AR42J cells. Journal of Physiology and Pharmacology. 2005;56(3):471–481. [PubMed] [Google Scholar]
- 45.Cabrera J, Quintana J, Reiter RJ, Loro J, Cabrera F, Estévez F. Melatonin prevents apoptosis and enhances HSP27 mRNA expression induced by heat shock in HL-60 cells: possible involvement of the MT2 receptor. Journal of Pineal Research. 2003;35(4):231–238. doi: 10.1034/j.1600-079x.2003.00071.x. [DOI] [PubMed] [Google Scholar]


