Abstract
Objective
To assess the association of total isoflavone intake with ovulatory function, including sporadic anovulation in healthy premenopausal women.
Design
Prospective cohort study.
Setting
University.
Patient(s)
Participants included 259 healthy regularly menstruating women aged 18–44 years.
Intervention(s)
None.
Main Outcome Measure(s)
Serum concentrations of E2, free E2, P, LH, FSH, and SHBG and sporadic anovulation in healthy premenopausal women.
Result(s)
Isoflavone intake was not associated with E2, free E2, P, LH, and FSH concentrations. Consumption in the highest quartile (Q4: 1.6–78.8 mg/d) was significantly associated with greater SHBG concentrations (β = 0.09; 95% confidence interval [CI] 0.02–0.16), compared with the first quartile (Q1: 0.0–0.3 mg/d).
Conclusion(s)
Isoflavone intake was not associated with sporadic anovulation (Q4 vs. Q1: odds ratio 0.87, 95% CI 0.32–1.66). Dietary isoflavone intake among young premenopausal women was not related to sex hormone concentrations or anovulation, but was associated with minimally increased SHBG concentrations. These results suggest potential endocrine effects with no subsequent effects on ovulation, easing concerns regarding their impacts on fertility.
Keywords: Isoflavone, anovulation, sex hormones, nutrition
Soy products are the major food source of isoflavones, a class of phytoestrogens, and the most abundant group of plant polyphenolics. Consumption of soy foods has increased in the United States owing to potential health benefits associated with isoflavone intake, such as lower incidences of coronary heart disease, atherosclerosis, and type 2 diabetes and decreased risk of certain hormone-dependent cancers (1). Because soy flour and soy protein isolates may be added to processed meats, meat substitutes, breads, and protein bars, isoflavones are also found in >60% of processed foods (2).
Isoflavone intake is of particular interest to pre- and postmenopausal women, because isoflavones are structurally similar to endogenous estrogen and have weak estrogenic and antiestrogenic effects. Isoflavones can bind and activate both estrogen receptors α and β, which are found in various reproductive tissues (3). Once bound, isoflavones may influence estrogen action by directly affecting the transcription of estrogen-regulated gene products (4), or by affecting various enzymes involved in steroid metabolism, such as aromatase, 17β-hyroxysteroid dehydrogenases, or steroid sulfatases and sulfotransferases (5). Increased exposure to exogenous estrogenic compounds such as isoflavones could result in increased signaling in these tissues, promoting the positive and negative feedback actions of estrogen and other sex hormones.
Numerous epidemiologic and clinical studies in women have evaluated the relationship between isoflavone consumption and circulating sex hormones (5). Available data suggest that isoflavones may have potential inhibitory effects on the likelihood of ovulation through reductions in circulating LH concentrations (5). However, available data are inconsistent, and studies among premenopausal women with longitudinal measures of sex hormones across the menstrual cycle are sparse. Therefore, the objective of the present study was to evaluate the association of usual dietary isoflavone consumption with sex hormone concentrations and SHBG as well as incident anovulation in a cohort of 259 healthy premenopausal women in the BioCycle study with hormone measurements timed to specific phases of the menstrual cycle.
MATERIALS AND METHODS
Study Design
The BioCycle study was a prospective cohort study of 259 regularly menstruating, healthy, premenopausal women aged 18–44 years. Participants were recruited from the western New York region and followed for up to two menstrual cycles. Of 449 women who were screened, 318 met the eligibility criteria, of which 276 enrolled. Seventeen women (6%) withdrew before completing the study (6). Reasons for withdrawal included scheduling conflicts (n = 8), lost to follow-up (n = 4), inability to tolerate blood draws (n = 3), inability to participate owing to illness (n = 1), or loss of interest (n = 1). Exclusion criteria included: use of oral contraceptives currently or in the 3 months before study entry; chronic use of certain medications, e.g., lipid-lowering drugs, antihypertensive medications, and/or aspirin; plans to consume a restricted diet in the next 3 months; diagnosis of certain chronic conditions, including history of menstrual and ovulation disorders; and a self-reported body mass index (BMI) of <18 kg/m2 or >35 kg/m2 at screening. Women could not be planning pregnancy over the study time period. Further details have been reported elsewhere (6).
Women were seen in the clinic at up to eight visits per cycle for up to two cycles, with visits timed with the use of fertility monitors (Clearblue Easy Fertility Monitor; Inverness Medical) to correspond to menstruation, midfollicular, late follicular, and LH/FSH surges; ovulation; and early luteal, midluteal, and late luteal phases (7). Study visits were scheduled with the use of an algorithm accounting for each woman’s self-reported cycle length, with the day of the midcycle visits adjusted based on indications of peak fertility on the monitor. Participants were highly compliant with the study protocol, with 94% completing seven or eight clinic visits per cycle. Data collection occurred from 2005 to 2007 at the University at Buffalo in New York. The University at Buffalo Health Sciences Institutional Review Board (IRB) approved the study and served as the IRB designated by the National Institutes of Health for this study under a reliance agreement. Each of the participants provided written informed consent.
Dietary Assessment
Dietary intake was assessed with the use of multiple 24-hour dietary recalls (24HDR), with information collected up to four times per cycle, corresponding to menses, midfollicular phase, ovulation, and midluteal phase. Dietary intake data were collected and analyzed with the use of the Nutrition Data System for Research (NDSR) software, version 2005, developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis. This program computed food sources (e.g., polyunsaturated vegetable fat-filled milk), food components (e.g., soy milk), and nutrients (e.g., isoflavones). The NDSR uses the USDA Database for the Isoflavone Content of Selected Foods, release 2.0 (8), for food and nutrient composition of isoflavones. Values in the database were converted to milligrams of aglycone of the following isoflavones: daidzein, genistein, glycitein, biochanin A, and formonontein. Total isoflavone intake refers to the sum of daidzein, genistein, glycitein, biochanin A, and formonontein. Ninety-six percent of the participants completed four 24HDRs in at least one of their cycles under study; 99% completed at least three 24HDRs, and all of the women completed at least two 24HDRs in both cycles.
Hormone Assessment
Total E2, P, LH, FSH, and SHBG were measured in fasting serum samples with the use of solid-phase competitive chemiluminescent enzymatic immunoassay (Immulite 2000). The albumin assay was tested with the Beckman LX20 autoanalyzer using bromocresol purple methods. Calculation of free E2 (i.e., bioavailable E2) was performed via the equation proposed by Sodergard et al. (9) using total E2, SHBG, and albumin concentrations. All hormonal assays were conducted by Kaleida Health Laboratories (6). Across the study period, the interassay coefficients of variation reported by the laboratory for E2, SHBG, LH, FSH, albumin, and P were < 10%, < 10%, < 5%, < 5%, < 5%, and < 14%, respectively.
Cycles were classified as anovulatory if the P concentration was ≤ 5 ng/mL throughout the entire cycle and if no serum LH peak was observed on the later cycle phase visits (10). This approach was used as a means of conservatively defining anovulation in this cohort. If P levels were low, and there was a chance of cycle visit misclassification and that the P assessment was not made during the luteal phase, then that cycle was classified as ovulatory. Based on that definition, 35 of the 259 (14%) women were considered to be anovulatory, including 28 (11%) who had one anovulatory cycle and seven (3%) who had two anovulatory cycles.
Covariate Assessment
At study enrollment, measured height and weight were obtained by trained study staff using standardized protocols and used to calculate BMI, and data on potential confounders, such as age, race, physical activity, past oral contraceptive use, education, smoking, and reproductive history, were obtained via questionnaires (6). All covariates had < 5% total missing data. Cycle length was defined as the number of days from the first day of bleeding until the day before the next onset of bleeding after confirming two consecutive days of bleeding to differentiate from spotting. Day 1 of the menstrual cycle was defined as menstruating by 4:00 p.m. on that day.
To account for possible effects of overall diet quality, we calculated the alternative Mediterranean Diet (aMED) Score (11) for each woman in the study. The aMED score, a scale adapted from the traditional Mediterranean Diet Score developed by Trichopoulou et al., is based on the dietary intake of nine components, including vegetables (excluding potatoes), legumes, fruit, nuts, whole grains, red and processed meat, fish, alcohol, and the ratio of monounsaturated fat to saturated fat. Possible scores on the aMED range from 0 to 9; higher scores correspond to greater adherence. Because each participant had more than one dietary recall, we calculated the average of each of the aMED dietary components across all of the recalls per participant to calculate the aMED scores (12, 13).
Statistical Analysis
Linear mixed models were used to compare dietary intake by visit to determine whether isoflavone intake changed significantly over the cycle. No significant differences in isoflavone intake were observed across each cycle; therefore, the mean daily intake per cycle was calculated for isoflavone intake and other dietary variables. Isoflavone intake was assessed continuously and as quartiles. Descriptive statistics for continuous and categorical covariates were compared between quartiles of intake and ovulatory status with the use of analysis of variance, chi-square tests, or Fisher exact test as appropriate. Sex hormones and SHBG were log transformed for statistical analyses. Geometric means and inter-quartile ranges (IQRs) were calculated for isoflavone intake and sex hormones across the cycle. Analysis of P was restricted to the luteal phase owing to its low variability during the follicular phase, and FSH and LH were restricted to the three visits around ovulation.
Linear mixed models on the log scale of the hormones were used to evaluate the association between hormone concentrations (E2, free E2, luteal P, ovulatory LH and FSH, and SHBG) and quartile of isoflavone intake per cycle (14). Random intercepts accounted for the variation in baseline hormone concentrations between women and the correlation between visits of the same woman. Potential confounders were identified with the use of directed acyclic graphs and a change in point estimates approach (15). Covariates were included in the model if they changed the exposure coefficient by >15%. Age, BMI, race (white, black, Asian, other), fiber, energy, vegetable protein, and omega-3 intakes were included in the final models. Additional covariates, including physical activity, past oral contraceptive use, dietary intakes such as animal protein, cholesterol, fat (polyunsaturated fatty acid [PUFA], monoun-saturated fatty acid [MUFA]), and the aMED score were considered but did not appreciably alter the estimates.
We present three models: model 1 (unadjusted), model 2 (adjusted for age, race, BMI, fiber, energy, vegetable protein, and omega-3 intakes), and model 3 (adjusted for variables in models 2 as well as other sex hormones through the use of inverse probability of exposure weights). Owing to the time-dependent confounding nature of sex hormones over the cycle, we used marginal structural models in model 3 to evaluate the association between isoflavone intake and log serum hormone concentrations after adjusting for the complex feedback mechanisms of the other hormones (16). Weighted linear mixed models with inverse probability of exposure weights were used to estimate the parameters of the marginal structural model. Stabilized weights for each cycle visit when diet information was collected were obtained with the use of ordinal logistic regression for the exposure quartiles of isoflavone intake.
Generalized estimating equations were used to model the association between quartiles of isoflavone intake and the odds of anovulation both unadjusted and adjusted for age, BMI, race (white, black, Asian/other), fiber, energy, vegetable protein, and omega-3 intakes. Women in the other and Asian race categories were collapsed for the generalized estimating equations owing to model instability (the “Asian/other” category was 72% Asian). All analyses were carried out with the use of SAS version 9.2 (SAS Institute).
RESULTS
Women in the BioCycle study were on average 27.3 years old with an average BMI of 24.1 kg/m2, moderate to highly physically active (90.5%), and nonsmokers (96.1%), and they had an arithmetic mean total isoflavone intake of 2.8 mg/d (geometric mean 0.86 mg/d, IQR 0.33–1.9; Table 1). There were no significant differences in age, BMI, years of education, past oral contraceptive use, or history of smoking status across quartiles of isoflavone intake. Women who identified as Asian were more likely to consume higher intakes of isoflavones, with 44% of Asians categorized in the highest quartile of intake. There were no significant differences between most dietary variables and isoflavone intake. However, women in the highest quartile of total isoflavone intake had significantly lower total dietary cholesterol, and significantly higher levels of total fiber compared with the lowest quartile. Daidzein and genistein were the predominant isoflavones consumed in the diet (Table 1) and came primarily from soy milk, vegetarian meat substitutes (e.g., veggie burgers, soybeans, and tofu) and granola/meal replacement bars (Fig. 1).
TABLE 1.
Characteristics of women enrolled in the BioCycle study by quartile of total dietary isoflavone intake.
| Total | Quartile of isoflavone intake | P value | ||||
|---|---|---|---|---|---|---|
| Q1 (0.0–0.3 mg/d) | Q2 (0.3–0.6 mg/d) | Q3 (0.6–1.6 mg/d) | Q4 (1.6–78.8 mg/d) | |||
| No. of participants | 259 | 64 | 65 | 65 | 65 | |
| Demographics/lifestyle | ||||||
| Age (y) | 27.3 ± 8.2 | 27.1 ± 8.7 | 26.4 ± 7.9 | 28.4 ± 8.1 | 27.3 ± 8.1 | .59 |
| BMI (kg/m2) | 24.1 ± 3.9 | 24.4 ± 3.8 | 23.9 ± 3.7 | 24.5 ± 4.5 | 23.4 ± 3.3 | .32 |
| Physical activity [n (%)] | .94 | |||||
| Low | 25 (9.7) | 6 (9.4) | 5 (7.7) | 6 (9.2) | 8 (12.3) | |
| Moderate | 92 (35.5) | 22 (34.4) | 24 (36.9) | 21 (32.3) | 25 (38.5) | |
| High | 142 (54.8) | 36 (56.2) | 36 (55.4) | 38 (58.5) | 32 (49.2) | |
| Race | .02 | |||||
| White | 154 (59.4) | 32 (50.0) | 43 (66.1) | 41 (63.0) | 38 (58.4) | |
| Black | 51 (19.7) | 21 (32.8) | 13 (20.0) | 10 (15.4) | 7 (10.8) | |
| Asian | 39 (15.1) | 8 (12.5) | 7 (10.8) | 7 (10.8) | 17 (26.2) | |
| Other | 15 (5.8) | 3 (4.7) | 2 (3.1) | 7 (10.8) | 3 (4.6) | |
| Years of education | .33 | |||||
| ≤ High school | 33 (12.7) | 9 (14.1) | 10 (15.4) | 10 (15.4) | 4 (6.2) | |
| Postsecondary | 226 (87.3) | 55 (85.9) | 55 (84.6) | 55 (84.6) | 61 (93.4) | |
| History of smoking | 10 (3.9) | 1 (1.6) | 1 (1.5) | 5 (7.7) | 3 (4.6) | .29 |
| Pact OC use | 140 (54.9) | 32 (50.0) | 35 (56.5) | 43 (67.2) | 30 (46.2) | .08 |
| Dietary variables | ||||||
| Isoflavones (mg/d) | ||||||
| Daidzein | 0.31 (0.11–0.69) | 0.06 (0.04–0.09) | 0.17 (0.13–0.23) | 0.33 (0.27–0.48) | 2.35 (1.24–4.43) | <.0001 |
| Genistein | 0.34 (0.11–0.88) | 0.07 (0.05–0.12) | 0.19 (0.14–0.27) | 0.33 (0.24–0.55) | 3.02 (1.62–5.41) | <.0001 |
| Glycitein | 0.06 (0.02–0.20) | 0.01 (0.01–0.02) | 0.02 (0.01–0.04) | 0.04 (0.02–0.09) | 0.50 (0.24–1.14) | <.0001 |
| Biochanin | 0.11 (0.04–0.37) | 0.04 (0.02–0.01) | 0.06 (0.03–0.14) | 0.22 (0.09–0.64) | 0.16 (0.07–0.42) | <.0001 |
| Formononetin | 0.005 (0.001–0.01) | 0.004 (0.001–0.01) | 0.004 (0.001–0.01) | 0.006 (0.002–0.01) | 0.007 (0.002–0.02) | .15 |
| Total isoflavones | 0.86 (0.33–1.90) | 0.17 (0.13–0.25) | 0.47 (0.39–0.58) | 1.02 (0.82–1.23) | 6.31 (3.18–10.49) | <.0001 |
| Total energy (kcal) | 1607.4 ± 354.3 | 1530.9 ± 303.7 | 1612.1 ± 350.7 | 1600.7 ± 363.5 | 1684.9 ± 385.0 | .11 |
| Total fat (%) | 33.9 ± 5.4 | 34.5 ± 5.5 | 34.8 ± 5.0 | 33.3 ± 6.0 | 33.0 ± 5.1 | .14 |
| Carbohydrate (%) | 50.8 ± 7.1 | 51.3 ± 6.7 | 49.1 ± 6.4 | 50.5 ± 8.3 | 54.4 ± 6.7 | .06 |
| Protein (%) | 15.7 ± 2.9 | 15.0 ± 2.7 | 16.0 ± 2.8 | 16.1 ± 2.9 | 15.9 ± 3.3 | .10 |
| Total fat (g) | 62.1 ± 18.5 | 60.6 ± 17.9 | 63.8 ± 18.2 | 60.3 ± 17.9 | 63.9 ± 20.2 | .55 |
| SFA | 21.3 ± 7.5 | 20.9 ± 7.4 | 22.0 ± 7.1 | 20.8 ± 6.9 | 21.4 ± 8.7 | .81 |
| MUFA | 23.1 ± 7.0 | 22.4 ± 6.9 | 23.8 ± 6.8 | 22.4 ± 7.1 | 23.7 ± 7.3 | .50 |
| PUFA | 12.8 ± 4.3 | 21.3 ± 4.2 | 13.0 ± 4.3 | 12.2 ± 3.9 | 13.9 ± 4.7 | .10 |
| Omega-3 | 1.4 ± 0.5 | 1.3 ± 0.6 | 1.4 ± 0.5 | 1.3 ± 0.4 | 1.5 ± 0.6 | .05 |
| Total carbohydrate (g) | 201.1 ± 49.0 | 192.8 ± 40.2 | 195.0 ± 49.3 | 199.9 ± 52.0 | 216.6 ± 50.9 | .02 |
| Total protein (g) | 62.2 ± 15.6 | 56.5 ± 13.6 | 63.4 ± 13.4 | 63.4 ± 17.3 | 65.4 ± 16.7 | .007 |
| Animal protein | 40.7 ± 14.4 | 38.1 ± 13.7 | 43.8 ± 10.4 | 43.0 ± 15.7 | 37.7 ± 16.4 | .02 |
| Vegetable protein | 21.4 ± 7.4 | 18.3 ± 5.8 | 19.4 ± 5.1 | 20.3 ± 6.0 | 27.5 ± 8.5 | <.0001 |
| Cholesterol (g) | 209.8 ± 98.0 | 189.9 ± 89.3 | 234.6 ± 106.4 | 216.5 ± 94.1 | 198.0 ± 97.5 | .04 |
| Alcohol (g) | 2.7 ± 5.2 | 1.2 ± 2.4 | 3.5 ± 5.3 | 3.7 ± 7.2 | 2.2 ± 4.3 | .02 |
| Fructose (g) | 16.9 ± 9.0 | 17.9 ± 10.3 | 17.5 ± 9.2 | 16.2 ± 9.1 | 16.2 ± 7.2 | .61 |
| Total fiber (g) | 13.6 ± 5.6 | 11.5 ± 4.8 | 12.4 ± 3.9 | 13.0 ± 4.5 | 17.3 ± 6.8 | <.0001 |
| Soluble fiber | 3.8 ± 1.3 | 3.3 ± 1.1 | 3.5 ± 1.1 | 3.6 ± 1.0 | 4.6 ± 1.5 | <.0001 |
| Insoluble fiber | 9.6 ± 4.4 | 8.1 ± 3.8 | 8.6 ± 3.0 | 9.3 ± 3.7 | 12.4 ± 5.4 | <.0001 |
| Hormones | ||||||
| E2 (pg/mL) | 82.4 (65.2–107.5) | 86.4 (69.2–112.5) | 81.1 (67.2–102.9) | 83.9 (64.9–110.9) | 82.8 (60.8–98.3) | .44 |
| Luteal P (ng/mL) | 4.4 (3.4–7.3) | 3.9 (3.4–6.2) | 4.6 (4.0–8.1) | 5.1 (4.1–8.4) | 3.9 (2.4–7.0) | .17 |
| Ovulatory LH (ng/mL) | 10.1 (7.7–13.3) | 9.6 (7.4–12.3) | 10.9 (8.2–15.1) | 10.4 (7.7–13.5) | 9.5 (7.0–11.9) | .17 |
| Ovulatory FSH (mIU/mL) | 6.7 (5.4–8.0) | 6.5 (5.3–7.6) | 7.1 (5.6–8.2) | 7.0 (5.4–8.8) | 6.3 (5.2–7.6) | .11 |
| SHBG (nmol/L) | 43.0 (31.9–60.2) | 41.5 (30.4–57.5) | 43.0 (34.0–62.0) | 43.7 (34.4–58.6) | 43.6 (33.1–63.7) | .92 |
| Free E2 (pg/mL) | 1.3 (1.1–1.6) | 1.4 (1.1–1.7) | 1.3 (1.0–1.5) | 1.3 (1.0–1.6) | 1.2 (1.0–1.4) | .10 |
| Cycle characteristics | ||||||
| Cycle length (d) | 28.9 ± 3.6 | 28.5 ± 3.6 | 29.5 ± 4.3 | 28.2 ± 3.1 | 29.3 ± 3.3 | .14 |
| Anovulatory cyclea [n (%)] | 35 (13.5) | 9 (14.1) | 5 (7.7) | 8 (12.3) | 13 (20.0) | .23 |
Note: P values for continuous variables calculated with the use of analysis of variance and chi-square tests and for categoric variables with the use of Fisher exact test. All comparisons take repeated measures and correlations between cycles into account. Sex hormones and SHBG were log transformed for normality for statistical analyses. Values are presented as mean ± SD, geometric mean (interquartile range), or n (%).
BMI = body mass index; MUFA = monounsaturated fatty acid; OC = oral contraceptive; PUFA = polyunsaturated fatty acid; SFA = saturated fatty acid.
Defined as having at least one anovulatory cycle over the two-cycle study period: 28 had one anovulatory cycle; 7 had two anovulatory cycles.
Filiberto. Isoflavones and sex hormones. Fertil Steril 2013.
FIGURE 1.
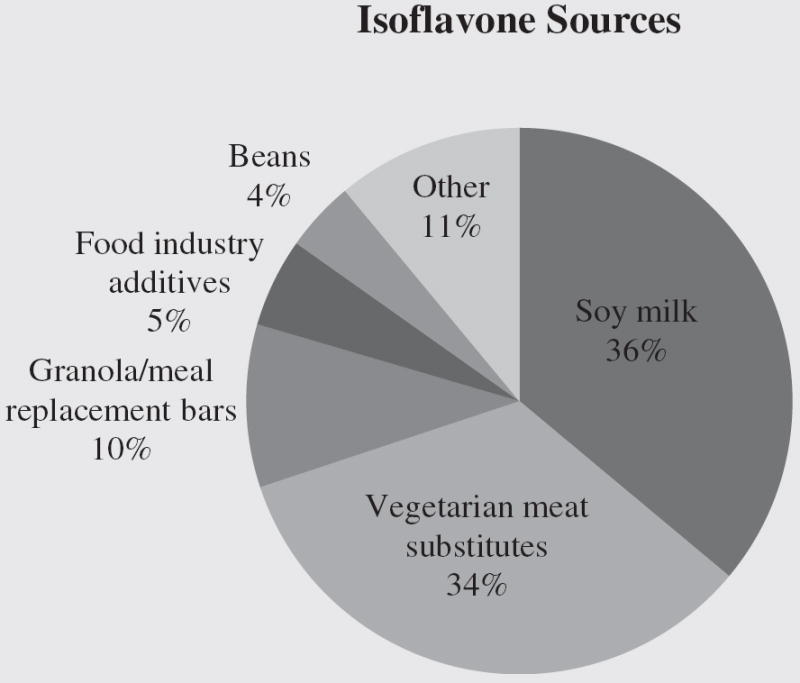
Main food sources of isoflavone intake, based on total isoflavones captured by eight 24-hour dietary recalls across two menstrual cycles (n = 259).
Filiberto. Isoflavones and sex hormones. Fertil Steril 2013.
There were no statistically significant associations between isoflavone intake and total E2, free E2, or ovulatory LH or FSH (Table 2). Although we saw a modest and positive association with luteal P in the second and third quartiles compared with the first quartile of models 1 and 2 and an association in the third quartile of model 3, there were no significant trends. We observed a positive association between SHBG and isoflavone intake in the highest quartile compared with the lowest quartile (model 3: β = 0.09; 95% confidence interval [CI] 0.02–0.16; Table 2). Isoflavone intake was not associated with incident anovulation in either unadjusted or fully adjusted models (fully adjusted model for Q4 vs. Q1: odds ratio [OR] 0.87, 95% CI 0.32–1.66; Table 3). Isoflavone intake was also considered as a continuous variable in analyses of both hormone concentrations and incident anovulation, yielding similar results (data not shown).
TABLE 2.
Quartiles of isoflavone intake and serum concentrations of sex hormones and SHBG.
| Log hormone | Modela | β (95% CI) | |||
|---|---|---|---|---|---|
| Q1 (0.0, 0.3 mg/d) | Q2 (0.3, 0.6 mg/d) | Q3 (0.6, 1.6 mg/d) | Q4 (1.6, 78.8 mg/d) | ||
| E2 (pg/mL) | Unadjusted | REF | −0.03 (−0.10-0.04) | −0.01 (−0.09-0.06) | 0.02 (−0.07-0.10) |
| Adjusted | REF | −0.02 (−0.09-0.06) | 0.006 (−0.07-0.08) | 0.04 (−0.05-0.12) | |
| MSM | REF | −0.02 (−0.09-0.04) | 0.002 (−0.08-0.08) | 0.03 (−0.07-0.13) | |
| Luteal P (ng/mL) | Unadjusted | REF | 0.24 (0.05-0.43) | 0.31 (0.11-0.51) | 0.15 (−0.05-0.36) |
| Adjusted | REF | 0.22 (0.03-0.41) | 0.29 (0.09-0.48) | 0.14 (−0.08-0.36) | |
| MSM | REF | 0.14 (−0.10-0.38) | 0.25 (0.02-0.47) | 0.10 (−0.17-0.37) | |
| Ovulatory FSH (mIU/mL) | Unadjusted | REF | 0.02 (−0.06-0.11) | −0.02 (−0.10-0.07) | 0.006 (−0.09-0.10) |
| Adjusted | REF | 0.01 (−0.07-0.94) | −0.02 (−0.11-0.06) | 0.03 (−0.06-0.13) | |
| MSM | REF | 0.05 (−0.04-0.13) | −0.01 (−0.10-0.08) | 0.04 (−0.06-0.14) | |
| Ovulatory LH (ng/mL) | Unadjusted | REF | 0.07 (−0.06-0.19) | 0.04 (−0.09-0.17) | 0.05 (−0.09-0.18) |
| Adjusted | REF | 0.03 (−0.10-0.17) | 0.01 (−0.12-0.14) | 0.06 (−0.09-0.20) | |
| MSM | REF | 0.07 (−0.05-0.18) | 0.01 (−0.11-0.14) | 0.07 (−0.07-0.22) | |
| SHBG (nmol/L) | Unadjusted | REF | 0.005 (−0.02-0.03) | 0.03 (0.003-0.06) | 0.07 (0.04-0.10) |
| Adjusted | REF | 0.008 (−0.02-0.03) | 0.04 (0.01-0.06) | 0.08 (0.05-0.11) | |
| MSM | REF | 0.03 (−0.04-0.09) | 0.05 (−0.01-0.11) | 0.09 (0.02-0.16) | |
| Free E2 (pg/mL) | Unadjusted | REF | −0.05 (−0.12-0.02) | −0.04 (−0.12-0.03) | −0.05 (−0.13-0.03) |
| Adjusted | REF | −0.03 (−0.10-0.04) | −0.02 (−0.10-0.05) | −0.02 (−0.10-0.06) | |
| MSM | REF | −0.04 (−0.11-0.03) | −0.03 (−0.11-0.06) | −0.03 (−0.13-0.07) | |
Note: Analyses were performed with the use of linear mixed models on the log scale of hormones.
β = effect estimate; CI = confidence interval; MSM = marginal structural model.
Adjusted: adjusted for age (continuous), race (white, black, Asian, other), body mass index (continuous), and fiber, energy, vegetable protein, and omega-3 intakes (continuous). MSM: adjusted for age (continuous), race (white, black, Asian, other), body mass index (continuous), fiber, energy, vegetable protein, and omega-3 intakes (continuous), and relevant phase-specific hormone concentrations with the use of MSMs with inverse probability of exposure weights.
Filiberto. Isoflavones and sex hormones. Fertil Steril 2013.
TABLE 3.
Quartiles of isoflavone intake and risk of anovulation.
| Model | Odds ratio (95% CI) | |||
|---|---|---|---|---|
| Q1 (0.0–0.3 mg/d) | Q2 (0.3–0.6 mg/d) | Q3 (0.6–1.6 mg/d) | Q4 (1.6–78.8 mg/d) | |
| Unadjusted | REF | 0.69 (0.25–1.87) | 1.33 (0.50–3.58) | 1.23 (0.50–3.04) |
| Adjusteda | REF | 0.69 (0.21–1.83) | 1.38 (0.50–1.68) | 0.87 (0.32–1.66) |
Note: Anovulation was defined as any cycle with a peak P concentration ≤ 5 ng/mL and no observed serum LH peak at the mid- or late-luteal phase visits. Analyses were performed with the use of generalized estimating equations.
Adjusted for age (continuous), race (white, black, Asian/other), body mass index (continuous), and fiber, energy, vegetable protein, and omega-3 intakes (continuous).
Filiberto. Isoflavones and sex hormones. Fertil Steril 2013.
DISCUSSION
Usual dietary intake of isoflavones was not significantly associated with sex hormone concentrations across the menstrual cycle, or with incident anovulation among a population of healthy regularly menstruating women. However, isoflavone intake was significantly and positively associated with serum concentrations of SHBG. These results suggest potential beneficial endocrine effects with no observed effects on ovulation at usual dietary intakes of isoflavones, lessening concerns regarding the effects of isoflavone intake on fertility.
The lack of association between isoflavone intake and E2 and free E2 is consistent with a recent meta-analysis of 11 dietary intervention studies involving premenopausal women (5). However, intervention trials attempt to emulate diets characteristic of the Asian population, with isoflavone supplementation of 23–84.4 mg/d, whereas isoflavone consumption of women in the BioCycle study was comparable to diets of American women (BioCycle women arithmetic mean 2.8 mg/d vs. National Health and Nutrition Examination Survey women arithmetic mean 1.3 mg/d [15]). Furthermore, although a few studies have shown an association between interventional isoflavone intake and E2 (18-20), the effects were modest and based on small studies (n = 6–14), and biospecimen collection was not well timed to the menstrual cycle.
It is important to evaluate the effects of isoflavone intake in premenopausal women on the LH surge, because this is the biologic trigger for ovulation which is necessary to achieve pregnancy. We did not observe a significant association of isoflavone intake with midcycle FSH or LH, which differs from some earlier studies which reported decreased midcycle concentrations of FSH and LH after soy isoflavone intervention diets (18, 21). These inconsistencies may be due to differences in timing of sample collection or in the amount of isoflavone intake, because this was not an intervention study. None of the earlier studies timed sample collection to capture the rapid surge in all hormones, especially LH, but evaluated LH concentrations 5–7 days after ovulation (21, 22). This may not be the optimal time to evaluate LH concentrations or ovulation, because there is very little biologic variability in LH concentrations during the luteal phase. Fertility monitors capture the LH surge in >75% of cycles, whereas traditional algorithms to time sample collection with the use of calendar time or cycle length are prone to mistiming and only detect the LH surge in 37%–57% of cycles (7). Our study used fertility monitors to time hormone measurements to specific cycle phases, and we observed no association between isoflavones and LH when evaluating LH concentrations around ovulation and the timing of the LH surge. Even when we evaluated associations with LH during the luteal phase for comparison with earlier work, we observed no significant associations (data not shown).
Because earlier studies observed effects on midcycle LH and FSH concentrations, it is plausible that effects on ovulation would be observed, though no studies have directly evaluated this association. We did not observe similar associations with sex hormones, and thus no subsequent effects on ovulation. This is the first study to directly link usual dietary isoflavone intake in a given cycle with ovulation, an important outcome for couples trying to conceive. Moreover, given the complex feedback mechanisms between the hormones that are time-dependent confounders as well as intermediates, it is necessary to evaluate these associations simultaneously. Our study evaluated the associations with hormone concentrations while accounting for the effects of the feedback mechanisms. Thus, our results add strong evidence that usual dietary intakes of isoflavones are not associated with concentrations of circulating hormones or ovulation among young healthy women without special dietary constraints.
We observed a significant and positive association between isoflavone intake and SHBG concentrations. This association remained significant even after controlling for other dietary covariates such as protein (animal, vegetable), cholesterol, fat (PUFA, MUFA), and/or aMED score (data not shown). Therefore, the observed significant association is not likely to be explained by these other nutrients commonly found in foods high in isoflavone content, although residual confounding can not be excluded. Our results are consistent with two earlier intervention trials in premenopausal women (23, 24) and in vitro studies (25, 26) which observed positive correlations between SHBG and isoflavone intake. Although earlier studies were intervention trials where intakes were 10–100 times the amount of isoflavones consumed by women in the BioCycle study (5), we still observed significant associations between usual intakes of isoflavones and SHBG concentrations.
The observed association between isoflavones and SHBG is biologically plausible. SHBG is a protein carrier for the majority of estrogens circulating in the blood. Isoflavones can bind to SHBG and stimulate its synthesis in the liver, thereby reducing the bioavailability of sex hormones transported to hormone-dependent tissues by blood (27). Fluctuations in SHBG can result in important changes in the unbound concentrations of sex hormones, which may largely determine the biologic effects of the major sex steroids. However, analyses using equation-based estimates of free E2 from total E2, SHBG, and albumin data yielded similar results. A measured value of free E2 may have been more sensitive in detecting changes. Despite the lack of clear association between isoflavone intake and E2, the finding of increased SHBG remains important, because SHBG concentrations are associated with a more favorable metabolic profile and decreased risk for type 2 diabetes (28, 29). Of note, as shown in Figure 1, soy milk and vegetarian meat substitutes constituted the primary sources of isoflavone intake among the women in our study. These foods may represent potential dietary sources for increasing SHBG, which may yield long-term positive health benefits.
The BioCycle study had a number of strengths. The 24HDRs included detailed information on almost all habitually consumed bread, nut and seed products, and vegetable and fruit items, which are the food groups most likely to contain isoflavones in Western diets. The use of multiple validated 24HDRs improved our ability to assess usual intake, reducing the potential for exposure misclassification. Additionally, our study used the NDSR, which matched food consumption data to the United States Department of Agriculture–Iowa State University Database on the Isoflavone Content of Foods, which was generated by extensive sampling of 108 soy-containing foods. Most analytic food composition databases report only isoflavone content of traditional soy foods, which are of limited use in Western countries where the consumption of traditional soy foods is low but other isoflavone-containing foods may be widespread (30). Furthermore, intensive monitoring throughout two cycles on a relatively large number of women with multiple clinic visits timed with the use of fertility monitors allowed us to ascertain more accurate menstrual cycle hormonal profiles and evaluate phase-specific effects and ovulation. Standardized assessment of a wide variety of participant and dietary characteristics allowed us to control for potential confounding variables, including time-dependent confounders such as other hormones and other dietary components with known impacts on hormone concentrations. Additionally, this study reports dietary intakes of isoflavones characteristic of the American population (17), making results generalizable to women of reproductive age.
Nevertheless, there are several limitations of this study. Usual isoflavone consumption was relatively low, and only a small number of women were consuming diets rich in isoflavones. Therefore, we were limited in our ability to assess dietary intakes of women consuming isoflavone-rich diets. Furthermore, although numerous epidemiologic and clinical studies in women have evaluated the relationship between isoflavone consumption and circulating sex hormones, studies among premenopausal women with longitudinal measures of sex hormones across the menstrual cycle are limited. Therefore, all available literature, including intervention studies among different populations with different dietary isoflavone intakes, were used to illustrate comparisons among findings. It should be noted that findings from a cross-sectional study can not be directly compared with that of an intervention study with different populations and dietary intakes. Additionally, the Institute of Medicine (31) notes that insufficient data are currently available to warrant inclusion of isoflavones in the report of Dietary Reference Intakes, owing to inadequate food composition data that are required to assess dietary intakes in a population (32). Thus, there is no established recommended daily intake of isoflavones to use as a reference for women in the BioCycle study and women of reproductive age. As mentioned above, isoflavone intake was assessed using up to four 24HDRs per cycle; however, we did not measure urinary metabolites of isoflavones and were unable to validate dietary isoflavone consumption data. Furthermore, because free E2 concentrations reported in this study were derived, a measured value of free E2 may have been more sensitive in detecting changes.
In conclusion, our data suggest no significant association with sex hormone concentrations or incident anovulation among women consuming usual intakes of isoflavones. The positive association between SHBG and isoflavone intake suggests potential beneficial endocrine effects with no observed subsequent effects on ovulation. However, additional studies are warranted to replicate our novel findings with respect to ovulation, and extend to prospective time-to-pregnancy studies for evaluation of potential impacts on fertility. These results will be critical for informing recommendations for isoflavone intake among reproductive-age women.
Acknowledgments
The authors are indebted to all of the investigators and staff at the Epidemiology Branch, Eunice Kennedy Shriver, National Institute of Child Health and Human Development, and the University at Buffalo for their roles in the study. The authors also recognize the BioCycle participants for their extraordinary commitment to the study.
Supported by the Intramural Research Program at the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Health.
Footnotes
A.C.F. has nothing to disclose. S.L.M. has nothing to disclose. A.Z.P. has nothing to disclose. C.Z. has nothing to disclose. E.H.Y. has nothing to disclose. K.C.S. has nothing to disclose. N.J.P. has nothing to disclose. J.W.-W. has nothing to disclose. E.F.S. has nothing to disclose.
References
- 1.Xiao CW. Health effects of soy protein and isoflavones in humans. J Nutr. 2008;138:1244S–9S. doi: 10.1093/jn/138.6.1244S. [DOI] [PubMed] [Google Scholar]
- 2.Setchell KD, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr. 1999;129:758S–67S. doi: 10.1093/jn/129.3.758S. [DOI] [PubMed] [Google Scholar]
- 3.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–63. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 4.Hwang CS, Kwak HS, Lim HJ, Lee SH, Kang YS, Choe TB, et al. Isoflavone metabolites and their in vitro dual functions: they can act as an estrogenic agonist or antagonist depending on the estrogen concentration. J Steroid Biochem Mol Biol. 2006;101:246–53. doi: 10.1016/j.jsbmb.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 5.Hooper L, Ryder JJ, Kurzer MS, Lampe JW, Messina MJ, Phipps WR, et al. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Hum Reprod Update. 2009;15:423–40. doi: 10.1093/humupd/dmp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wactawski-Wende J, Schisterman EF, Hovey KM, Howards PP, Browne RW, Hediger M, et al. Biocycle study: design of the longitudinal study of the oxidative stress and hormone variation during the menstrual cycle. Paediatr Perinat Epidemiol. 2009;23:171–84. doi: 10.1111/j.1365-3016.2008.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howards PP, Schisterman EF, Wactawski-Wende J, Reschke JE, Frazer AA, Hovey KM. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the Biocycle Study. Am J Epidemiol. 2009;169:105–12. doi: 10.1093/aje/kwn287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nutrient Data Laboratory, Agricultural Research Service, United States Department of Agriculture. USDA–Iowa State University database on the isoflavone content of selected foods, release 2.0. 2008 Available at: http://www.ars.usda.gov/nutrientdata.
- 9.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–10. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 10.Gaskins AJ, Mumford SL, Zhang C, Wactawski-Wende J, Hovey KM, Whitcomb BW, et al. Effect of daily fiber intake on reproductive function: the Biocycle study. Am J Clin Nutr. 2009;90:1061–9. doi: 10.3945/ajcn.2009.27990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 12.Boghossian NS, Yeung EH, Mumford SL, Zhang C, Gaskins AJ, Wactawski-Wende J, et al. Biocycle Study Group. Adherence to the Mediterranean diet and body fat distribution in reproductive aged women. Eur J Clin Nutr. 2013;67:289–94. doi: 10.1038/ejcn.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaskins AJ, Rovner AJ, Mumford SL, Yeung E, Browne RW, Trevisan M, et al. Biocycle Study Group. Adherence to a Mediterranean diet and plasma concentrations of lipid peroxidation in premenopausal women. Am J Clin Nutr. 2010;92:1461–7. doi: 10.3945/ajcn.110.000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Littell RC, Henry PR, Ammerman CB. Statistical analysis of repeated measures data using SAS procedures. J Anim Sci. 1998;76:1216–31. doi: 10.2527/1998.7641216x. [DOI] [PubMed] [Google Scholar]
- 15.Weng HY, Hsueh YH, Messam LL, Hertz-Picciotto I. Methods of covariate selection: directed acyclic graphs and the change-in-estimate procedure. Am J Epidemiol. 2009;169:1182–90. doi: 10.1093/aje/kwp035. [DOI] [PubMed] [Google Scholar]
- 16.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–64. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chun OK, Chung SJ, Song WO. Estimated dietary flavonoid intake and major food sources of U.S. adults. J Nutr. 2007;137:1244–52. doi: 10.1093/jn/137.5.1244. [DOI] [PubMed] [Google Scholar]
- 18.Cassidy A, Bingham S, Setchell KD. Biological effects of a diet of soy protein rich in isoflavones on the menstrual cycle of premenopausal women. Am J Clin Nutr. 1994;60:333–40. doi: 10.1093/ajcn/60.3.333. [DOI] [PubMed] [Google Scholar]
- 19.Lu LJ, Anderson KE, Grady JJ, Nagamani M. Effects of soya consumption for one month on steroid hormones in premenopausal women: implications for breast cancer risk reduction. Cancer Epidemiol Biomarkers Prev. 1996;5:63–70. [PubMed] [Google Scholar]
- 20.Petrakis NL, Barnes S, King EB, Lowenstein J, Wiencke J, Lee MM, et al. Stimulatory influence of soy protein isolate on breast secretion in pre- and post-menopausal women. Cancer Epidemiol Biomarkers Prev. 1996;5:785–94. [PubMed] [Google Scholar]
- 21.Duncan AM, Merz BE, Xu X, Nagel TC, Phipps WR, Kurzer MS. Soy isoflavones exert modest hormonal effects in premenopausal women. J Clin Endocrinol Metab. 1999;84:192–7. doi: 10.1210/jcem.84.1.5387. [DOI] [PubMed] [Google Scholar]
- 22.Maskarinec G, Williams AE, Inouye JS, Stanczyk FZ, Franke AA. A randomized isoflavone intervention among premenopausal women. Cancer Epidemiol Biomarkers Prev. 2002;11:195–201. [PubMed] [Google Scholar]
- 23.Kumar NB, Cantor A, Allen K, Riccardi D, Cox CE. The specific role of isoflavones on estrogen metabolism in premenopausal women. Cancer. 2002;94:1166–74. doi: 10.1002/cncr.10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu AH, Stanczyk FZ, Hendrich S, Murphy PA, Zhang C, Wan P, et al. Effects of soy foods on ovarian function in premenopausal women. Br J Cancer. 2000;82:1879–86. doi: 10.1054/bjoc.1999.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loukovaara M, Carson M, Palotie A, Adlercreutz H. Regulation of sex hormone-binding globulin production by isoflavonoids and patterns of isoflavonoid conjugation in HepG2 cell cultures. Steroids. 1995;60:656–61. doi: 10.1016/0039-128x(95)00089-9. [DOI] [PubMed] [Google Scholar]
- 26.Mousavi Y, Adlercreutz H. Genistein is an effective stimulator of sex hormone-binding globulin production in hepatocarcinoma human liver cancer cells and suppresses proliferation of these cells in culture. Steroids. 1993;58:301–4. doi: 10.1016/0039-128x(93)90088-5. [DOI] [PubMed] [Google Scholar]
- 27.Kurzer MS. Hormonal effects of soy in premenopausal women and men. J Nutr. 2002;132:570S–3S. doi: 10.1093/jn/132.3.570S. [DOI] [PubMed] [Google Scholar]
- 28.Ding EL, Song Y, Manson JE, Hunter DJ, Lee CC, Rifai N, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med. 2009;361:1152–63. doi: 10.1056/NEJMoa0804381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onat A, Hergenc G, Karabulut A, Albayrak S, Can G, Kaya Z. Serum sex hormone-binding globulin, a determinant of cardiometabolic disorders independent of abdominal obesity and insulin resistance in elderly men and women. Metabolism. 2007;56:1356–62. doi: 10.1016/j.metabol.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 30.Cotterchio M, Boucher BA, Kreiger N, Mills CA, Thompson LU. Dietary phytoestrogen intake—lignans and isoflavones—and breast cancer risk (Canada) Cancer Causes Control. 2008;19:259–72. doi: 10.1007/s10552-007-9089-2. [DOI] [PubMed] [Google Scholar]
- 31.Food and Nutrition Board, Institute of Medicine. Dietary reference intakes: proposed definition and plan for review of dietary antioxidants and related compounds. Washington, D.C.: National Academies Press; 1998. [PubMed] [Google Scholar]
- 32.Chun OK, Chung SJ, Song WO. Urinary isoflavones and their metabolites validate the dietary isoflavone intakes in US adults. J Am Diet Assoc. 2009;109:245–54. doi: 10.1016/j.jada.2008.10.055. [DOI] [PubMed] [Google Scholar]


