Abstract
αLβ2 integrin (LFA-1) has an important role in the formation of T cell and NK cell cytotoxic immunological synapses and in target cell killing. Binding of LFA-1 to ICAM on target cells promotes not only adhesion, but also polarization of cytolytic granules in NK cells. Here we tested whether LFA-1-dependent NK cell responses are regulated by the distribution and mobility of ICAM at the surface of target cells. We show that depolymerization of F-actin in NK-sensitive target cells abrogated LFA-1-dependent conjugate formation and granule polarization in primary NK cells. Degranulation, which is not controlled by LFA-1, was not impaired. Fluorescence recovery after photobleaching experiments and particle tracking by total internal reflection fluorescence microscopy revealed that ICAM-1 and ICAM-2 were distributed in largely immobile clusters. ICAM clusters were maintained and became highly mobile after actin depolymerization. Moreover, reducing ICAM-2 mobility on an NK-resistant target cell through expression of ezrin, an adapter molecule that tethers proteins to the actin cytoskeleton, enhanced LFA-1-dependent adhesion and granule polarization. Finally, while NK cells kept moving over freely diffusible ICAM-1 on a lipid bilayer, they bound and spread over solid-phase ICAM-1. We conclude that tethering, rather than clustering of ICAM promotes proper signaling by LFA-1 in NK cells. Our findings suggest that the lateral diffusion of integrin ligands on cells may be an important determinant of susceptibility to lysis by cytotoxic lymphocytes.
Keywords: NK cell, adhesion, LFA-1, granule polarization, cytoskeleton, ezrin
Introduction
The αLβ2 integrin LFA-1 (a heterodimer of CD11a/CD18) binds to inter-cellular adhesion molecules (ICAM) and mediates arrest of rolling leukocytes in blood vessels (1), and tight adhesion of cytotoxic lymphocytes to target cells, which is essential for the cytotoxic activity of T cells and NK cells (2-4). LFA-1 plays a central role in the organization of immunological synapses formed by T cells and NK cells (5-10). LFA-1 on resting T cells is kept in an inactive, low affinity conformation and changes into a higher affinity conformation through “inside-out” signals delivered by other receptors (11). In contrast, resting NK cells bind directly to ICAM-1 in a signaling dependent way (12, 13). Furthermore, LFA-1 binding to ICAM-1 and ICAM-2 is sufficient to induce polarization of cytolytic granules in NK cells (12, 14, 15). In mouse NK cells, talin is required for outside-in signaling by LFA-1, which, together with signaling by NKG2D, induces granule polarization (16).
Whereas polarization of cytolytic granules is induced by LFA-1 in NK cells, degranulation is triggered by the low affinity Fcγ receptor CD16, or by synergistic combinations of co-activation receptors, independently of LFA-1 (10, 13, 14, 17). This uncoupling of signals for granule polarization and degranulation observed in NK cells is very different from cytotoxic T cells, in which these two functions are controlled centrally by the TCR, and are co-stimulated by other receptors including CD28 and LFA-1 (9, 18, 19). The ability of LFA-1 in NK cells to signal autonomously make NK cells an excellent tool to study LFA-1-dependent functions.
Lateral segregation of proteins within cell membranes leads to functional subcompartementalization of the lipid bilayer (20, 21). How receptor distribution at the plasma membrane and receptor binding to the cytoskeleton determines signaling output has been examined (16, 22-28). In contrast, little is known about how receptor function is controlled by the distribution of ligands on target cells. A few studies have suggested that ligand distribution on target cells may be important for proper recognition by T cells and NK cells. A striking finding was that the cytoplasmic tail of CD80 (B7-1), a ligand of CD28 expressed on APC, is required for the proper segregation of CD28 at immunological synapses and for full T cell activation, highlighting an unexpected contribution of the intracellular portion of a ligand to synapse organization, and to signaling in another cell (29). A mutation in the acylation site of MICA, an NKG2D receptor ligand on target cells, resulted in diminished NKG2D-dependent killing by NK cells, suggesting that sorting of NKG2D ligands into cholesterol-enriched membrane domains of target cells improves signaling by NKG2D in NK cells (30). An earlier study suggested that polarization of ICAM-2 to the uropod of a target cell through expression of the ERM protein ezrin rendered the cells more sensitive to lysis by NK cells (31). However, whether it is distribution, clustering, or mobility of ICAM at the surface of target cells that is critical for LFA-1 dependent responses is not known.
Here we tested how the distribution of ICAM on target cells affects NK cell function. We focused on LFA-1-dependent functions, namely adhesion to target cells and polarization of cytolytic granules. We have used several approaches to manipulate the attachment of ICAM-1 and ICAM-2 to the cytoskeleton or to artificial surfaces. Our results show that it is the immobilization of ICAM, rather than polarization to one end of the cell or clustering, that is required for functional interaction with LFA-1.
Materials and Methods
Cells
Human NK cells were isolated from peripheral blood cells by negative selection using an NK cell isolation kit (Miltenyi Biotec). Resting NK cells (95-99% CD3-CD56+) were resuspended in Iscove’ s modified Dulbecco medium (IMDM, Invitrogen) supplemented with 10% human serum (Valley Biomedical) and used 1 - 2 days after isolation. Polyclonal IL-2 activated NK cells were expanded in the presence of feeder cells (0.5 × 106 PBL/ml, g-irradiated with 4.5k) with IMDM supplemented with 10% human serum, 100 IU/ml recombinant IL-2 (Hoffmann-La Roche), and 10% purified human IL-2 (Hemagen). IL-2 activated cells were used 2 – 3 weeks after isolation. The B cell lymphoma line 721.221 was cultivated in IMDM medium supplemented with 10% heat inactivated fetal bovine serum (FBS, Thermo Scientific). The mouse thymoma cell line BW5147 (kind gift by T. Kamala, National Institute of Allergy and Infectious Diseases, National Institutes of Health) was cultivated in IMDM medium supplemented with 10% heat inactivated FBS.
Transfection of BW5147 cells with hEzrin-EGFP
BW5147 cells were transfected with hEzrin-EGFP (pHJ421: human ezrin coding sequence subcloned into pEGFP-N1 eukaryotic expression vector, (gift from J.-J. Hao and S. Shaw, National Cancer Institute, National Institutes of Health (32)) using the BTX machine (ECM830, Harvard Aparatus, settings: 230 V, 10 ms, 1 pulse). Cells were recovered for 16 h at 37°C and 5% CO2 and hEzrin-EGFP positive cells were selected by fluorescence activated cell sorting (FACS). Stable transfectants were generated from the FACS sorted cells by cultivating them in IMDM/10% FBS/1mg/ml Geneticin (Gibco) and subcloning.
Pre-treatment of target cells with inhibitors
All inhibitors were dissolved in DMSO (Sigma). For Latrunculin A or Jasplakinolide (both Calbiochem) treatment 10 × 106 721.221 or K562 target cells were resuspended in 1 ml IMDM supplemented with 10% FBS containing 2% DMSO as a negative control to match the final concentration of carrier in treated cells. For the experiments cells were treated 0.5 μM, 3 μM, or 20 μM Latrunculin A, or 0.5 μM, 1 μM, or 2 μM Jasplakinolide. Staining F-actin with phalloidin revealed that 3 μM of LatA was required to disrupt F-actin completely, and that the effect of Jasp was evident at a concentration as low as 0.5 μM (data not shown). Cells were incubated for 40 min at 37°C. Inhibitors were washed away prior mixing with NK cells for the different assays.
Conjugation assay
Conjugate formation between NK cells and target cells was determined as previously described (12) with minor modifications. Briefly, NK cells were labeled with 1 μg/ml Cell Tracker Green CMFDA (Invitrogen) for 30 min at 37°C and 5% CO2, washed and incubated for another 30 min at 37°C and 5% CO2. If BW5147 cells were used as targets, NK cells were labeled with 20 μl/ml anti-CD56 APC (Becton Dickinson) for 15 min at 37°C and 5% CO2. Target cells were labeled with PKH26 Red (721.221, K562) or PKH67 Green (BW5147 cells) (both Sigma) for 5 min at RT, washed extensively, and recovered for 30 min at 37°C and 5% CO2. NK cells and target cells were mixed at a 1:2 effector to target (E:T) cell ratio with 1 × 105 NK cells and 2 × 105 target cells at 4°C. Cells were spun down at 20 g for 3 min and conjugate formation was stopped by vortexing and fixation of cells using 0.5% paraformaldehyde solution (Electron Microscopy Sciences) after 0, 5, 10, 20, 30 or 60 min incubation at 37°C. Conjugate formation was determined by flow cytometry (FACSCalibour, Becton Dickinson) and is represented as the fraction of NK cells that shifted into two-color conjugates. For blocking of LFA-1 on NK cells, 1 × 106 Cell Tracker Green labeled NK cells were pre-treated with 20 μg/ml IgG2a (HOPC-1, Sigma) or anti-human CD18 (Calbiochem) for 15 min at 4°C.
Degranulation assay
The degranulation assay was performed as previously described (14). Briefly, 2 × 105 NK cells were added to 4 × 105 721.221 or K562 cells in a total volume of 200 μl IMDM medium supplemented with 10% heat inactivated FBS. Cells were mixed and incubated for 1 h at 37°C and 5% CO2. Afterwards the cells were spun down and stained with PE-conjugated anti-CD56 (Becton Dickinson) and FITC-conjugated anti-CD107a (Becton Dickinson) antibody for 45 min at 4°C. Degranulation of CD56 positive NK cells was analyzed by flow cytometry.
Perforin polarization assay
The polarization assay was performed as previously described (15). Briefly, NK cells and target cells (721.221, K562, or BW5147) were mixed at a 1:1 E:T ratio, with 1 × 106 of each cell type per sample. Cells were incubated for 20 min at 37°C and 5% CO2. Cells were resuspended and put onto a Poly-D-Lysine coated 2-well Culture Slide (BD Biosciences). Cells were allowed to settle down for 1 h at room temperature and fixed using 4% paraformaldehyde (Electron Microscopy Sciences). Cells were permeabilized using 0.5% Triton X-100 and stained using an anti-human perforin antibody (Pierce) and an Alexa 488-conjugated secondary goat anti-mouse IgG antibody (Molecular Probes). Cells were imaged by confocal microscopy (LSM510, Zeiss) with a 40x, 1.3 NA Plan Neofluar (Zeiss) oil immersion objective lens. 50-200 NK cells in contact with target cells were analyzed for polarization of perforin containing granules.
Fluorescence recovery after photobleaching (FRAP) analysis
ICAM-1 on 721.221 cells was stained using a PE-conjugated anti-ICAM-1 antibody (Becton Dickinson). FRAP was performed using a confocal microscope (LSM510, Zeiss). A region of the cell surface was bleached using the 488 nm laser. The recovery of the bleached region was captured at one frame per 30 seconds for 10 frames. During the assay cells were maintained at 37°C. The fluorescence intensities of the bleached region and an unbleached region of the same cell over time were analyzed using the LSM510 software (Zeiss). The medians of the relative intensities of different cells were plotted over time.
TIRF microscopy
Endogenous cell-surface ICAM-1 or ICAM-2 on 721.221 or BW5147 cells was fluorescently labeled with PE-conjugated anti-human ICAM-1 (Becton Dickinson), anti-human ICAM-2 (Beckman Coulter), or anti-mouse ICAM-2 (BD Pharmingen) antibody. TIRF imaging was performed using an Olympus IX81 TIRF microscope equipped with a 561 nm diode-pumped laser (Cobolt), 100x 1.45 NA Olympus TIRFM lens, and a Cascade II 1024B EM-CCD camera (Photometrics). Cells were maintained at 37°C using a LiveCell environmental chamber (Pathology Devices). Images were captured at 32 frames per second using MetaMorph software (Molecular Devices) for 250 frames. The movement of labeled ICAM molecules was automatically tracked using code developed for MatLab software ((33); http://physics.georgetown.edu/matlab/). The code was modified to refine particle positioning with a 2D gaussian fit (34) and to determine intensities of individual particles. Mean square displacements were determined from positional coordinates to calculate short-range diffusion coefficients of individual particles for five time intervals. The resulting diffusion coefficients were plotted as a cumulative distribution function (CDF). A CDF plots the same information as a histogram for a given data set; however, where the histogram plots the frequency distribution (y-axis) as a function of the binned data set (x-axis), the data is not binned for a CDF plot. The CDF plot is generated as follows: The data set is sorted from the smallest to greatest value. Next, the number of data points is summed and the value of the position of a data point on the sorted list is divided by the sum to yield the Probability value (y-axis). Finally, based on its position on sorted list, the Probability is plotted against the value of the data point. The CDF displays the distribution of the data set from the smallest (from left on the x-axis) to greatest value (at the right of the x-axis) and provides the probability (y-axis) of whether a particular value will occur at or less than a specified point on the x-axis. Moreover, a CDF plot allows for the rapid identification of the median value of the data set by interpolation at 50% on the y-axis, it is not prone to binning artifacts, and the separation of the data set by log scale is more apparent for visual inspection. In addition, graphical comparisons between data sets are generally more easily interpretable relative to overlaying histograms.
Imaging of NK cells on ICAM-1 bound on lipid bilayers or coverslips
Planar lipid bilayers carrying human ICAM-1 tagged with six histidines were formed between a coverslip and the microaqueduct slide of a Bioptechs parallel plate flow chamber-FCS2 (Bioptechs, Butler, PA) as described (10, 11). A human ICAM-1-Fc fusion protein was coated on coverslips in 100 mM sodium bicarbonate, pH 9.2, at 10 ng/ml, 100 ng/ml, 1 μg/ml, and 10 μg/ml, at 4°C overnight. 5% FBS-containing culture media was used to block non-specific binding. The ICAM-1-Fc fusion protein cloned in the CD5lneg1 vector (35) was produced by transfection into 293T cells, as described (36). To avoid stimulation of NK cells via CD16 by the human IgG1 Fc portion of the ICAM-1-Fc fusion protein, the CD16 binding site was mutated in the CD5lneg1 vector, as described (14). Briefly, amino acids 235 and 236 of human IgG1 within the Fc domain were mutated as Leu235 to Gly235 and Gly236 to Leu236. Whereas unmutated ICAM-1-Fc fusion protein that was attached to plates triggered degranulation in NK cells, the ICAM-1-Fc protein carrying the mutated CD16 binding site did not. The movement of NK cells was measured 20 min after injection and tracked for 25 frames with a 10 s interval between frames by Dynamic Image Analysis System (DIAS) (37).
Results
Disruption of actin filaments in target cells prevents conjugate formation with NK cells
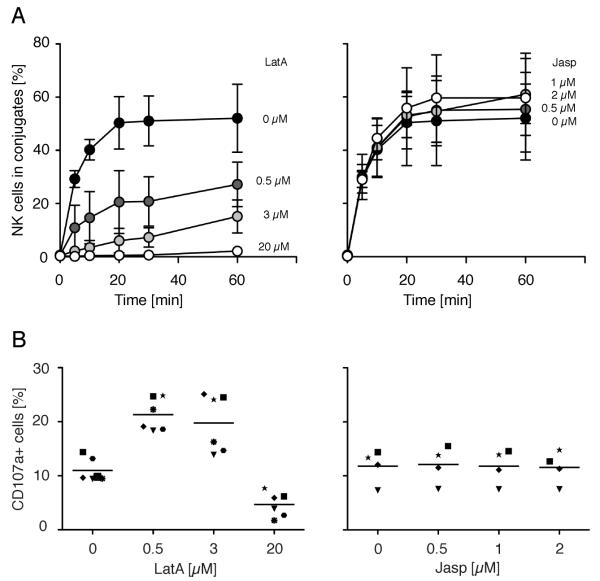
Two functions of NK cells for which LFA-1 signaling is sufficient are adhesion and granule polarization. The B cell lymphoma cell line 721.221 (38), which does not express HLA-A, HLA-B, and HLA-C, is sensitive to lysis by NK cells. To test if conjugate formation of NK cells is dependent on an intact cytoskeleton in target cells, actin filaments (F-actin) were disrupted in 721.221 cells with different concentrations of Latrunculin A (0.5 μM, 3 μM, and 20 μM) prior to washing and mixing those cells with NK cells in a conjugation assay. Conjugate formation of freshly isolated, primary NK cells, was inhibited by Latrunculin A treatment of 721.221 cells (Fig. 1A, left). Moreover, conjugate formation of IL-2-activated NK cells with 721.221 cells was also prevented by pre-treatment of target cells with Latrunculin A (Fig. S1). These results suggest that either an intact actin cytoskeleton or actin cytoskeleton remodeling is required for proper conjugate formation.
FIGURE 1.
Disruption of actin filaments in target cells prevents conjugate formation with NK cells. A, 721.221 cells pre-treated with DMSO carrier alone (filled circles), Latrunculin A (left side), or Jasplakinolide (right side) at indicated concentrations for 40 min at 37°C were tested for conjugate formation with resting NK cells. Bars indicate the SD (n = 3 individual NK cell donors in three independent experiments). B, 721.221 target cells treated as in A were mixed with resting NK cells and degranulation was measured after 1 h at 37°C by staining for CD107a at the surface of NK cells. Each symbol indicates one of the six (left side) or four (right side) individual NK cell donors. Six (left side) or four (right side) independent experiments were performed.
To distinguish between these two possibilities, F-actin filaments in target cells were stabilized by treatment with Jasplakinolide (Fig. 1A, right). Jasplakinolide stabilizes existing F-actin but prevents actin cytoskeleton remodeling. As Jasplakinolide had no effect on conjugate formation with NK cells (Fig. 1A, right), cytoskeleton dynamics in target cells do not seem to be important for conjugate formation of NK cells. In addition, disruption of microtubules in target cells with Nocodazole had no effect on conjugate formation with resting NK cells (data not shown). We conclude that an intact cytoskeleton, but not cytoskeleton dynamics, in target cells is important for NK cell conjugate formation.
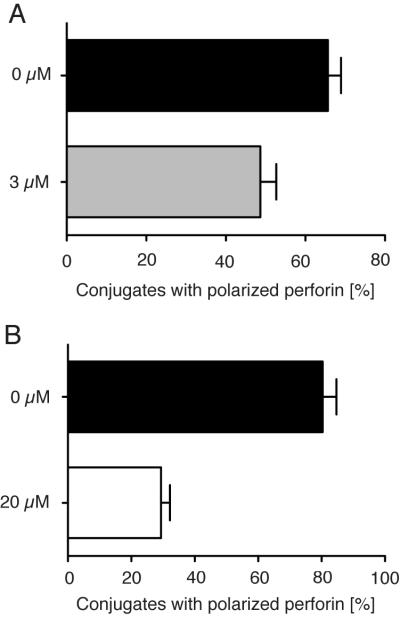
To test whether disruption of F-actin in target cells was preventing conjugate formation specifically, or whether it had more global inhibitory effects, the ability of NK cells to degranulate in response to target cells was evaluated. Whereas conjugate formation of NK cells with ICAM-expressing target cells is strictly dependent on LFA-1 ((39); and Fig. S2), degranulation is LFA-1-independent (13, 14). 721.221 cells treated with lower doses of Latrunculin A induced about twice as much degranulation by NK cells as untreated 721.221 cells (Fig. 1B, left). Our laboratory has observed that, under certain conditions, engagement of LFA-1 by ICAM-1 reduces the amount and delays the onset of degranulation induced by activation receptors (10, 14). The enhanced degranulation seen here after Latrunculin treatment of target cells may be due to a reversal of LFA-1-mediated inhibition of degranulation. In agreement with this hypothesis, blocking of LFA-1 with an Ab resulted in a 2-fold increase of degranulation by NK cells (Fig. S3). At the highest dose (20 μM), however, Latrunculin A blocked degranulation by NK cells (Fig. 1B, left). Stabilization of F-actin in 721.221 cells by Jasplakinoloide (Fig. 1B, right), or disruption of microtubules with Nocodazole (data not shown), had no effect on degranulation by NK cells. We conclude that LFA-1-dependent conjugate formation, but not LFA-1-independent degranulation, requires an intact cytoskeleton on target cells.
Disruption of F-actin in target cells inhibits polarization of cytolytic granules in NK cells
The sensitivity of conjugate formation but not degranulation of NK cells to the disruption of F-actin in target cells suggested that a functional LFA-1 interaction with ICAM is dependent on intact F-actin in target cells. To test this possibility, we evaluated the importance of an intact cytoskeleton in target cells for another LFA-1-dependent function in NK cells —granule polarization toward target cells (14, 15). Pre-treatment of 721.221 cells with Latrunculin A resulted in diminished granule polarization in NK cells (Fig. 2). As expected, due to inhibition of tight conjugate formation, fewer NK cells were found in conjugates with 721.221 cells in the presence of Latrunculin A. However, the reduction of polarization was not a consequence of reduced conjugate formation as polarization was scored only in NK cells that had formed tight contact with target cells. Therefore, in addition to the LFA-1-dependent conjugate formation, another LFA-1-dependent process, polarization of cytolytic granules, is dependent on an intact cytoskeleton in target cells.
FIGURE 2.
Disruption of actin filaments in 721.221 target cells inhibits polarization of cytolytic granules in NK cells. 721.221 cells incubated either with DMSO carrier (A, B, filled histograms) or with 3 μM (A) or 20 μM (B) Latrunculin A were mixed with NK cells for 20 min at 37°C. The cells were transferred to poly-D-lysine coated culture slides and incubated for 1 h at room temperature before fixation, permeabilization, and staining for perforin. Granule polarization is expressed as the fraction of cells in contact with perforin clustered at the NK-target cell interface. Bars indicate the SD (n = 3 individual donors in three independent experiments).
Disruption of F-actin changes the lateral mobility of ICAM-1 and ICAM-2
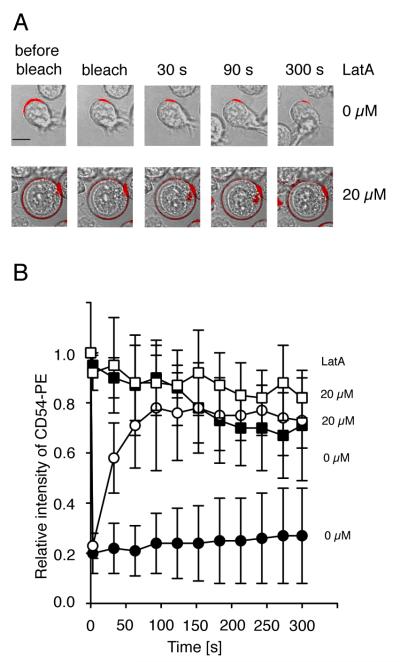
How could disruption of F-actin in target cells result in defective ICAM interactions with LFA-1 on NK cells? As ICAM is linked to the actin cytoskeleton by direct binding to α-actinin (α-actinin-1, α-actinin-4) (40-42) and to the ezrin/radixi/moesin (ERM) protein ezrin (31, 43, 44), we tested whether treatment of 721.221 cells with Latrunculin A, had an effect on ICAM-1 mobility at the plasma membrane by fluorescence recovery after photobleaching (FRAP) (Fig. 3, video1, video2). ICAM-1 molecules on 721.221 cells were stained using a PE-conjugated anti-ICAM-1 antibody and a region of the cell surface was bleached using a 488 nm laser (Fig. 3A, white arrow). The recovery of the bleached region of untreated (Fig. 3A, upper panel, Fig. 3B, filled circles, video 1) and Latrunculin A-treated (Fig. 3A, lower panel, Fig. 3B, open circles, video 2) 721.221 cells was captured during 5 minutes. Photobleaching due to image acquisition was monitored in untreated and Latrunculin A-treated 721.221 cells to normalize recovery curves (Fig. 3B, closed squares and open squares, respectively). On untreated 721.221 cells, no recovery of the bleached region was observed (Fig. 3A, upper panel, Fig. 3B, filled circles, video 1), suggesting that ICAM-1 is immobilized on 721.221 cells. However, after disruption of F-actin with 20 μM Latrunculin A, recovery of the bleached region (Fig. 3A, lower panel, Fig. 3B, open circles, video 2) occurred over 90 sec, indicating a release of ICAM-1 from the cytoskeleton. Moreover, ICAM-1, which was polarized to one side of untreated 721.221 cells (Fig. 3A, upper panel, video 1), was evenly distributed after disruption of F-actin with Latrunculin A (Fig. 3A, lower panel, video 2).
FIGURE 3.
Disruption of actin filaments in target cells increases ICAM-1 mobility. ICAM-1 mobility was determined by Fluorescence Recovery after Photobleaching (FRAP). Cells were stained with a PE-labeled anti-ICAM-1 antibody. Fluorescence recovery in the bleached area of 721.221 cells either untreated (A, upper panel, bar indicates 5 μm, B, filled circles) or Latrunculin A-treated (A, lower panel, B, open circles) is displayed. Fluorescence intensity of a non-bleached area of the 721.221 cells either untreated (B, filled squares) or Latrunculin A-treated (B, open squares) served as a control. Bars indicate the SD (untreated cells: n = 7, Latrunculin-A treated cells: n = 8).
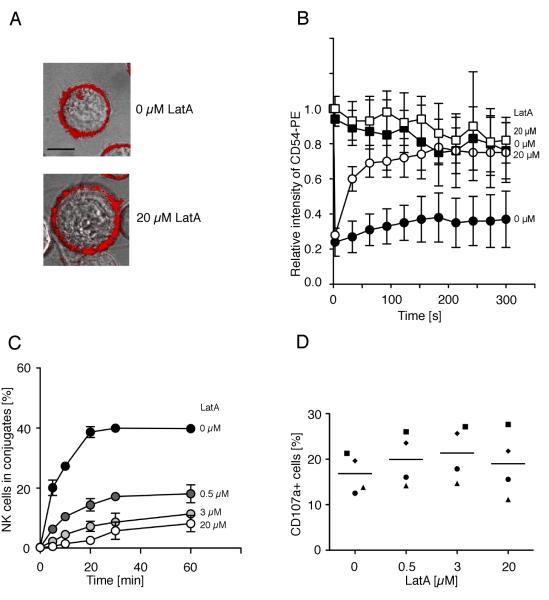
To test whether polarization of ICAM-1 to one side of target cells, rather than or in addition to ICAM-1 tethering, was important for LFA-1-dependent adhesion of NK cells, we examined the cell line K562, which does not exhibit polarity of ICAM-1 at the cell surface (Fig. 4A). Treatment of K562 cells with Latrunculin A released ICAM-1 form the cytoskeleton and increased its mobility (Fig. 4B). In accordance with the 721.221 data, the release of ICAM-1 from the cytoskeleton of K562 target cells prevented conjugate formation in freshly isolated primary NK cells (Fig. 4C) and polarization of cytolytic granules (data not shown) indicating that ICAM-1 tethering rather than polarization is important for LFA-1 dependent signaling in NK cells. The LFA-1 independent degranulation of NK cells was not inhibited by pre-treatment of K562 target cells with different concentrations of Latrunculin A (Fig. 4D) excluding more global inhibitory effects of the drug.
FIGURE 4.
Disruption of actin filaments increases the mobility of ICAM-1 on K562 cells and decreases conjugate formation with NK cells. The mobility of ICAM-1 was determined by FRAP. Cells were stained with a PE-labeled anti-ICAM-1 antibody. Fluorescence recovery in the bleached area of K562 cells either untreated (A, upper panel, bar indicates 5 μm, B, filled circles) or Latrunculin A-treated (A, lower panel, B, open circles) is displayed. Fluorescence intensity of a non-bleached area of the K562 cells either untreated (B, filled squares) or Latrunculin A-treated (B, open squares) served as a control. Bars indicate the SD (untreated cells: n = 9, Latrunculin-A treated cells: n = 6). (C) K562 cells pre-treated with DMSO carrier alone (filled circles), Latrunculin A at indicated concentrations for 40 min at 37°C were tested for conjugate formation with resting NK cells. Bars indicate the SD (n = 3 individual NK cell donors in three independent experiments). (D) K562 target cells treated as in C were mixed with resting NK cells and degranulation was measured after 1 h at 37°C by staining for CD107a at the surface of NK cells. Each symbol indicates one of the four individual NK cell donors. (n = 4 independent experiments).
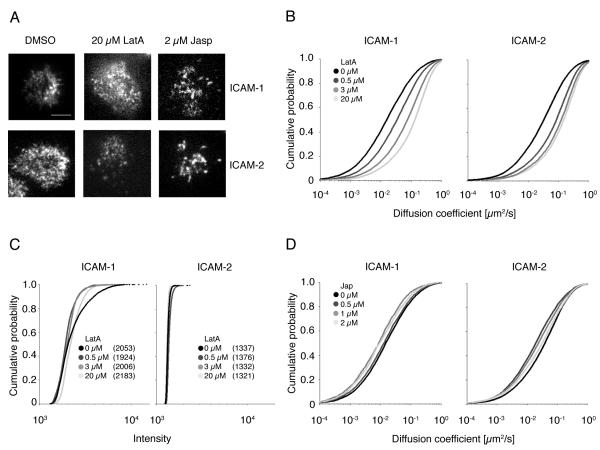
We next used total internal reflection fluorescence (TIRF) microscopy to visualize the distribution and mobility of ICAM molecules in the plasma membrane of target cells, and to understand how the actin cytoskeleton affects ICAM mobility by treating target cells with either Latrunculin A or Jasplakinolide to disrupt or stabilize the actin cytoskeleton, respectively. TIRF microscopy is a spatially-limited, high-contrast technique that eliminates interference from bulk fluorescence that may be present within cells to allow for the detection of fluorophores proximal to and within the plasma membrane of cells adhered to glass coverslips (45). Endogenous, cell-surface ICAM-1 or ICAM-2 on 721.221 cells were fluorescently labeled with PE-conjugated anti-ICAM antibodies. While individual ICAM proteins were labelled with a single PE-fluorophore (see Materials and Methods), photobleaching characteristics (the presence of multiple-step bleaching events over long track lengths; not shown) of fluorescent PE-labeled particles suggest that ICAM proteins were mostly observed as clusters and not single molecules (data not shown).
The lateral movement of labeled ICAM-1 (Fig. 5A, upper panel, Fig. 5B, left, video 3, 4, and 5) and ICAM-2 (Fig. 5A, lower panel, Fig. 5B, right) particles recorded by TIRF microscopy was automatically tracked using an algorithm developed for MatLab software (33), which was further modified to refine particle positioning with a 2D Gaussian fit (34). Short-range mean square displacements (MSDs) were determined from positional coordinates of particles tracked for five frames (over 160 ms; (34)) and were linearly dependent on time under all conditions measured, consistent with a simple diffusion model. MSD vs time plots clearly showed that diffusion increases, as indicated by the slope of the line, with Latrunculin A concentration (Fig. S4). Short-range diffusion coefficients were then determined for thousands of particles in multiple cells and graphed as a cumulative distribution function (CDF) to represent the frequency of diffusion coefficients for the entire population of tracked particles (Fig. 5B; (34). Consistent with our FRAP data above, disrupting the actin cytoskeleton with Latrunculin A incurred a dose-dependent shift toward the mobile population for both ICAM-1 and ICAM-2 particles (Fig. 5B, video 3 and 4). The median diffusion coefficient for ICAM-1 and ICAM-2 in untreated control cells was calculated to be 0.013 μm2/s and 0.030 μm2/s respectively and after the maximum dose of Latrunculin A treatment, mobility increased ~10 and 5 fold, respectively (0.132 μm2/s for ICAM-1 and 0.146 μm2/s for ICAM-2; Fig. 5B). In accordance with these results, binding of NK cells to 721.221 target cells was inhibited by disruption of F-actin filaments in 721.221 cells (Fig1A, left). Although Latrunculin A reduced the intensity of the brightest ICAM-1 clusters, it did not appreciably alter the median intensity of either ICAM-1 or ICAM-2 particles (Fig. 5C), suggesting that ICAM cluster size on target cells does not affect NK cell response.
FIGURE 5.
Disruption of actin filaments increases the mobility of ICAM-1 and ICAM-2. The mobility of ICAM-1 (A, upper panel, bar indicates 5 μm, B and D, left side) and ICAM-2 (A, lower panel, B and D, right side) in 721.221 cells treated with the indicated concentrations of Latrunculin A (A, middle, B) and Jasplakinolide (A, right, D) was determined by Total Internal Reflection Fluorescence (TIRF) microscopy. The movement of ICAM particles labeled with ICAM-1 and ICAM-2 PE-conjugated antibodies was tracked by capturing TIRF images at 32 frames per second for 250 frames. Diffusion coefficients of ICAM-1 (B, and D, left side) or ICAM-2 (B and D, right side) particles are shown in CDF plots. Plots represent one representative experiment out of 2. The total number of ICAM-1 particles analyzed over the drug concentration range is ~40,500 for Latrunculin treated cells and ~15,400 for Jasplakinolide treated cells. The p-values for the Latrunculin treated cells are the following: p = 4.98 × 10−95 between DMSO and 0.5 μM LatA, p = 7.81 × 10−141 between 0.5 μM LatA and 3 μM LatA, and p = 7.86 × 10−116 between 3 μM and 20 μM LatA. All determined by Kolmogorov-Smirnov test. Similarly, ~32,700 ICAM-2 particles in Latrunculin treated cells and ~23,600 particles in Jasplakinolide treated cells were analyzed. C, The average intensity of ICAM-1 and ICAM-2 particles was measured in a 5×5 pixel grid centered over the peak of the Gaussian distribution calculated for the particle in the first frame in which it appeared in the tracking algorithm. Average particle intensities are shown in CDF plots. Median intensities are indicated in parenthesis.
Both our FRAP and TIRF microscopy results indicate that ICAM-1 and ICAM-2 are tethered to the actin cytoskeleton, which, in turn, slows their lateral mobility in the plasma membrane; if so, then one would predict that ICAM mobility would not increase, and may be reduced even further, after treatment with the actin-stabilizing drug Jasplakinolide. Indeed, the mobility of ICAM-1 (Fig. 5D, left, video 5) and ICAM-2 (Fig. 5D, right) was slightly reduced with Jasplakinolide and stabilization of F-actin in target cells had little effect on LFA-1 dependent signaling (Fig. 1A, right). In addition, disruption of the microtubule network with Nocodazole did not affect the mobility of ICAM-1 (data not shown).
Expression of ezrin in BW5147 cells restores ICAM-2 tethering and functional interaction with LFA-1
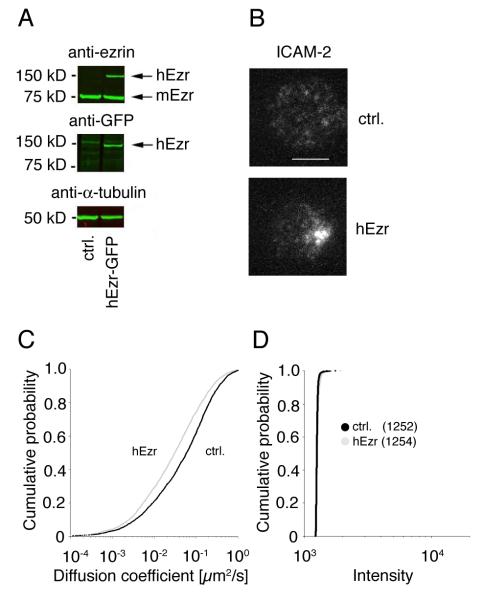
Our results so far indicate that a higher mobility of ICAM in the plasma membrane hinders its interaction with LFA-1 on NK cells. However, the contribution of other changes induced by Latrunculin A in target cells that could impact on LFA-1–ICAM interactions cannot be ruled out. We therefore carried out gain-of-function experiments using a cellular system in which ICAM mobility could be reversed. A change in the distribution of ICAM-2 on the mouse BW5147 thymoma cell line from evenly distributed to polarized after transient expression of human ezrin has been reported (31). We generated BW5147 cells that stably express human ezrin tagged with GFP (Fig. 6A) and monitored the mobility of ICAM-2 by TIRF microcopy (Fig. 6B and C, video 6 and 7). As shown in Fig. 6B and video 6, ICAM-2 was evenly distributed and mobile on BW5147 cells. Expression of GFP-tagged ezrin (Fig. 6A, 150 kDa band) in BW5147 cells decreased the mobility of ICAM-2 (Fig. 6C, video 6 and 7) from 0.053 μm2/s to 0.027 μm2/s. Moreover, ICAM-2 became polarized (Fig. 6B) and co-localized with the distribution of ezrin-GFP (data not shown). However, clustering of ICAM-2 molecules did not change (Fig. 6D).
FIGURE 6.
Expression of human ezrin in BW5417 cells reduces the mobility of mouse ICAM-2. A, Lysates of BW5147 cells and BW5147 cells transfected with human ezrin-EGFP where probed with anti-ezrin, anti-GFP, and anti-a-tubulin antibody. B, C, The mobility of ICAM-2 on BW5147 cells (C, black line) and BW5147 cells expressing human ezrin (C, grey line) was determined by TIRF microscopy as described in Figure 5 using a PE labeled anti-mouse ICAM-2 antibody. Bar indicates 5 μm. Plots represent the cumulative frequency of diffusion coefficients of ICAM-2 particles of one representative experiment out of two. ~3100 and 2700 particles in untransfected and ezrin transfected cells, respectively, were analyzed. D, The intensity of ICAM-2 clusters on BW5147 cells (black line) and BW5147 cells expressing human ezrin (grey line) was analyzed as described in Figure 5. Median intensities are indicated in parenthesis.
To test if the reduced mobility of ICAM-2 due to ezrin expression had an impact on functional interaction with LFA-1 on NK cells, conjugate assays were performed. Conjugate formation between human primary IL-2-activated NK cells and BW5147 cells was increased (Fig. 7A) when ICAM-2 mobility was reduced through ezrin expression (Fig. 6C). Moreover, expression of human ezrin in BW5147 target cells increased polarization of cytolytic granules in IL-2 activated NK cells (Fig. 7B). We conclude that tethering of ICAM-2 to the cytoskeleton of BW5147 cells by expression of human ezrin restores functional interaction with LFA-1.
FIGURE 7.
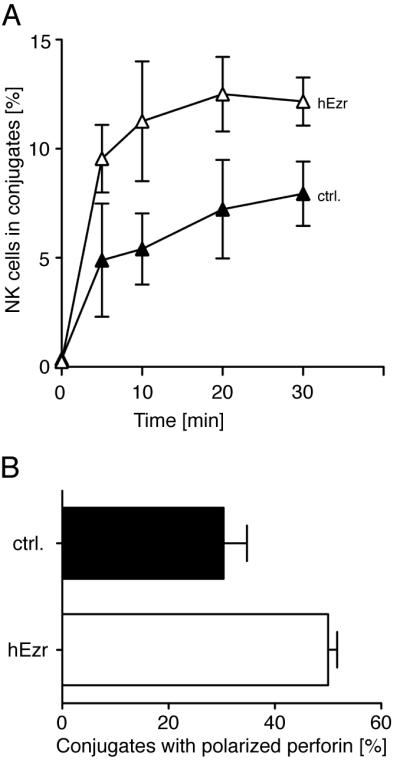
Expression of human ezrin in BW5147 cells restores functional interaction with LFA-1. A, Conjugate formation of IL-2-activated NK cells with untransfected BW5147 cells (filled triangles) or with BW5147-hEzrin target cells (open triangles) was determined by flow cytometry. B, Polarization of cytolytic granules in IL-2-activated NK cells mixed with either BW5147 (filled bar) or BW5147-hEzrin (open bar) cells was measured as described in Figure 2. Bars indicate the SD (n = 3 individual NK cell donors in three independent experiments).
Rapid movement of NK cells over ICAM-1 inserted into lipid bilayers
We next tested the interaction of LFA-1 on NK cells with ICAM-1 in the absence of other receptor-ligand interactions, and compared interactions with mobile versus immobile ICAM-1. To visualize binding of NK cells to mobile ICAM-1, a histidine-tagged form of ICAM-1 was inserted into artificial planar lipid bilayers at the physiological density of 250 molecules/μm2. Under these conditions, ICAM-1 exhibits high lateral mobility (10, 46). Primary resting NK cells on ICAM-1-coated lipid bilayers displayed rapid movement and failed to stop (video 8). A large fraction of NK cells displayed directed movement (Fig. 8A, video 8), while some moved randomly. The moving NK cells have a distinguished morphology consisting of a uropod and a dominant pseudopod at the leading edge of the cell (Fig. 8A, video 8). NK cells did not contact lipid bilayers in the absence of ICAM-1 (data not shown). At concentrations of 200 and 500 ICAM-1 molecules/μm2 NK cells showed a similar phenotype than at 250 molecules per μm2. The inability of NK cells to stop over lipid bilayers carrying ICAM-1 is not a general feature of their interaction with diffusible ligands, as the CD16 ligand IgG1 Fc inserted into lipid bilayers caused NK cell arrest and accumulation of IgG1 Fc into tight clusters (10). To monitor interaction with immobile ICAM-1, an ICAM-1-Fc fusion protein, in which the CD16 binding site had been mutated, was attached to coverslips. NK cells did not contact the coverslips in the absence of ICAM-1 or coverslips that had been coated with ICAM-1 at a concentration of 10 ng/ml and 100 ng/ml (data not shown). However, ICAM-1 coating at concentrations of 1 μg/ml and 10 μg/ml resulted in NK cells that were either attached and spread on the coverslips (Fig. 8B, left, video 9), or attached and spread NK cells that moved a little (Fig. 8B, right, video 10). In contrast to the persistent movement over ICAM-1-coated lipid bilayers, movement of NK cells over ICAM-1-coated coverslips was non-directional and slower (Fig. 8B, video 9 and 10). A recent study reported that the NK cell line NKL moved over plate-bound ICAM-1-Fc (47). The difference may be due to the cells, as in our hands, NKL cells do not behave like primary NK cells when placed over lipid bilayers carrying ligands of NK cell receptors (D. L., unpublished). Taken together our results indicate that diffusible ICAM-1 interacts with receptors on NK cells but does not promote the arrest of cell movement, whereas immobile ICAM-1 promotes stable binding of primary NK cells.
FIGURE 8.
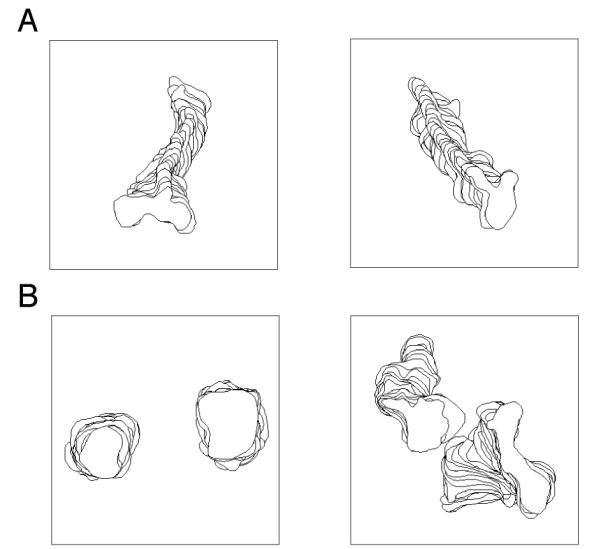
Movement of human resting NK cells over mobile and immobile ICAM-1. NK cells were visualized 20 minutes after injection into chambers containing ICAM-1 bound to either lipid bilayers or glass surfaces, respectively. The movement of NK cells was tracked for 25 frames with a 10 s interval between frames by Dynamic Image Analysis System (DIAS). The cell at the top of the stack is the cell from the last frame. A, Engagement of LFA-1 by diffusible ICAM-1 on lipid bilayer promoted active movement of NK cells. B, Engagement of LFA-1 by solid phase ICAM-1 resulted in arrest and stable binding (left) or spreading and slow movement of NK cells (right).
Discussion
Lateral mobility and clustering of transmembrane receptors and their segregation within membrane domains are often essential for proper signaling (26-28). Less is known about the importance of receptor ligand distribution at the surface of opposing cells (48). We addressed the question of how the distribution or mobility of ligands affect receptor signaling in the context of β2 integrin LFA-1 in NK cells and its functional interaction with ligands ICAM-1 and ICAM-2 on target cells. Earlier work established that the binding of LFA-1 on NK cells to ICAM-1 on target cells was sufficient for the formation of tight conjugates and polarization of cytolytic granules towards the NK–target cell interface (4, 12, 14, 15, 39). As LFA-1 signaling for adhesion and granule polarization in human NK cells is uncoupled from signaling by other activation receptors (13, 14), it was possible to focus on NK cell functions controlled uniquely by LFA-1.
Two techniques, FRAP and TIRF microscopy, were used to visualize and quantify the distribution and movement of ICAM-1 and ICAM-2 on target cells in which the cytoskeleton was disrupted by Latrunculin A. The two-dimensional mobility of ICAM-1 and ICAM-2 at the surface of target cells was greatly enhanced after depolymerization of F-actin, and correlated with reduced LFA-1-dependent NK cell responses. ICAM mobility, rather than clustering or polarization, was the key parameter for adhesion and granule polarization induced by LFA-1. Latrunculin A had minimal effect on the intensity of ICAM-1 and ICAM-2 clusters. Polarization of ICAM to one end of the cell, such as the one induced by ezrin, is not necessary for functional LFA-1-dependent responses because the target cell K562, which is highly sensitive to lysis by NK cells, does not display polarized ICAM-1 and stimulates strong LFA-1-dependent responses, which were lost upon release of ICAM-1 from cytoskeletal constraints.
To address the possibility that the reduction in NK cell adhesion and granule polarization after Latrunculin A treatment of target cells was due to a change other than ICAM distribution, gain-of-function experiments were carried out with the NK-sensitive mouse thymoma cell line BW5147, which expresses ICAM-2. Transfection of human ezrin into BW5147 causes a redistribution of ICAM-2 into a uropod-like cell extension, and an increase in sensitivity to killing by NK cells (31). As reported here, the lateral mobility of ICAM-2, but not the intensity of ICAM-2 clusters, was reduced by transfection of ezrin, which correlated with increased NK cell adhesion and granule polarization. Although ICAM clustering and polarization to one end of target cells may well contribute to NK cell activation, our data show that tethering and reduced mobility of ICAM are required for proper signaling by LFA-1.
To test the interaction of NK cells with either mobile or immobile ICAM-1 in the complete absence of other receptor–ligand interactions, purified recombinant ICAM-1 was inserted into artificial planar lipid bilayers, on which it is freely diffusible, or attached to glass. Primary, resting NK cells readily bound and formed tight contact with ICAM-1 on the solid support, but moved continuously on lipid bilayers carrying diffusible ICAM-1. Therefore, ICAM-1 tethering is required for primary NK cells to form stable contacts, such as those occurring during NK–target cell interactions.
Adhesion of NK cells to target cells is accompanied by the formation of a cytotoxic immunological synapse, in which ICAM-1 on target cells segregates into a ring that surrounds a central region (10, 49, 50). At inhibitory NK cell immunological synapses, LFA-1 on NK cells and ICAM-1 on target cells are excluded from a central region where inhibitory receptors accumulate (8, 51). These results suggest that ICAM-1 acquires mobility during synapse formation. Engagement of ICAM-1 initiates signaling, which leads to recruitment of lipid rafts and MHC molecules (48). Whether or not ICAM-1 signaling is required for its peripheral distribution at NK cell cytotoxic immunological synapses remains to be tested.
Cellular signaling in response to mechanical forces exerted through cell surface receptors and the cytoskeleton, referred to as “mechanotransduction”, has been gaining recognition (52-54). Cells sense force at points of attachment, such as β1 integrin binding to fibronectin (55). Force contributes to conformational and affinity changes in integrins (56, 57). As force sensing by LFA-1 would depend on tethering of its ligands, it is possible that a loss of mechanotransduction due to release of ICAM from the cytoskeleton in target cells is at the base of adhesion and granule polarization defects in NK cells.
We have shown that low mobility of ICAM on target cells is important for conjugate formation and adhesion of NK cells, suggesting that changes in the cytoskeleton could render target cells less sensitive to killing by NK cells. Changes in ICAM-1 mobility, such as those occurring during cell senescence (58), could have a direct impact on the sensitivity of cells to killing by NK cells. The importance of the cytoskeleton in cells that activate lymphocytes was illustrated recently by the effect of Schistosoma mansoni T2 ribonuclease on dendritic cells, in which cytoskeletal changes were accompanied by deficient interaction with CD4+ T cells (59).
Supplementary Material
Acknowledgements
We thank J.-J. Hao and S. Shaw for the hEzrin-EGFP construct, T. Kamala for the BW5147 cells, M. Peterson for mutagenesis of the Fc fusion vector, M. March, P. Sun, and S. Rajagopalan for advice and comments, and P. Tolar for the MatLab code and technical discussions.
This research was supported by the Intramural Research Program of the NIAID/NIH.
Abbreviations used in this paper
- ERM
ezrin/radixin/moesin
- FRAP
fluorescence recovery after photobleaching
- TIRF
total internal reflection fluorescence
References
- 1.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 2.Davignon D, Martz E, Reynolds T, Kurzinger K, Springer TA. Lymphocyte function-associated antigen 1 (LFA-1): a surface antigen distinct from Lyt-2,3 that participates in T lymphocyte-mediated killing. Proc Natl Acad Sci U S A. 1981;78:4535–4539. doi: 10.1073/pnas.78.7.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helander T, Timonen T, Kalliomaki P, Schroder J. Recognition of chromosome 6-associated target structures by human lymphokine-activated killer cells. J Immunol. 1991;147:2063–2067. [PubMed] [Google Scholar]
- 4.Krensky AM, Sanchez-Madrid F, Robbins E, Nagy JA, Springer TA, Burakoff SJ. The functional significance, distribution, and structure of LFA-1, LFA-2, and LFA-3: cell surface antigens associated with CTL-target interactions. J Immunol. 1983;131:611–616. [PubMed] [Google Scholar]
- 5.Somersalo K, Anikeeva N, Sims TN, Thomas VK, Strong RK, Spies T, Lebedeva T, Sykulev Y, Dustin ML. Cytotoxic T lymphocytes form an antigen-independent ring junction. J Clin Invest. 2004;113:49–57. doi: 10.1172/JCI200419337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki J, Yamasaki S, Wu J, Koretzky GA, Saito T. The actin cloud induced by LFA-1-mediated outside-in signals lowers the threshold for T-cell activation. Blood. 2007;109:168–175. doi: 10.1182/blood-2005-12-020164. [DOI] [PubMed] [Google Scholar]
- 7.Sims TN, Dustin ML. The immunological synapse: integrins take the stage. Immunol Rev. 2002;186:100–117. doi: 10.1034/j.1600-065x.2002.18610.x. [DOI] [PubMed] [Google Scholar]
- 8.Almeida CR, Davis DM. Segregation of HLA-C from ICAM-1 at NK cell immune synapses is controlled by its cell surface density. J Immunol. 2006;177:6904–6910. doi: 10.4049/jimmunol.177.10.6904. [DOI] [PubMed] [Google Scholar]
- 9.Anikeeva N, Somersalo K, Sims TN, Thomas VK, Dustin ML, Sykulev Y. Distinct role of lymphocyte function-associated antigen-1 in mediating effective cytolytic activity by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 2005;102:6437–6442. doi: 10.1073/pnas.0502467102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu D, Bryceson YT, Meckel T, Vasiliver-Shamis G, Dustin ML, Long EO. Integrin-dependent organization and bidirectional vesicular traffic at cytotoxic immune synapses. Immunity. 2009;31:99–109. doi: 10.1016/j.immuni.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dustin ML, Springer TA. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341:619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- 12.Barber DF, Long EO. Coexpression of CD58 or CD48 with Intercellular Adhesion Molecule 1 on Target Cells Enhances Adhesion of Resting NK Cells. J Immunol. 2003;170:294–299. doi: 10.4049/jimmunol.170.1.294. [DOI] [PubMed] [Google Scholar]
- 13.Bryceson YT, Ljunggren HG, Long EO. Minimal requirement for induction of natural cytotoxicity and intersection of activation signals by inhibitory receptors. Blood. 2009;114:2657–2666. doi: 10.1182/blood-2009-01-201632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med. 2005;202:1001–1012. doi: 10.1084/jem.20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barber DF, Faure M, Long EO. LFA-1 contributes an early signal for NK cell cytotoxicity. J Immunol. 2004;173:3653–3659. doi: 10.4049/jimmunol.173.6.3653. [DOI] [PubMed] [Google Scholar]
- 16.Mace EM, Monkley SJ, Critchley DR, Takei F. A dual role for talin in NK cell cytotoxicity: activation of LFA-1-mediated cell adhesion and polarization of NK cells. J Immunol. 2009;182:948–956. doi: 10.4049/jimmunol.182.2.948. [DOI] [PubMed] [Google Scholar]
- 17.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107:159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mor A, Dustin ML, Philips MR. Small GTPases and LFA-1 reciprocally modulate adhesion and signaling. Immunol Rev. 2007;218:114–125. doi: 10.1111/j.1600-065X.2007.00538.x. [DOI] [PubMed] [Google Scholar]
- 19.Yokosuka T, Saito T. Dynamic regulation of T-cell costimulation through TCR-CD28 microclusters. Immunol Rev. 2009;229:27–40. doi: 10.1111/j.1600-065X.2009.00779.x. [DOI] [PubMed] [Google Scholar]
- 20.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 21.Lillemeier BF, Mortelmaier MA, Forstner MB, Huppa JB, Groves JT, Davis MM. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat Immunol. 11:90–96. doi: 10.1038/ni.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watzl C, Long EO. Natural killer cell inhibitory receptors block actin cytoskeleton-dependent recruitment of 2B4 (CD244) to lipid rafts. J Exp Med. 2003;197:77–85. doi: 10.1084/jem.20020427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marwali MR, MacLeod MA, Muzia DN, Takei F. Lipid rafts mediate association of LFA-1 and CD3 and formation of the immunological synapse of CTL. J Immunol. 2004;173:2960–2967. doi: 10.4049/jimmunol.173.5.2960. [DOI] [PubMed] [Google Scholar]
- 24.Sheets ED, Holowka D, Baird B. Critical role for cholesterol in Lyn-mediated tyrosine phosphorylation of FcOERI and their association with detergent-resistant membranes. J.Cell Biol. 1999;145:877–887. doi: 10.1083/jcb.145.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tolar P, Sohn HW, Pierce SK. Viewing the antigen-induced initiation of B-cell activation in living cells. Immunol Rev. 2008;221:64–76. doi: 10.1111/j.1600-065X.2008.00583.x. [DOI] [PubMed] [Google Scholar]
- 26.Dykstra M, Cherukuri A, Sohn HW, Tzeng SJ, Pierce SK. Location is everything: lipid rafts and immune cell signaling. Annu Rev Immunol. 2003;21:457–481. doi: 10.1146/annurev.immunol.21.120601.141021. [DOI] [PubMed] [Google Scholar]
- 27.Harder T, Rentero C, Zech T, Gaus K. Plasma membrane segregation during T cell activation: probing the order of domains. Curr Opin Immunol. 2007;19:470–475. doi: 10.1016/j.coi.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Sengupta P, Baird B, Holowka D. Lipid rafts, fluid/fluid phase separation, and their relevance to plasma membrane structure and function. Semin Cell Dev Biol. 2007;18:583–590. doi: 10.1016/j.semcdb.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tseng SY, Liu ML, Dustin ML. CD80 cytoplasmic domain controls localization of CD28, CTLA-4, and protein kinase Ctheta in the immunological synapse. J Immunol. 2005;175:7829–7836. doi: 10.4049/jimmunol.175.12.7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eleme K, Taner SB, Onfelt B, Collinson LM, McCann FE, Chalupny NJ, Cosman D, Hopkins C, Magee AI, Davis DM. Cell surface organization of stress-inducible proteins ULBP and MICA that stimulate human NK cells and T cells via NKG2D. J Exp Med. 2004;199:1005–1010. doi: 10.1084/jem.20032194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helander TS, Carpen O, Turunen O, Kovanen PE, Vaheri A, Timonen T. ICAM-2 redistributed by ezrin as a target for killer cells. Nature. 1996;382:265–268. doi: 10.1038/382265a0. [DOI] [PubMed] [Google Scholar]
- 32.Hao JJ, Liu Y, Kruhlak M, Debell KE, Rellahan BL, Shaw S. Phospholipase C-mediated hydrolysis of PIP2 releases ERM proteins from lymphocyte membrane. J Cell Biol. 2009;184:451–462. doi: 10.1083/jcb.200807047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Douglass AD, Vale RD. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell. 2005;121:937–950. doi: 10.1016/j.cell.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tolar P, Hanna J, Krueger PD, Pierce SK. The constant region of the membrane immunoglobulin mediates B cell-receptor clustering and signaling in response to membrane antigens. Immunity. 2009;30:44–55. doi: 10.1016/j.immuni.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 36.Winter CC, Long EO. Binding of soluble KIR-Fc fusion proteins to HLA class I. Methods Mol Biol. 2000;121:239–250. doi: 10.1385/1-59259-044-6:239. [DOI] [PubMed] [Google Scholar]
- 37.Wessels DJ, Kuhl S, Soll DR. Light microscopy to image and quantify cell movement. Methods Mol Biol. 2009;571:455–471. doi: 10.1007/978-1-60761-198-1_30. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu Y, Geraghty DE, Koller BH, Orr HT, DeMars R. Transfer and expression of three cloned human non-HLA-A,B,C class I major histocompatibility complex genes in mutant lymphoblastoid cells. Proc Natl Acad Sci USA. 1988;85:227–231. doi: 10.1073/pnas.85.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burshtyn DN, Shin J, Stebbins C, Long EO. Adhesion to target cells is disrupted by the killer cell inhibitory receptor. Curr Biol. 2000;10:777–780. doi: 10.1016/s0960-9822(00)00568-6. [DOI] [PubMed] [Google Scholar]
- 40.Carpen O, Pallai P, Staunton DE, Springer TA. Association of intercellular adhesion molecule-1 (ICAM-1) with actin-containing cytoskeleton and alpha-actinin. J Cell Biol. 1992;118:1223–1234. doi: 10.1083/jcb.118.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heiska L, Kantor C, Parr T, Critchley DR, Vilja P, Gahmberg CG, Carpen O. Binding of the cytoplasmic domain of intercellular adhesion molecule-2 (ICAM-2) to alpha-actinin. J Biol Chem. 1996;271:26214–26219. doi: 10.1074/jbc.271.42.26214. [DOI] [PubMed] [Google Scholar]
- 42.Celli L, Ryckewaert JJ, Delachanal E, Duperray A. Evidence of a functional role for interaction between ICAM-1 and nonmuscle alpha-actinins in leukocyte diapedesis. J Immunol. 2006;177:4113–4121. doi: 10.4049/jimmunol.177.6.4113. [DOI] [PubMed] [Google Scholar]
- 43.Heiska L, Alfthan K, Gronholm M, Vilja P, Vaheri A, Carpen O. Association of ezrin with intercellular adhesion molecule-1 and -2 (ICAM-1 and ICAM-2). Regulation by phosphatidylinositol 4, 5-bisphosphate. J Biol Chem. 1998;273:21893–21900. doi: 10.1074/jbc.273.34.21893. [DOI] [PubMed] [Google Scholar]
- 44.Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol. 2002;157:1233–1245. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Axelrod D. Total internal reflection fluorescence microscopy in cell biology. Traffic. 2001;2:764–774. doi: 10.1034/j.1600-0854.2001.21104.x. [DOI] [PubMed] [Google Scholar]
- 46.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 47.Culley FJ, Johnson M, Evans JH, Kumar S, Crilly R, Casasbuenas J, Schnyder T, Mehrabi M, Deonarain MP, Ushakov DS, Braud V, Roth G, Brock R, Kohler K, Davis DM. Natural killer cell signal integration balances synapse symmetry and migration. PLoS Biol. 2009;7:e1000159. doi: 10.1371/journal.pbio.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lebedeva T, Dustin ML, Sykulev Y. ICAM-1 co-stimulates target cells to facilitate antigen presentation. Curr Opin Immunol. 2005;17:251–258. doi: 10.1016/j.coi.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 49.Vyas YM, Mehta KM, Morgan M, Maniar H, Butros L, Jung S, Burkhardt JK, Dupont B. Spatial organization of signal transduction molecules in the NK cell immune synapses during MHC class I-regulated noncytolytic and cytolytic interactions. J Immunol. 2001;167:4358–4367. doi: 10.4049/jimmunol.167.8.4358. [DOI] [PubMed] [Google Scholar]
- 50.Nedvetzki S, Sowinski S, Eagle RA, Harris J, Vely F, Pende D, Trowsdale J, Vivier E, Gordon S, Davis DM. Reciprocal regulation of human natural killer cells and macrophages associated with distinct immune synapses. Blood. 2007;109:3776–3785. doi: 10.1182/blood-2006-10-052977. [DOI] [PubMed] [Google Scholar]
- 51.Schleinitz N, March ME, Long EO. Recruitment of Activation Receptors at Inhibitory NK Cell Immune Synapses. PLoS ONE. 2008;3:e3278. doi: 10.1371/journal.pone.0003278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giannone G, Sheetz MP. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol. 2006;16:213–223. doi: 10.1016/j.tcb.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz MA. Cell biology. The force is with us. Science. 2009;323:588–589. doi: 10.1126/science.1169414. [DOI] [PubMed] [Google Scholar]
- 54.Kim ST, Takeuchi K, Sun ZY, Touma M, Castro CE, Fahmy A, Lang MJ, Wagner G, Reinherz EL. The alphabeta T cell receptor is an anisotropic mechanosensor. J Biol Chem. 2009;284:31028–31037. doi: 10.1074/jbc.M109.052712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls alpha5beta1 function. Science. 2009;323:642–644. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- 56.Astrof NS, Salas A, Shimaoka M, Chen J, Springer TA. Importance of force linkage in mechanochemistry of adhesion receptors. Biochemistry. 2006;45:15020–15028. doi: 10.1021/bi061566o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alon R, Dustin ML. Force as a facilitator of integrin conformational changes during leukocyte arrest on blood vessels and antigen-presenting cells. Immunity. 2007;26:17–27. doi: 10.1016/j.immuni.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 58.Zhou X, Perez F, Han K, Jurivich DA. Clonal senescence alters endothelial ICAM-1 function. Mech Ageing Dev. 2006;127:779–785. doi: 10.1016/j.mad.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 59.Steinfelder S, Andersen JF, Cannons JL, Feng CG, Joshi M, Dwyer D, Caspar P, Schwartzberg PL, Sher A, Jankovic D. The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1) J Exp Med. 2009;206:1681–1690. doi: 10.1084/jem.20082462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.