Abstract
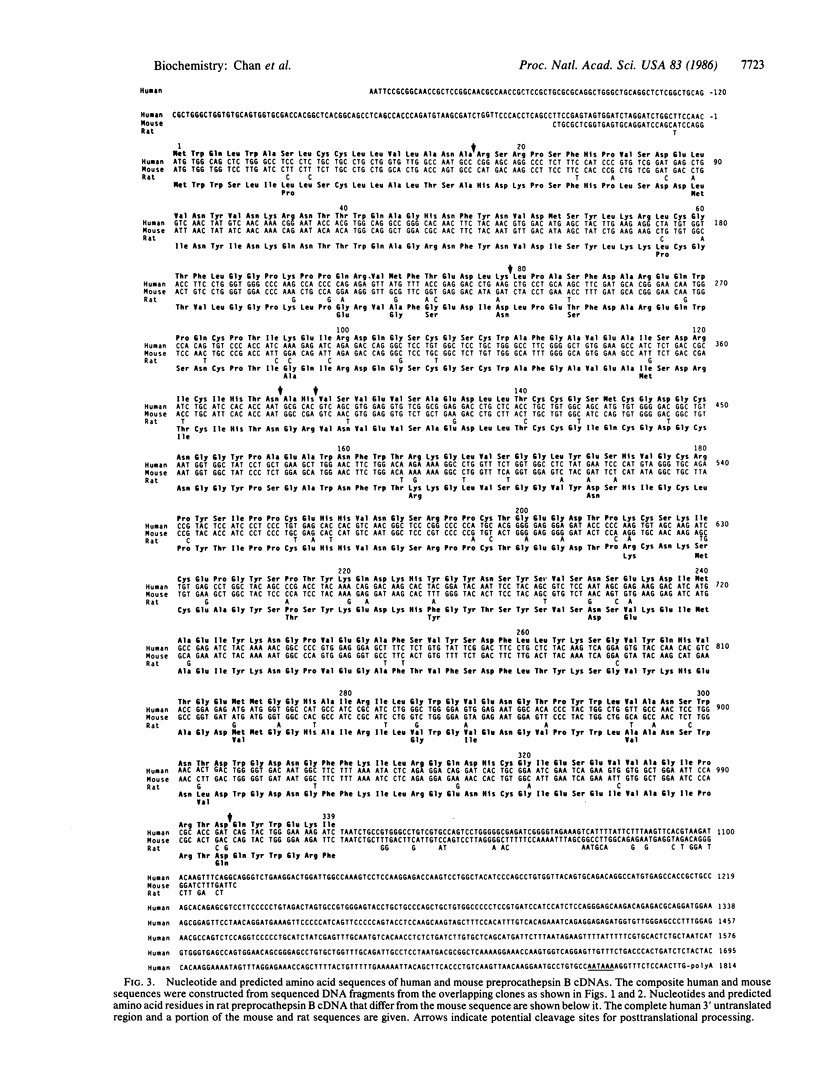
Cathepsin B is a lysosomal thiol proteinase that may have additional extralysosomal functions. To further our investigations on the structure, mode of biosynthesis, and intracellular sorting of this enzyme, we have determined the complete coding sequences for human and mouse preprocathepsin B by using cDNA clones isolated from human hepatoma and kidney phage libraries. The nucleotide sequences predict that the primary structure of preprocathepsin B contains 339 amino acids organized as follows: a 17-residue NH2-terminal prepeptide sequence followed by a 62-residue propeptide region, 254 residues in mature (single chain) cathepsin B, and a 6-residue extension at the COOH terminus. A comparison of procathepsin B sequences from three species (human, mouse, and rat) reveals that the homology between the propeptides is relatively conserved with a minimum of 68% sequence identity. In particular, two conserved sequences in the propeptide that may be functionally significant include a potential glycosylation site and the presence of a single cysteine at position 59. Comparative analysis of the three sequences also suggests that processing of procathepsin B is a multistep process, during which enzymatically active intermediate forms may be generated. The availability of the cDNA clones will facilitate the identification of possible active or inactive intermediate processive forms as well as studies on the transcriptional regulation of the cathepsin B gene.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bienkowski R. S. Intracellular degradation of newly synthesized secretory proteins. Biochem J. 1983 Jul 15;214(1):1–10. doi: 10.1042/bj2140001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carne A., Moore C. H. The amino acid sequence of the tryptic peptides from actinidin, a proteolytic enzyme from the fruit of Actinidia chinensis. Biochem J. 1978 Jul 1;173(1):73–83. doi: 10.1042/bj1730073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. J., Episkopou V., Zeitlin S., Karathanasis S. K., MacKrell A., Steiner D. F., Efstratiadis A. Guinea pig preproinsulin gene: an evolutionary compromise? Proc Natl Acad Sci U S A. 1984 Aug;81(16):5046–5050. doi: 10.1073/pnas.81.16.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty K., Carroll R., Steiner D. F. Identification of a 31,500 molecular weight islet cell protease as cathepsin B. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3245–3249. doi: 10.1073/pnas.80.11.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty K., Hutton J. C., Steiner D. F. Cathepsin B-related proteases in the insulin secretory granule. J Biol Chem. 1984 May 25;259(10):6041–6044. [PubMed] [Google Scholar]
- Docherty K., Steiner D. F. Post-translational proteolysis in polypeptide hormone biosynthesis. Annu Rev Physiol. 1982;44:625–638. doi: 10.1146/annurev.ph.44.030182.003205. [DOI] [PubMed] [Google Scholar]
- Dufek V., Matous B., Král V., Bures L. Characterization of cathepsin B-like proteinase from ascitic fluid of patients with primary liver cancer. Neoplasma. 1984;31(5):581–590. [PubMed] [Google Scholar]
- Fong D., Calhoun D. H., Hsieh W. T., Lee B., Wells R. D. Isolation of a cDNA clone for the human lysosomal proteinase cathepsin B. Proc Natl Acad Sci U S A. 1986 May;83(9):2909–2913. doi: 10.1073/pnas.83.9.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf M., Baici A., Sträuli P. Histochemical localization of cathepsin B at the invasion front of the rabbit V2 carcinoma. Lab Invest. 1981 Dec;45(6):587–596. [PubMed] [Google Scholar]
- Lawrence J. B., Singer R. H. Quantitative analysis of in situ hybridization methods for the detection of actin gene expression. Nucleic Acids Res. 1985 Mar 11;13(5):1777–1799. doi: 10.1093/nar/13.5.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mort J. S., Leduc M. S., Recklies A. D. Characterization of a latent cysteine proteinase from ascitic fluid as a high molecular weight form of cathepsin B. Biochim Biophys Acta. 1983 Feb 22;755(3):369–375. doi: 10.1016/0304-4165(83)90240-4. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. The pathway of eukaryotic mRNA formation. Annu Rev Biochem. 1983;52:441–466. doi: 10.1146/annurev.bi.52.070183.002301. [DOI] [PubMed] [Google Scholar]
- Ohno S., Emori Y., Imajoh S., Kawasaki H., Kisaragi M., Suzuki K. Evolutionary origin of a calcium-dependent protease by fusion of genes for a thiol protease and a calcium-binding protein? Nature. 1984 Dec 6;312(5994):566–570. doi: 10.1038/312566a0. [DOI] [PubMed] [Google Scholar]
- Olstein A. D., Liener I. E. Comparative studies of mouse liver cathepsin B and an analogous tumor thiol proteinase. J Biol Chem. 1983 Sep 25;258(18):11049–11056. [PubMed] [Google Scholar]
- Poole A. R., Tiltman K. J., Recklies A. D., Stoker T. A. Differences in secretion of the proteinase cathepsin B at the edges of human breast carcinomas and fibroadenomas. Nature. 1978 Jun 15;273(5663):545–547. doi: 10.1038/273545a0. [DOI] [PubMed] [Google Scholar]
- Ritonja A., Popovic T., Turk V., Wiedenmann K., Machleidt W. Amino acid sequence of human liver cathepsin B. FEBS Lett. 1985 Feb 11;181(1):169–172. doi: 10.1016/0014-5793(85)81136-4. [DOI] [PubMed] [Google Scholar]
- San Segundo B., Chan S. J., Steiner D. F. Differences in cathepsin B mRNA levels in rat tissues suggest specialized functions. FEBS Lett. 1986 Jun 9;201(2):251–256. doi: 10.1016/0014-5793(86)80618-4. [DOI] [PubMed] [Google Scholar]
- San Segundo B., Chan S. J., Steiner D. F. Identification of cDNA clones encoding a precursor of rat liver cathepsin B. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2320–2324. doi: 10.1073/pnas.82.8.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Urdea M. S. Use of unpurified synthetic deoxynucleotide primers for rapid dideoxynucleotide chain termination sequencing. DNA. 1984 Aug;3(4):339–343. doi: 10.1089/dna.1.1984.3.339. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sloane B. F., Dunn J. R., Honn K. V. Lysosomal cathepsin B: correlation with metastatic potential. Science. 1981 Jun 5;212(4499):1151–1153. doi: 10.1126/science.7233209. [DOI] [PubMed] [Google Scholar]
- Sly W. S., Fischer H. D. The phosphomannosyl recognition system for intracellular and intercellular transport of lysosomal enzymes. J Cell Biochem. 1982;18(1):67–85. doi: 10.1002/jcb.1982.240180107. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Docherty K., Carroll R. Golgi/granule processing of peptide hormone and neuropeptide precursors: a minireview. J Cell Biochem. 1984;24(2):121–130. doi: 10.1002/jcb.240240204. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Dehdarani A. H., Schmidt P. G., Tang J. Cathepsins B and H from porcine spleen. Purification, polypeptide chain arrangements, and carbohydrate content. J Biol Chem. 1984 Aug 10;259(15):9874–9882. [PubMed] [Google Scholar]
- Takio K., Towatari T., Katunuma N., Teller D. C., Titani K. Homology of amino acid sequences of rat liver cathepsins B and H with that of papain. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3666–3670. doi: 10.1073/pnas.80.12.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet J. R., Fukushima H., Dewji N. N., Wilcox E., O'Brien J. S., Helinski D. R. Chromogenic immunodetection of human serum albumin and alpha-L-fucosidase clones in a human hepatoma cDNA expression library. DNA. 1984 Dec;3(6):437–447. doi: 10.1089/dna.1.1984.3.437. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]



