Summary
Chronic airway infection with Pseudomonas aeruginosa (PA) causes morbidity and mortality in patients with cystic fibrosis (CF). Additional anti-PA therapies are needed to improve health status and health-related quality of life. AIR-CF3 was an international 18-month, open-label study to evaluate the safety and efficacy of repeated courses of aztreonam for inhalation solution (AZLI, now marketed as Cayston®) in patients aged ≥6 years with CF and PA infection who previously participated in one of two Phase 3 studies: AIR-CF1 or AIR-CF2. Patients received up to nine courses (28 days on/28 days off) of 75 mg AZLI two (BID) or three times daily (TID) based on randomization in the previous trials. 274 patients, mean age 28.5 years (range: 8–74 years), participated. Mean treatment adherence was high (92.0% BID group, 88.0% TID group). Hospitalization rates were low and adverse events were consistent with CF With each course of AZLI, FEV1 and scores on the Cystic Fibrosis Questionnaire-Revised Respiratory Symptomscale improved and bacterial density in sputum was reduced. Benefits waned in the 28 days off therapy, but weight gain was sustained over the 18months. There were no sustained decreases in PA susceptibility. A dose response was observed; AZLI TID-treated patients demonstrated greater improvements in lung function and respiratory symptoms over 18 months. Repeated intermittent 28-day courses of AZLI treatment were well tolerated. Clinical benefits in pulmonary function, health-related quality of life, and weight were observed with each course of therapy. AZLI is a safe and effective new therapy in patients with CF and PA airway infection.
Keywords: antibiotic, Pseudomonas aeruginosa, pulmonary function, quality of life
INTRODUCTION
Chronic, suppressive antibiotic therapy has become the standard of care for patients with cystic fibrosis (CF) and chronic Pseudomonas aeruginosa (PA) infection.1 Inhaled antibiotics may be preferred to systemic antibiotics in the treatment of chronic endobronchial infection in patients with CF because of better tolerability, increased airway concentrations of antibiotic to ventilated regions of the lung compared with systemic administration, and the minimization of systemic effects and drug-drug interactions.
Aztreonam for inhalation solution (AZLI) is an aerosolized formulation of the monobactam antibiotic, aztreonam, with lysine as an excipient.2 The intravenous (IV) aztreonam formulation contains arginine, which can cause airway inflammation after repeated inhalation in patients with CF.3,4
Two published placebo-controlled studies of AZLI showed benefit in patients with CF and PA infection.5,6 AIR-CF1 demonstrated that a 28-day course of AZLI given three times daily (TID) resulted in improved respiratory symptoms as measured by the cystic fibrosis questionnaire-revised (CFQ-R), increased forced expiratory volume in 1 sec (FEV1), and decreased bacterial density in sputum.6 AIR-CF2 showed that a 28-day course of AZLI immediately following a 28-day course of tobramycin inhalation solution (TIS) delayed the timeto-need for additional inhaled or systemic anti-PA antibiotics.5 It also showed an increase in FEV1 and improved respiratory symptom scores on the CFQ-R at the end of the AZLI treatment course compared to placebo. No safety concerns emerged with the two short-term studies. However, the safety and efficacy of long-term AZLI therapy remained untested.
The current protocol, AIR-CF3, was an 18-month openlabel study to evaluate the safety and efficacy of two dose regimens of AZLI in patients with CF and PA airway infection using the accepted treatment paradigm of month on/month off therapy.7
MATERIALS AND METHODS
Study Design
Patients who previously participated in either AIR-CF1 or AIR-CF2 were eligible to enroll in this open-label, follow-on study conducted at 71 CF centers (Australia, Canada, New Zealand, US; August 2005–November 2008). Patients received up to 9 courses, 28 days of AZLI followed by 28 days off therapy. Additional specialized CF care continued throughout the study period as prescribed by each patient’s primary treating CF care provider. The original protocol was designed to have patients receive two courses with an optional third course; the protocol was amended to extend the treatment period to nine courses in order to provide long-term safety and efficacy data and satisfy clinical demand for continued therapy. Patients attended up to 20 scheduled investigational visits. Patients received open-label AZLI 75 mg TID (via an investigational nebulizer (PARI eFlow® Electronic Nebulizer, branded Altera® in the European Union, manufactured by PARI Innovative Manufacturers, Midlothian, Virginia),8 except for those who had originally been randomized to the 75mg twice daily (BID) dosing regimen arm of AIR-CF2. All 85 patients receiving BID therapy in AIR-CF3 were from the BID arm of the AIR-CF2 study. Patients were off AIR-CF1 or AIR-CF2 study drug for at least 28 days before starting AZLI in AIR-CF3. The drug-free interval varied and may have been longer at some sites due to prolonged Institutional Review Board/Ethics Committee review timelines. Patients were instructed to use an inhaled bronchodilator prior to each dose of AZLI. The bronchodilator used was based on each patient’s routine (i.e., long-acting versus short-acting) and used to avoid any potential bronchospasm associated with inhaled medication use. Patients were also instructed to take the doses of AZLI a minimum of 4 hr apart. Patients attended a follow-up visit 28 days after completing the last course of AZLI.
A physical exam was performed at screening, subsequent visits, and at follow up. Spirometry (American Thoracic Society standards) was performed at every visit before, and 30 minutes after, receiving a dose of AZLI.9 FEV1 % predicted values were calculated using the Knudson equation.10 The age-appropriate CFQ-R was administered at each study visit prior to collection of any other data.11 Study medication was dispensed at the beginning of each course of treatment; used and unused vials were collected to assess treatment adherence.
This study was conducted in compliance with the Declaration of Helsinki, the International Conference on Harmonization guideline for Good Clinical Practices, and the applicable regulations for each participating country. Institutional Review Boards (US) and Ethics Committees (Canada, Australia, and New Zealand) approved the study for each site, and all patients or their guardians provided written informed consent or assent prior to any study procedures. The ClinicalTrials.gov accession number is NCT00128492.
Study Population
Patients were ≥6 years of age with a documented CF diagnosis (as evidenced by one or more clinical features consistent with the CF phenotype and one or more of the following criteria: sweat chloride ≥60 mEq/L by quantitative pilocarpine iontophoresis test, or two well characterized mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, or abnormal nasal potential difference).12 All patients had completed either Study AIR-CF1 or AIR-CF2 or had withdrawn from either of these studies due to need for antipseudomonal antibiotics or due to an adverse event (AE) unrelated to study medication tolerability.
Exclusion criteria included: the use of any investigational medication or device between the last visit of Studies AIR-CF1 or AIR-CF2 and Visit 1 (baseline) of AIR-CF3; concurrent participation in a study of another investigational medication or device; current oral corticosteroid use equivalent to> 10 mg prednisone daily; airway cultures yielding Burkholderia cepacia complex (previous 2 years); daily continuous oxygen supplementation of>2 L/min at night; inability to tolerate study medication in Studies AIR-CF1 or AIR-CF2; monobactam antibiotic hypersensitivity; intolerance to inhaled short-acting β2-agonists; aspartate aminotransferase (AST) or alanine aminotransferase (ALT) >5 times upper limit of normal at most recent test prior to enrolling in AIR-CF3; pregnancy; lactation; or, in the opinion of the investigator, medical or psychiatric illness interfering with study participation.
Safety Measures
Adverse events and changes in clinical laboratory values, vital signs, and airway reactivity were monitored. Worsening CF symptoms were classified as treatment-emergent adverse events.
Disease-Related Endpoint Measures
FEV1 was recorded at all scheduled and unscheduled visits.
The CFQ-R was administered at baseline and every visit thereafter prior to other study procedures and AZLI treatment. The endpoint was change in respiratory symptoms from baseline, assessed with the CFQ-R Respiratory Symptom (CFQ-R-Respiratory) scale (range of scores: 0–100; higher scores indicate fewer symptoms). The minimal clinically important difference (MCID) corresponds to the smallest change in symptoms that a patient can detect and is used to interpret responses to patient-reported outcomes(PROs).13,14 An MCID score of five was used in previous studies;5,6 however, a score of 4 has been determined for the CFQ-R-Respiratory scale in stable patients.11 Thus four-point change in scores reflected improved or worsened respiratory symptoms as reported by patients.
The following non-respiratory quality of life domains were measured by the CFQ-R on a standardized scale of 0–100 by both the patient and parent/caregiver: Physical Functioning, Emotional Functioning, Social Functioning, Body Image, Eating Disturbances, Role Limitations/ School Performance, Weight, Vitality, Treatment Burden, Digestive Symptoms, and Health Perceptions.
Other disease-related endpoints included sputum PA density (colony forming units (CFU)/g sputum, log10 transformed), the percent of days and number of days hospitalized, time to first respiratory hospitalization, percent change in weight, and the time to use of IV antibiotics.
Microbiology Endpoints
Sputum samples were collected at all visits for qualitative and quantitative culture for PA, Burkholderia spp., Stenotrophomonas maltophilia, Achromobacter xylosoxidans, Staphylococcus aureus, Candida spp., and Aspergillus spp. If a patient was unable to produce sputum, an oropharyngeal swab was collected for qualitative culture only. Microbiologic testing and analyses were conducted at two central laboratories: Covance Central Laboratory Services (for specimens collected in North America); and SydPath Central Laboratory (for specimens collected in Australia/New Zealand). Sputum and swab specimens were collected prior to the in-clinic administration of AZLI and at least 4 hr after an at-home AZLI administration. Sputum PA density (CFU/g sputum) was determined using serial sputum dilutions plated onto MacConkey agar. The minimum inhibitory concentration (MIC) of aztreonam to PA isolated from subject specimens was determined using a microbroth dilution technique. Twofold dilutions of aztreonam in Mueller Hinton broth spanned from 2,048 to 1 µg/ml. A ≥4-fold change in MIC50 or MIC90 from the baseline value was considered an increase or decrease.15 The presence of methicillinsensitive (MSSA) and methicillin-resistant (MRSA) S. aureus was determined by testing the susceptibility of S. aureus isolates to cefoxitin.16
Statistical Analyses
Descriptive statistics for all patients receiving ≥ 1 dose of AZLI were summarized for the safety, microbiology, and disease-related endpoints. No formal hypothesis tests were planned.
The percent of patients experiencing at least one AE was summarized.
Percent changes from baseline in FEV1 percent predicted and actual changes from baseline in CFQ-R-Respiratory scores and log10 PA CFUs in sputum were summarized.
Rate of hospitalizations were calculated as the total number of hospitalizations divided by the sum total of years patients were on study. The time to first respiratory hospitalization and the time to first use of IV antipseudomonal antibiotics were summarized by regimen based on Kaplan–Meier analyses. Actual changes from baseline in weight (kg) were summarized for the BID and TID regimens.
Statistical analyses used Statistical Analysis Software version 9.1 (SAS®, SAS Institute Inc., Cary, NC).
RESULTS
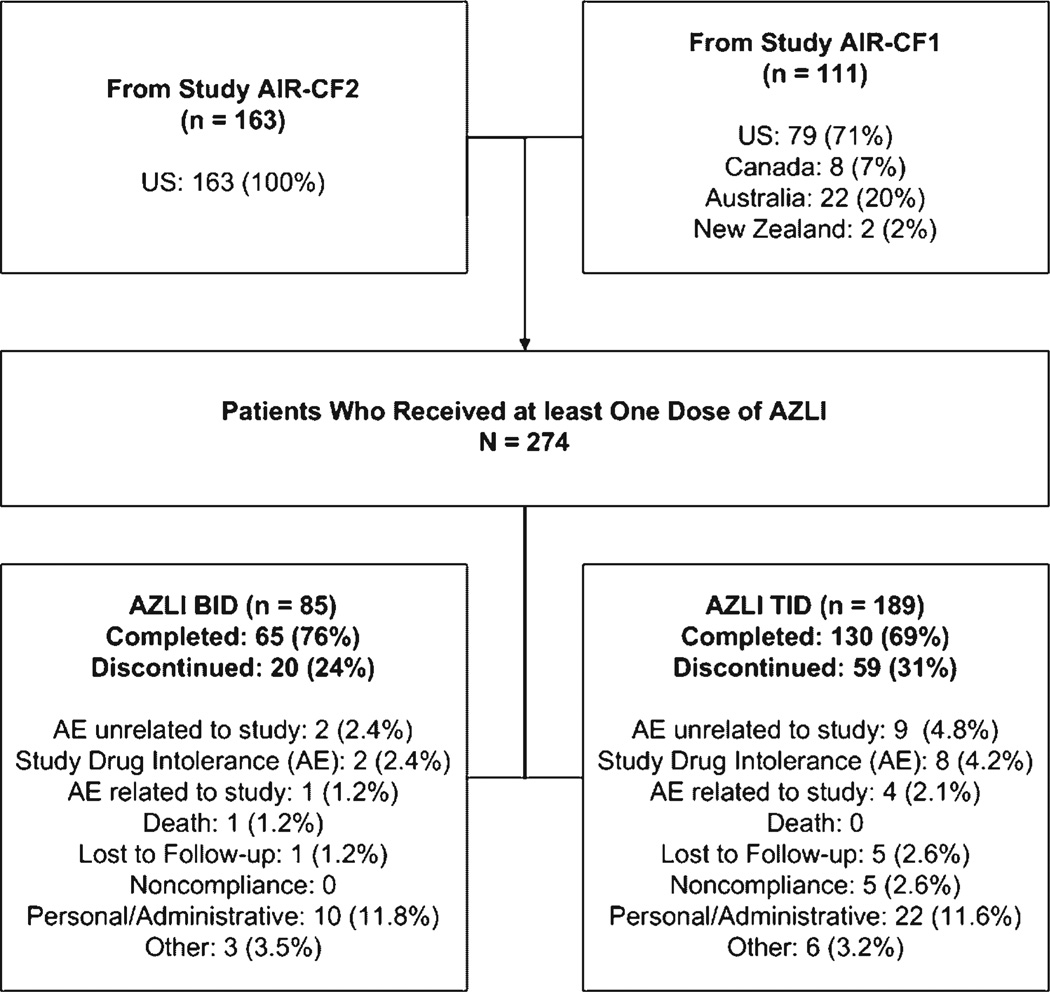
Two hundred seventy-four patients were enrolled; 85 patients received AZLI BID and 189 patients received AZLI TID. Of the 274 patients enrolled, 195 patients (71.2%; Fig. 1) completed the study: 26 patients (18 in the BID group and 8 in the TID group) completed the study after 3 planned treatment courses (Visit 7), prior to the trial extension; 166 (60.6%) patients completed nine planned courses (Visit 20).
Fig. 1.
Study design and patient disposition.
Drop out rates were similar for pediatric (25%) versus adult (30%) patients. The most common reason for discontinuation was personal or administrative reasons (32 patients [11.7%]) followed by adverse events judged by the investigator to be unrelated to the study drug (11 patients [4.0%]). The remaining reasons for discontinuation included: study drug intolerance (adverse event) (10 patients [3.6%]); the patient being lost to followup (6 patients [2.2%]), adverse events related to the study drug (5 patients [1.8%]), nonadherence to study protocol (5 patients 1.8%]), other reasons (9 patients [3.3%]), and 1 death judged by the investigator to be unrelated to study drug.
Patient Characteristics
Mean age was 28.5 years (range: 8–74 years) and most patients (79.9%) were ≥18 years of age (Table 1). At baseline, mean FEV1 % predicted was 55.6% and the mean CFQ-R-Respiratory score was 61.9. Concomitant medications used by ≥40% of patients in either group at baseline included vitamins (91.2%), pancreatic enzymes (88.0%), salbutamol (84.7%), dornase alfa (79.9%), fluticasone propionate with salmeterol xinafoate (55.5%), and azithromycin (53.6%). Hypertonic saline was used by 16.8% of patients. Of these concomitant medications, the use of fluticasone propionate with salmeterol xinafoate and azithromycin were different (≥5%) between the BID and TID groups (60.0% vs. 53.4% and 60.0% vs. 50.8%, respectively). Over the course of the study 51.5% of subjects had at least one course of inhaled tobramycin (Table 2). For patients administered tobramycin during the study, the median number of days on inhaled tobramycin was 78 days.
TABLE 1.
Patient Demographics and Baseline Characteristics1
| AZLI BID (N=85) | AZLI TID (N=189) | Total (N=274) | |
|---|---|---|---|
| Country, n (%) | |||
| US and Canada | 85 (100.0) | 165 (87.3) | 250 (91.2) |
| Australia and New Zealand | 0 | 24 (12.7) | 24 (8.8) |
| Age, years; mean (range) | 27.3 (11.4) | 29.0 (13.0) | 28.5 (12.5) |
| Age group, n (%) | |||
| <18 years | 19 (22.4) | 36 (19.0) | 55 (20.1) |
| ≥18 years | 66 (77.6) | 153 (81.0) | 219 (79.9) |
| Male; n (%) | 51 (60.0) | 100 (52.9) | 151 (55.1) |
| Weight, kg; mean (SD) | 59.5 (13.3) | 59.3 (15.8) | 59.4 (15.1) |
| Body mass index, kg/m2; mean (SD) | 21.3 (3.3) | 21.4 (4.0) | 21.4 (3.8) |
| CFTR genotype, n (%)2 | |||
| Homozygous for ΔF508 | 32 (52.5) | 71 (48.0) | 103 (49.3) |
| Heterozygous for ΔF508 | 16 (26.2) | 44 (29.7) | 60 (28.7) |
| Unidentified or other | 13 (21.3) | 33 (22.3) | 46 (22.0) |
| FEV1 % of predicted value; mean (SD) | 56.7 (17.5) | 55.1 (15.4) | 55.6 (16.1) |
| Patients with FEV1 ≤50% predicted value, n (%) | 36 (42.4) | 74 (39.2) | 110 (40.1) |
| CFQ-R-respiratory score; mean (SD) | 65.4 (16.9) | 60.3 (18.5) | 61.9 (18.1) |
| Log10 PA CFUs in sputum, mean (SD) | 5.7 (2.2) | 6.2 (1.9) | 6.0 (2.0) |
| MIC of aztreonam for all PA isolates, mg/ml | |||
| MIC50 | 4 | 4 | 4 |
| MIC90 | 128 | 128 | 128 |
| Minimum MIC | ≤1 | ≤1 | ≤1 |
| Maximum MIC | 2,048 | > 2,048 | > 2,048 |
| Number of isolates tested | 131 | 288 | 419 |
At baseline (Visit 1, Day 0).
Genotyping was performed at the beginning of AIR-CF1 and AIR-CF2. Not all patients underwent CFTR genotyping; percentages were calculated based upon the sample population (BID group, 61 patients; TID group 148 patients; total, 209 patients).
TABLE 2.
On-Study Use of Inhaled Tobramycin (≥300 mg) During the AZLI Off-Treatment Intervals
| AZLI BID (N = 85) |
AZLI TID (N=189) |
Total (N=274) |
||
|---|---|---|---|---|
| Number of tobramycin courses1 taken for all patients, n (%) | ||||
| 0 | 40 (47.1) | 93 (49.2) | 133 (48.5) | |
| >0–1 | 8 (9.4) | 21 (11.1) | 29 (10.6) | |
| >1–2 | 6 (7.1) | 23 (12.2) | 29 (10.6) | |
| >2–3 | 7 (8.2) | 14 (7.4) | 21 (7.7) | |
| >3–6 | 9 (10.6) | 14 (7.4) | 23 (8.4) | |
| >6–10 | 11 (12.9) | 21 (11.1) | 32 (11.7) | |
| >10 | 4 (4.7) | 3 (1.6) | 7 (2.6) | |
| Days on tobramycin for patients taking tobramycin | ||||
| N | 45 | 96 | 141 | |
| Mean | 123.0 | 98.2 | 106.1 | |
| Median | 93 | 60 | 78 | |
| SD | 99.8 | 92.4 | 95.2 | |
| Min | 1 | 1 | 1 | |
| Max | 350 | 488 | 488 | |
A tobramycin course was defined as ≥300mg dose of inhaled tobramycin for 28 days.
Safety
Treatment-emergent respiratory adverse events reported with an incidence rate ≥10% are summarized in Table 3. Non-respiratory events reported for ≥30% of BID- or TID-treated patients were pyrexia (45.9% and 45.5%), fatigue (37.6% and 43.9%), decreased appetite (30.6% and 45.5%), and headache (31.8% and 32.3%, respectively). The most common adverse events were ascribed to baseline disease and included cough (89.4%) and productive cough (80.3%). Over the 18-month study, serious adverse events occurred in 44.7% of BID-treated patients and 52.4% of TID-treated patients; respiratory symptoms were the primary cause of serious adverse events.
TABLE 3.
Treatment-Emergent Respiratory Adverse Events Reported by ≥10% of Patients in Either Treatment Group
| AZLI BID (N=85) |
AZLI TID (N=189) |
Total (N=274) |
||||
|---|---|---|---|---|---|---|
| Treatment-emergent adverse events1 | n | % | n | % | n | % |
| Cough | 74 | 87.1 | 171 | 90.5 | 245 | 89.4 |
| Productive cough | 58 | 68.2 | 162 | 85.7 | 220 | 80.3 |
| Respiratory tract congestion | 38 | 44.7 | 96 | 50.8 | 134 | 48.9 |
| Pharyngolaryngeal pain | 41 | 48.2 | 84 | 44.4 | 125 | 45.6 |
| Nasal congestion | 33 | 38.8 | 71 | 37.6 | 104 | 38.0 |
| Dyspnoea | 26 | 30.6 | 67 | 35.4 | 93 | 33.9 |
| Haemoptysis | 26 | 30.6 | 66 | 34.9 | 92 | 33.6 |
| Rhinorrhoea | 23 | 27.1 | 62 | 32.8 | 85 | 31.0 |
| Wheezing | 26 | 30.6 | 54 | 28.6 | 80 | 29.2 |
| Chest discomfort | 21 | 24.7 | 46 | 24.3 | 67 | 24.5 |
| Crackles lung | 27 | 31.8 | 38 | 20.1 | 65 | 23.7 |
| Pulmonary function test decreased | 12 | 14.1 | 50 | 26.5 | 62 | 22.6 |
| Non-cardiac chest pain | 18 | 21.2 | 36 | 19.0 | 54 | 19.7 |
| Sinus congestion | 10 | 11.8 | 41 | 21.7 | 51 | 18.6 |
| Sinus headache | 9 | 10.6 | 30 | 15.9 | 39 | 14.2 |
| Dyspnoea exacerbated | 6 | 7.1 | 31 | 16.4 | 37 | 13.5 |
| Dyspnoea exertional | 7 | 8.2 | 25 | 13.2 | 32 | 11.7 |
Treatment-emergent adverse events were coded using the Medical Dictionary for Regulatory Activities (MedDRA, Version 8.0) preferred term.
Clinically significant changes in vital signs or mean clinical laboratory values were not observed. There were no notable changes overall in heart rate, blood pressure, or respiratory rate related to AZLI treatment.
Disease-Related Endpoints
Analyses of the disease related endpoints, change from baseline FEV1 percent predicted, FEV1 absolute volume, CFQ-R-Respiratory scores, and density of PA in sputum, are presented in Table 4. Comparing the BID to TID group revealed an apparent dose response benefit. In both regimens, patients showed mean improvement from Visit 1 (baseline) at the end of each treatment course, and a return toward baseline at the end of the off-treatment intervals. For treatment courses 1–9, percent change in FEV1 (L) was positive at the end of each on-drug course, and generally a greater response was observed for the TID regimen. Additional pulmonary function measurements were obtained (forced vital capacity [FVC] and forced expiratory flow from 25% to 75% of the FVC [FEF25–75]). For FVC, mean change from baseline ranged from − 1.40% to 5.39% (BID) and from 0.97% to 6.18% (TID). For FEF25–75, mean change from baseline ranged from −4.20% to 16.05% (BID) and from −5.02% to 14.14% (TID). For the on-treatment months, the mean increase in CFQ-R-Respiratory score was >4, the established MCID.17 No response shift on the CFQ-R Respiratory Symptom scale (e.g., resetting symptom ratings to baseline) was observed over 18 monthly administrations, and no testing effects (remembering and recreating answers from last test) were apparent.
TABLE 4.
Change in Mean (±SD) FEV1 % Predicted, CFQ-R Respiratory Symptoms Scores, and Sputum PA Density: Change Over 18 months From Baseline to Study End (Visits 1 – 19)
| Percent change in FEV1 % predicted |
Percent change in FEV1 (L) |
Change in CFQ-R RSS |
Change in PA log10 CFUs in sputum |
|||||
|---|---|---|---|---|---|---|---|---|
| Treatment course | BID (N=85) | TID (N=189) |
BID (N=85) |
TID (N=189) |
BID (N=85) |
TID (N=189) |
BID (N=85) |
TID (N=189) |
| End C1 | ||||||||
| n | 83 | 185 | 83 | 185 | 81 | 187 | 58 | 129 |
| Mean (SD) | 4.9 (11.6) | 8.0 (16.5) | 4.9 (11.4) | 8.0 (16.5) | 3.5 (12.2) | 6.8 (17.4) | −0.2 (1.5) | −0.8 (1.8) |
| Start C2 | ||||||||
| n | 81 | 182 | 81 | 182 | 80 | 181 | 60 | 133 |
| Mean (SD) | 0.6 (11.0) | 0.7 (14.5) | 0.6 (11.0) | 0.7 (14.5) | 1.1 (15.0) | 1.3 (15.9) | −0.2 (1.7) | −0.3 (1.8) |
| End C2 | ||||||||
| n | 79 | 177 | 79 | 177 | 78 | 177 | 59 | 124 |
| Mean (SD) | 3.4 (11.0) | 7.4 (17.4) | 3.4 (11.0) | 7.4 (17.4) | 2.7 (13.8) | 6.5 (16.2) | −0.6 (1.7) | −0.8 (2.2) |
| Start C3 | ||||||||
| n | 75 | 171 | 75 | 171 | 75 | 173 | 52 | 127 |
| Mean (SD) | −0.4 (9.7) | 1.3 (14.2) | −0.4 (9.7) | 1.2 (14.1) | 0.2 (15.2) | 2.4 (17.2) | −0.7 (1.6) | −0.1 (1.7) |
| End C3 | ||||||||
| n | 76 | 165 | 76 | 165 | 75 | 163 | 56 | 111 |
| Mean (SD) | 3.5 (12.5) | 6.2 (16.6) | 3.6 (12.5) | 6.0 (16.5) | 0.4 (19.3) | 7.3 (18.5) | −0.4 (1.4) | −0.5 (2.1) |
| Start C4 | ||||||||
| n | 75 | 159 | 75 | 159 | 74 | 160 | 53 | 115 |
| Mean (SD) | −0.6 (11.4) | 0.8 (15.3) | −0.5 (11.4) | 0.7 (15.2) | −2.0 (13.5) | 3.1 (19.3) | −0.4 (2.0) | −0.1 (1.8) |
| End C4 | ||||||||
| n | 55 | 148 | 55 | 148 | 55 | 148 | 39 | 106 |
| Mean (SD) | 3.5 (13.5) | 4.8 (14.4) | 3.5 (13.4) | 4.7 (14.3) | −0.7 (19.8) | 7.7 (17.8) | −0.7 (2.0) | −0.7 (2.1) |
| Start C5 | ||||||||
| n | 56 | 147 | 56 | 147 | 56 | 149 | 43 | 113 |
| Mean (SD) | −1.1 (11.1) | 0.7 (14.2) | −1.0 (11.0) | 0.5 (14.1) | − 1.8 (18.0) | 3.3 (18.3) | −0.3 (1.7) | −0.3 (1.8) |
| End C5 | ||||||||
| n | 53 | 144 | 53 | 144 | 53 | 143 | 42 | 104 |
| Mean (SD) | 3.0 (10.3) | 4.4 (15.7) | 3.2 (10.3) | 4.1 (15.6) | 0.6 (17.1) | 5.2 (18.1) | −0.5 (2.0) | −0.5 (2.0) |
| Start C6 | ||||||||
| n | 52 | 138 | 52 | 138 | 54 | 136 | 41 | 100 |
| Mean (SD) | −2.6 (12.3) | 0.7 (16.8) | −2.4 (12.1) | 0.4 (16.7) | − 1.5 (16.6) | 2.4 (18.0) | −0.4 (2.0) | −0.3 (1.9) |
| End C6 | ||||||||
| n | 51 | 132 | 51 | 132 | 52 | 133 | 37 | 86 |
| Mean (SD) | 4.6 (12.7) | 5.1 (18.0) | 4.7 (12.6) | 4.8 (17.9) | 5.1 (17.7) | 5.3 (18.6) | −0.5 (1.9) | −0.6 (2.0) |
| Start C7 | ||||||||
| n | 51 | 130 | 51 | 130 | 50 | 132 | 34 | 92 |
| Mean (SD) | 0.6 (11.9) | −1.1 (16.1) | 0.7 (11.8) | −1.4 (16.0) | 3.4 (16.8) | 1.7 (18.6) | −0.3 (1.9) | −0.3 (2.0) |
| End C7 | ||||||||
| n | 51 | 128 | 51 | 128 | 51 | 131 | 39 | 92 |
| Mean (SD) | 4.1 (13.7) | 4.2 (13.8) | 4.2 (13.7) | 3.9 (13.5) | 4.9 (17.5) | 6.4 (19.0) | −0.6 (1.8) | −0.7 (2.3) |
| Start C8 | ||||||||
| n | 50 | 126 | 50 | 126 | 50 | 128 | 39 | 93 |
| Mean (SD) | −1.4 (13.3) | 1.3 (17.7) | −1.3 (13.2) | 1.1 (17.9) | 1.9 (15.8) | 2.5 (18.0) | −0.3 (2.3) | —0.3 (2.0) |
| End C8 | ||||||||
| n | 49 | 127 | 49 | 127 | 49 | 127 | 41 | 89 |
| Mean (SD) | 5.1 (14.8) | 5.5 (16.2) | 5.2 (14.5) | 5.3 (16.1) | 4.7 (12.9) | 8.3 (16.6) | −0.4 (1.8) | −0.7 (2.2) |
| Start C9 | ||||||||
| n | 48 | 123 | 48 | 123 | 47 | 122 | 39 | 86 |
| Mean (SD) | 0.0 (14.0) | 0.4 (17.9) | 0.1 (14.6) | 0.1 (17.6) | 0.3 (18.2) | 2.5 (19.6) | −0.4 (2.3) | −0.5 (1.8) |
| End C9 | ||||||||
| n | 46 | 122 | 46 | 122 | 47 | 122 | 36 | 85 |
| Mean (SD) | 1.2 (15.7) | 4.2 (18.0) | 1.3 (15.9) | 4.0 (17.9) | 0.3 (15.2) | 6.0 (17.9) | −0.5 (1.9) | −0.6 (2.1) |
| Follow-up | ||||||||
| n | 47 | 119 | 47 | 119 | 46 | 119 | 37 | 86 |
| Mean (SD) | 0.0 (15.3) | −0.7 (17.9) | 0.2 (16.1) | −1.1 (17.7) | 2.7 (13.8) | 3.8 (15.4) | −0.4 (1.9) | −0.5 (2.2) |
C1–C9 refer to treatment course number.
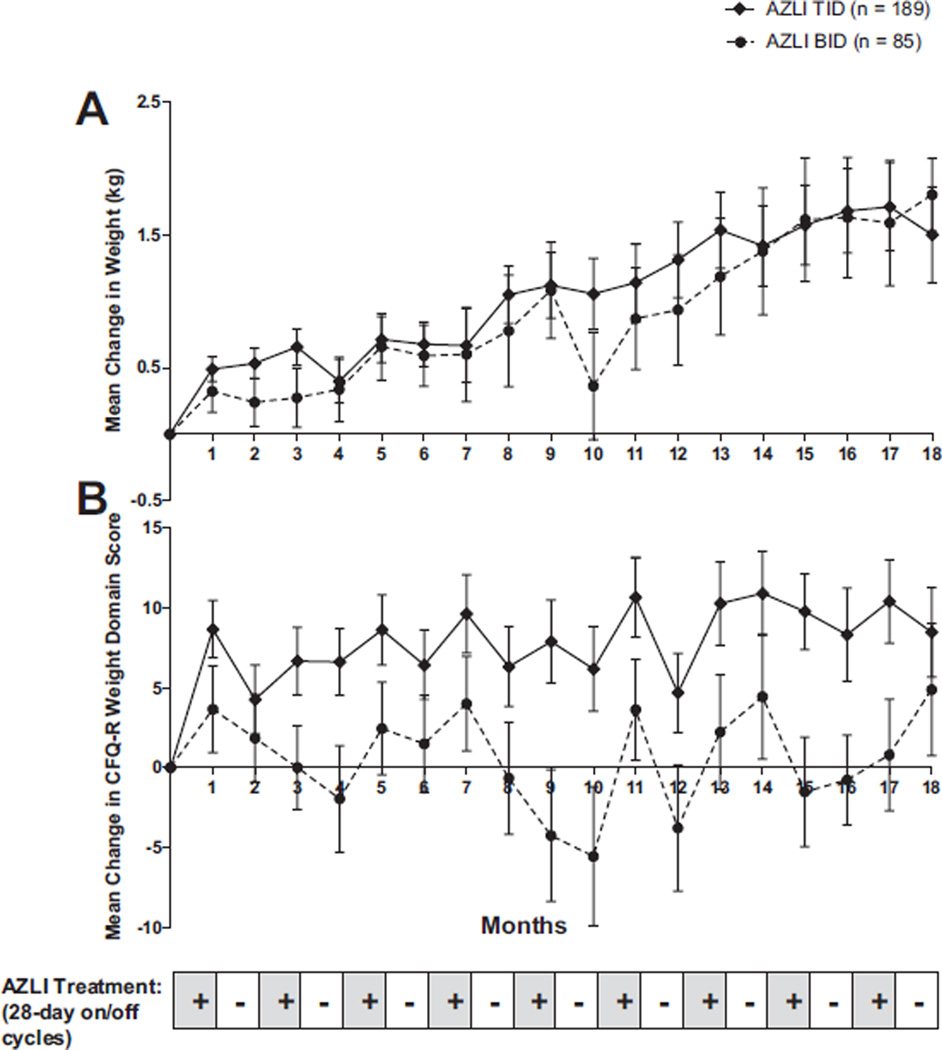
Changes on other symptom scales of the CFQ-R were consistent with treatment benefit, with greater improvements seen for the TID group compared to the BID group (data not shown). In the TID group, mean improvements from baseline for the Physical Functioning, Vitality and Health Perceptions domains tended to be greater during each of the intervals when the patient was on treatment and less during each of the intervals when the patient was off treatment; however, no MCID has been determined for these domains. For the TID group, mean scores for the Weight domain tended to be above baseline throughout the nine treatment courses. Of note, there was a small mean decline from baseline in the Treatment Burden score after nine treatment courses; however, the change (worsening) was similar for both treatment regimens. Absolute changes from baseline for the remaining domains (emotional functioning, social functioning, body image, eating disturbances, role limitations/school performance and digestion) were variable and showed no apparent dose response.
Hospitalization Rates and Use of Systemic Antipseudomonal Therapy
One hundred thirty-one patients (47.8%) were hospitalized at least once during the study, and the overall hospitalization rate per patient year was 0.897 (Table 5). The most frequent reason for hospitalization was the worsening or appearance of lower respiratory tract symptoms, and the hospitalization rate for respiratory events per patient year was 0.793. Median time to the first hospitalization for a respiratory event was 449 days (95% CI 347, NE) with median times of 31 and 449 days for the BID- and TID-treated groups, respectively.
TABLE 5.
Summary of Hospitalization1
| AZLI BID (N=85) | AZLI TID (N=189) | Total (N=274) | |
|---|---|---|---|
| Number of patients never hospitalized, n (%) | 49 (57.6) | 94 (49.7) | 143 (52.2) |
| Withdrew early, n (%) | 13 (15.3) | 26 (13.8) | 39 (14.2) |
| Completed study, n (%) | 36 (42.4) | 68 (36.0) | 104 (38.0) |
| Number of patients hospitalized at least once, n (%) | 36 (42.4) | 95 (50.3) | 131 (47.8) |
| Number of patient years2 | 95.46 | 221.02 | 316.48 |
| Number of hospitalizations | 65 | 219 | 284 |
| Hospitalization rate per patient year3 | 0.681 | 0.991 | 0.897 |
| Total number of respiratory hospitalizations | 60 | 191 | 251 |
| Respiratory hospitalization rate per patient year | 0.629 | 0.864 | 0.793 |
| Number of hospitalization days4 | |||
| Mean (SD) | 8.32 (27.59) | 12.46 (21.82) | 11.17 (23.78) |
| Median | 0.00 | 3.00 | 0.00 |
| Minimum | 0.0 | 0.0 | 0.0 |
| Maximum | 245.0 | 132.0 | 245.0 |
| n | 85 | 189 | 274 |
| % of days hospitalized5 | 2.03 | 2.92 | 2.65 |
| Time to first respiratory hospitalization (days)6 | |||
| Minimum | 4 | 1 | 1 |
| 25th percentile | 204 | 141 | 161 |
| Median | 431 | 449 | 449 |
| 95% CI for median | (294, NE) | (288, NE) | (347, NE) |
| 75th percentile | NE | NE | NE |
| Maximum | 431 | 609 | 609 |
| Number of censored values | 52 | 99 | 151 |
| Number of events | 33 | 90 | 123 |
NE, not estimable.
Hospitalization included all hospitalizations recorded as a serious adverse event lasting more than one calendar day, or any death (excl. hospitalizations after completion or >28 days after the date of the last dose).
Number of patient years is calculated as the sum of all days on study divided by 365.25.
Hospitalization rate is calculated as the number of hospitalizations divided by the number of patient years.
Number of hospitalization days for all patients (including zero days for patients who were not hospitalized).
Percent of days hospitalized is calculated as the sum of all hospitalization days divided by the sum of all patient study days.
Kaplan–Meier method is used to calculate statistics for time to first hospitalization.
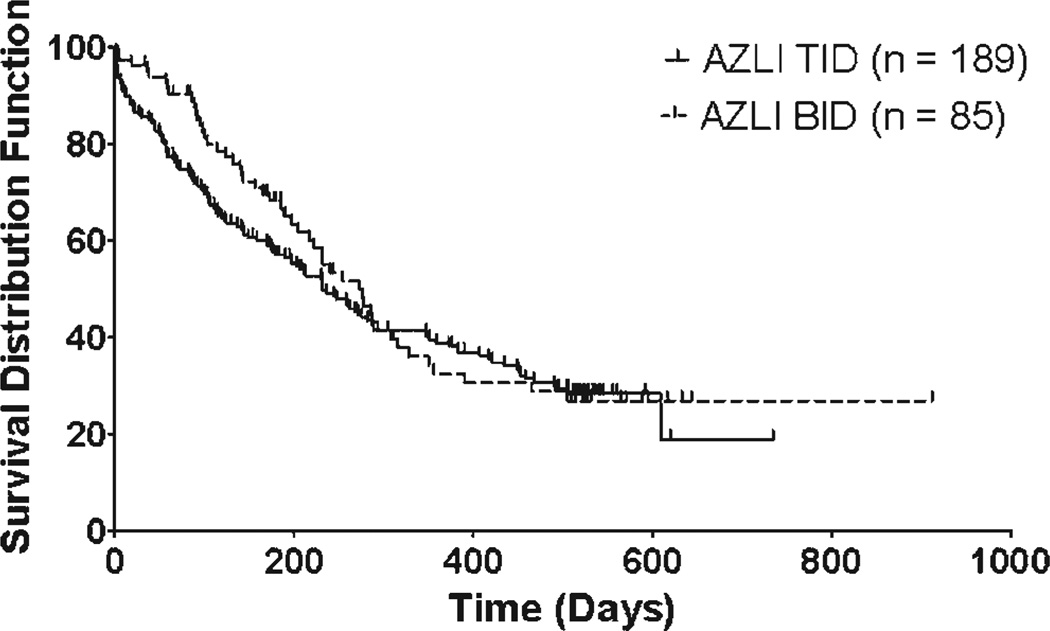
Median time to IV antipseudomonal antibiotics was 247 days (95% CI 210, 287), with similar times between the two regimen groups: 276 days for the BID-treated group (95% CI 217, 316) and 232 days for the TID group (95% CI 179, 288; Fig. 2).
Fig. 2.
Time to intravenous antipseudomonal antibiotics (days).
Changes in Weight
Change in weight (kg) from baseline and the mean change in the CFQ-R Weight Domain score is presented for each visit in Figure 3. Repeated courses of AZLI resulted in consistent weight gain, which were sustained over the 18-month period. Improvement was greater among patients receiving TID compared to BID treatment.
Fig. 3.
Mean change (±SE) in weight (A) and mean change in CFQ-R weight domain score (B) from baseline to study end.
Clinical Microbiology
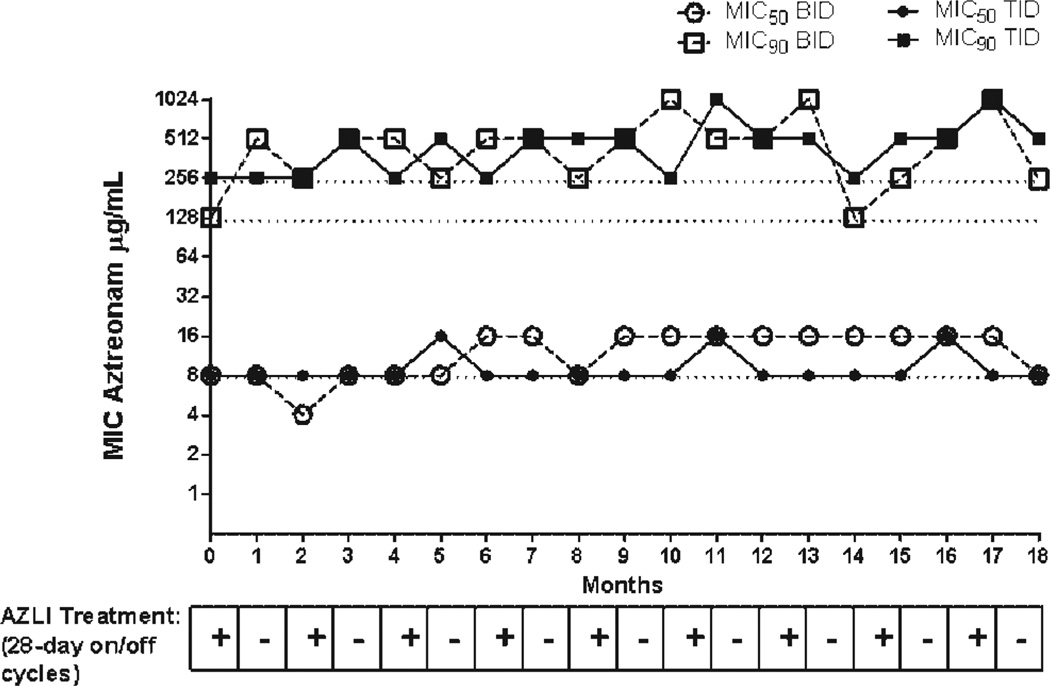
The baseline (Visit 1) MIC50 of aztreonam for PA isolates with the highest MIC was 8 ug/ml in both treatment groups, indicating susceptibility to aztreonam based on the parenteral breakpoint. The baseline MIC90 of aztreonam for PA isolates with the highest MIC was 128 µg/ml in the BID treatment group and 256 µg/ml in the TID treatment group. Throughout the study, the MIC50 of aztreonam for PA isolates with the highest MIC remained unchanged (±2-fold change) from baseline for the BID and TID regimens (Fig. 4). There were transient increases in MIC90 of aztreonam from baseline for PA isolates with the highest MIC in both treatment groups (Fig. 4). More increases were observed in the BID regimen than the TID regimen but both remained unchanged at the end of study.
Fig. 4.
MIC50 and MIC90 of aztreonam for Pseudomonas aeruginosa isolates with the highest MIC from each patient (µg/ml): baseline to study end.
As expected, sputum cultures remained positive for PA in a majority of patients. There was no evidence for increases in the isolation of S. maltophilia, MSSA, MRSA, A. xylosoxidans or Aspergillus spp. resulting from treatment with AZLI. Burkholderia spp. were isolated from five patients, all in the TID group, at four different study sites. In two patients, Burkholderia spp. were confirmed by the B. cepacia Research Laboratory and Repository prior to the patient receiving AZLI in Studies AIR-CF1, and AIR-CF2, and AIR-CF3; the remaining three patients had first time isolation but Burkholderia spp. confirmation was not undertaken. None of the five patients experienced B. cepacia syndrome. All isolates of presumptive Burkholderia spp. from four of the patients had an MIC ≤4 µg/ml to aztreonam, indicating susceptibility to AZLI. The isolate from the remaining patient had an MIC of 32 µg/ml.
Overall increases in the prevalence of Candida spp. were observed over repeated courses of AZLI; however, the majority of patients experienced no change in the presence or absence of Candida spp. through the nine courses.
Adherence
Adherence was assessed by the return of empty vials as a percent of total vials prescribed, and mean adherence was 92.0% in the BID group and 88.0% in the TID group.
DISCUSSION
The use of cyclic, suppressive inhaled antibiotic therapy with 28 days of therapy followed by 28 days off therapy has become the standard of care for CF patients greater than 6 years of age with chronic PA airway infection.1 AIR-CF1 and AIR-CF2 were designed to assess the safety and efficacy of a 28-day course of AZLI in patients with varying degrees of maintenance therapy. Results of these studies have demonstrated the safety and efficacy of single courses of AZLI. This open-label study provided long-term data on the use of nine courses (on/ off months) of AZLI used in conjunction with routine therapeutic regimens prescribed by each patient’s primary CF-care provider. AIR-CF3 provides information needed to evaluate the long-term clinical and microbiologic safety of AZLI, the durability of AZLI’s effect on a variety of clinical health measures, and AZLI’s optimum dosing regimen (BID vs. TID).
The safety profile observed in this study is consistent with previous AZLI studies5,6 and with expected symptoms of patients’ underlying CF lung disease. No new safety concerns were identified.
The endpoints of change in FEV1 % predicted and CFQR-Respiratory scores were used to assess different aspects of CF lung disease, pulmonary function and respiratory symptoms, respectively. The CFQ-R is a validated CFspecific PRO measuring both generic and CF-specific domains and directly measures patients’ perception of their respiratory symptoms. Change from baseline on the CFQ-R-Respiratory Symptom score is categorized as improved, stable, or worsened, depending on the magnitude and direction of change in relation to the MCID of 4 points.11 In this study, the durability of the efficacy of AZLI was evidenced by the sustained response in both disease-related endpoints (FEV1 and CFQ-R-Respiratory score), as well as weight, observed over multiple courses of therapy. In the off-treatment month, disease-related endpoints returned to near baseline but were still above baseline values at most end-of-course visits.
In contrast to the results of repeated courses of TIS in adult patients,18 mean FEV1 % predicted was above the baseline value at the end of each on-AZLI treatment period. In patients with CF, progressive loss of lung function averages 1–4% predicted each year;19,20 therefore, it would be expected that patients with CF would have at least a slight decline in FEV1 over an 18-month period rather than maintaining or improving FEV1 over this time period. However, the absence of a placebo group limits this interpretation. Additionally, in those patients treated with AZLI TID, mean FEV1 % predicted was above the baseline value at the end of each off-AZLI treatment period, with the exception of the sixth and ninth treatment courses. Thus, AZLI TID improves lung function over repeated courses of therapy, demonstrating sustained improvement over 18 months of treatment.
It is important to note that all subjects received AZLI in addition to the routine therapeutic regimens prescribed by each patient’s primary CF-care provider. However, improvements in FEV1 and CFQ-R-Respiratory score only occurred when patients were being treated with AZLI, and outcomes decreased during the off-treatment intervals. This suggests that AZLI can achieve improvements in FEV1 and CFQ-R when added to standard treatments.
This report is the first describing weight gain as an indicator of improved overall health in a clinical trial of an inhaled antibiotic. The relationship between lung function and nutritional status for patients with CF has been well established,21 with malnourished patients having worse lung function and more frequent infection with PA. In this study, repeated courses of AZLI resulted in consistent weight gain and the CFQ-R Weight scores, both of which were sustained in the TID group over the nine treatment courses. Again, although this was not a placebo-controlled trial, this finding has not been demonstrated in long-term studies of inhaled antibiotics such as TIS or colistin.
The most common cause of hospitalization during this study was the development of symptoms consistent with CF pulmonary exacerbation; however, a median time to first respiratory hospitalization of 449 days is notable for its length given the disease severity of this patient population. In addition, the rate of hospitalization in this study (0.897 hospitalizations per patient year) was lower than the rate reported in a case-matched control study of CF patients receiving standard of care therapy (1.26 hospitalizations per patient year).22 In this retrospective, matched case control study22 between a subset of patients receiving AZLI during the first year of AIR-CF3 versus patients with CF receiving standard of care therapy,23 patients receiving AZLI in addition to standard of care had significantly lower risk of hospitalization (28% less than matched-controls, P=0.020) compared to patients receiving standard of care therapy alone.22 The addition of new therapies, such as AZLI, may decrease the significant cost of hospitalizations and improve the overall health care in patients with CF.
Adherence to therapy was high in this study. Interpretation may be limited by the vial count method used to assess compliance, as well as the fact that follow-on trials may attract more motivated patients. Nonetheless, it is useful to note that the dosing regimen was not associated with differences in adherence. Treatment Burden scores on the CFQ-R were comparable between BID and TID dosing and did not change during the off-months. The high treatment adherence observed in this study may be due to improvement in lung function and respiratory symptoms when the patients were on treatment and due to the portability of the eFlow nebulizer and the rapid administration of AZLI (2–3 min per dose for AZLI administration24 vs. approximately 15–20 min per dose with TIS).18
A long-term suppressive effect of AZLI as an antipseudomonal agent was observed over the nine treatment courses in this study. A persistent reduction in PA CFUs from baseline was observed at each visit throughout the study, regardless of on-or off-treatment interval period. As expected, decreases in PA CFUs consistently occurred during the on-treatment courses throughout the study while increases toward baseline values were observed during the off-treatment courses; this trend was more clearly observed in patients treated with AZLI TID than BID. The fact that TID dosing appears to be a more efficacious dose is consistent with the mode of action of aztreonam; bacterial killing is dependent on time above the MIC.25
A theoretical concern of long-term antibiotic exposure is the development of antibiotic resistance and a possible decline in clinical efficacy. Increases in the MIC90, but not the MIC50, of aztreonam for PA isolates with the highest MIC were transiently observed in the BID and TID groups. The fact that increases were seen in the MIC90 but not the MIC50 suggests that bacterial growth advantages at the highest levels of resistance may confer a selective advantage to PA isolates during exposure to high antibiotic concentrations. In contrast, increases in resistance at lower levels, that is, MIC50, are unable to confer a selective advantage to PA during exposure to high antibiotic concentrations. During long-term antibiotic exposure in a disease characterized by chronic infection, aerosolized antibiotics like AZLI ideally achieve sputum drug concentrations close to the mutant prevention concentration (MPC) and thus suppress the development of resistance.26 Alternatively, acquisition of antibiotic resistance can confer a biologic cost to PA and this cost can become a disadvantage in the absence of selective pressure.27 Interestingly, immediate decreases in the MIC90 were observed during the off-treatment months in the TID group. The reasons for this are unknown. All of these observations support the use of 75 mg AZLI TID in an intermittent 1-month on-/1-month off-treatment paradigm.
In addition, there is a theoretical concern that an alteration in the bacterial microenvironment of the lung in a patient with CF by an antibiotic may promote growth of resistant pathogens other than PA. Accordingly, treatment emergence of other pathogens known to colonize the lung of patients with CF,for example, MRSAand Burkholderia spp., were intensively monitored before, during, and after long-term use of AZLI. Presumptive intermittent treatment-emergent infection with Burkholderia spp. was observed in three patients, but no patients experienced B. cepacia syndrome. Moreover, it is unknown if the finding of Burkholderia represents new acquisition of this fastidious pathogen, or in fact, the organism was isolated more easily due to the diminution of PA bacterial density. The treatment emergence of other pathogens was not observed, and the high prevalence of Candida spp. observed in this study is consistent with a report demonstrating detection of Candida albicans in sputum from 76% of adult patients with CF.28 It is possible that AZLI does not alter the microenvironment of the CF lung enough to promote emergence of other pathogens. Alternatively, the high concentrations of aztreonam achieved in the lung may exert antimicrobial affects on pathogens typically not considered targets of the drug.
The clinical management of cystic fibrosis has improved during the past 15 years, resulting in better outcomes and greater life expectancy for patients with CF. This is in large part due to the development of the Care Center network, and additional therapies such as dornase alfa, azithromycin, tobramycin solution for inhalation, and, most recently, hypertonic saline. Nevertheless, chronic airway infection with PA remains a primary source of morbidity and mortality. The management of PA lower respiratory tract disease has historically involved the use of repeated courses of IV antibiotics because of a lack of oral anti-pseudomonal agents. The introduction of TIS established the concept of cyclic, suppressive inhaled antibiotic therapy for chronic airway infection in patients with CF. Unfortunately, the combined effects of antibiotic resistance within the environment of frequent antibiotic use, cumulative toxicity associated with aminoglycoside use,29,30 off-cycle clinical deterioration, and patient sensitivity have meant that TIS may not be suitable for all patients. Clearly, there is a critical unmet medical need for additional inhaled antibiotic therapies for chronic use in patients with CF. Such therapies will be vital in maintaining or improving lung function and respiratory symptoms in CF patients with PA airway infection. The ideal therapy should be safe, effective over the long term, and involve minimal burden to patients to promote adherence to therapy. AZLI meets these criteria and represents an important new therapy for patients with CF and chronic PA airway infection.
ACKNOWLEDGMENTS
We thank the patients and their families as well as the participating study sites, site investigators (SI), and study research coordinators (RC), listed below. A. Bruce Montgomery is an employee of Gilead Sciences, Inc. The remaining authors were scientific advisors for this study; some were also clinical investigators, as listed below. We thank Robert Hill for writing assistance in the preparation of this manuscript. This study was funded by Gilead Sciences, Inc. and by NIH General Clinical Research Center grants M01 RR00188, M01 RR00037, M01 RR02172, M01 RR00043, M01 RR000489, M01 RR00034, M01 RR00039, M01 RR00750, M01 RR01066, M01 RR001070, M01 RR10733, M01 RR00070, M01 RR10710, M01 RR00069, M01 RR00827, M01 RR00082, M01 RR023940, M01 RR00042, M01 RR00400, and M01 RR00065.
Funding source: Grant sponsor: Gilead Sciences, Inc.; NIH General Clinical Research Center; M01 RR00188, M01 RR00037, M01 RR02172, M01 RR00043, M01 RR000489, M01 RR00034, M01 RR00039, M01 RR00750, M01 RR01066, M01 RR001070, M01 RR10733, M01 RR00070, M01 RR10710, M01 RR00069, M01 RR00827, M01 RR00082, M01 RR023940, M01 RR00042, M01 RR00400, M01 RR00065.
This study was funded by Gilead Sciences Inc. Preliminary results were presented at the 23rd Annual North American Cystic Fibrosis Conference in Minneapolis, MN.
ABBREVIATIONS
- AE
Adverse event
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- AZLI
Aztreonam for inhalation solution
- BID
Twice daily
- CF
Cystic fibrosis
- CFU
Colony forming units
- CFQ-R
Cystic fibrosis questionnaire-revised
- CFTR
Cystic fibrosis transmembrane conductance regulator gene
- CI
Confidence interval
- FEF25–75
Forced expiratory flow from 25%to 75% of the forced vital capacity
- FEV1
Forced expiratory volume in 1 sec
- FVC
Forced vital capacity
- IV
Intravenous
- MCID
Minimal clinically important difference
- MedDRA
Medical Dictionary for Regulatory Activities
- MIC
Minimum inhibitory concentration
- MIC50
Aztreonam concentration inhibiting the growth of 50% PA isolates
- MIC90
Aztreonam concentration inhibiting the growth of 90% PA isolates
- MPC
Mutant prevention concentration
- MRSA
Methicillin-resistant Staphylococcus aureus
- MSSA
Methicillin-sensitive Staphylococcus aureus
- n
Number of patients with an observation
- N
Number of patients in a specified group
- PA
Pseudomonas aeruginosa
- PRO
Patient-reported outcome
- SD
Standard deviation
- SE
Standard error
- SI
Site Investigator
- TID
Three times daily
- TIS
Tobramycin inhalation solution
- US
United States
APPENDIX
AUSTRALIA
Alfred Hospital, Melbourne, VIC; Site Investigator (SI): John Wilson, M.B.B.S.; RC: Denise Clark.
Children’s Hospital at Westmead, Sydney, NSW; SI: Peter J. Cooper, M.B., Ch.B.; RC: Karen McKay.
Princess Margaret Hospital for Children, Perth, WA; SI: Tonia A. Douglas, M.B., Ch.B. Barry Clements, M.D. (previous SI); RC: Charlotte Allen.
Royal Adelaide Hospital, Adelaide, SA; SI: Hugh W. Greville, M.B.B.S.; RC: Kirsty Herewane.
Royal Children’s Hospital (Herston), Brisbane, QLD; SI: Claire E. Wainwright, M.B.B.S.; RC: Aaron Buckner.
Sir Charles Gairdner Hospital, Perth, WA; SI: Gerard Ryan, M.B.B.S.; RC: Kerry Boughton.
Westmead Hospital, Sydney, NSW; SI: Peter G. Middleton, M.B.B.S.; RC: Karen Bovington.
CANADA
Children’s Hospital of Western Ontario, London ON; SI: Brian D. Lyttle, M.D.; RC: Anne-Marie Lyttle.
University of Alberta, Edmonton, AB; SI: Peter Zuberbuhler, M.D.; RC: Josette Salgado.
NEW ZEALAND
Greenlane Clinical Centre and Starship Children’s Health Centre, Auckland; SI: John Kolbe, M.B.B.S.; RC: Wendy Fergusson.
UNITED STATES
Alamo Clinical Research Associates, San Antonio, TX; SI: Peter S. Fornos, M.D.; RC: Terri Phillips.
Albany Medical Center, Albany, NY; SI: Jonathan M. Rosen, M.D.; RC: Paula Malone and Katharine Mokhiber.
Baptist Medical Center, Oklahoma City, OK; SI: Santiago Reyes, M.D.; RC: Teresa Orf.
Children’s Asthma Respiratory and Exercise Specialists, Glenview, IL; SI: Steven R. Boas, M.D.; RC: Melinda Bicchinella.
Children’s Hospital & Regional Medical Center, Seattle, WA; SI: Ronald L. Gibson, Jr., M.D., Ph.D.; RC: Sharon McNamara.
Children’s Hospital Boston, Boston, MA; SI: Thomas R. Martin, M.D. David A. Waltz, M.D.(Previous SI); RC: Summer Adams.
Children’s Hospital Los Angeles, Los Angeles, CA; SI: Marlyn S. Woo, M.D.; RC: Lynn Fukushima.
Children’s Hospital Medical Center of Akron, Akron, OH; SI: Gregory J. Omlor, M.D.; RC: Debbie Ouellette.
Children’s Hospital of Denver, Denver, CO; SI: Frank J. Accurso, M.D.; RC: Meg Anthony and Churee Pardee.
Children’s Hospital of Michigan and Wayne State University, Detroit, MI; SI: Debbie S. Toder, M.D.; RC: Catherine Van Wagnen.
Children’s Hospital of Orange County, Orange, CA; SI: Bruce G. Nickerson, M.D.; RC: Zona Lopez and Candice Ramos and Luis Valdez.
Children’s Hospital of Pittsburgh. Pittsburgh, PA; SI: Joseph M. Pilewski, M.D.; RC: Elizabeth Hartigan.
Children’s Lung Specialists, Las Vegas, NV; SI: Craig T. Nakamura, M.D.; RC: Tara Brascia.
Children’s Medical Center Dayton, Dayton, OH; SI: Robert J. Fink, M.D.; RC: Sandy Bartosik.
Children’s Memorial Hospital and Northwestern University, Chicago, IL; SI: Susanna A. McColley, M.D.; RC: Catherine Powers.
Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; SI: Bruce C. Trapnell, M.D., Cori L.
Daines, M.D. (Previous PI); RC: Lorrie Duan and Diana Kardous.
Columbia University Medical Center, New York, NY; SI: Emily DiMango, M.D.; RC: Jennifer Sormillon.
Columbus Children’s Hospital / Ohio State University, Columbus, OH; SI: Karen S. McCoy, M.D.; RC: M. Terri Johnson.
Connecticut Children’s Medical Center, Hartford, CT; SI: Craig D. Lapin, M.D.; RC: Ginny Drapau.
Drexel University College of Medicine, Philadelphia, PA; SI: Michael S. Sherman, M.D. William P. Sexauer, M.D. (Previous SI); RC: Judy Hillman.
Emory University Cystic Fibrosis Center, Atlanta, GA; SI: Daniel B. Caplan, M.D.; RC: Tedra Flynn.
Indiana University, Indianapolis, IN; SI: Aruna Sannuti, M.D.; RC: Annette Hempfling.
Kaiser Permanente Oakland Medical Center, Oakland, CA; SI: Gregory F. Shay, M.D.; RC: Julie Lee.
Long Island College Hospital, Brooklyn, NY; SI: Robert J. Giusti, M.D.; RC: Christine Mavaro.
Long Island Jewish Medical Center, New Hyde Park, NY; SI: Rubin I. Cohen, M.D.; RC: Maryanne Gannon.
Louisiana State University Health Sciences Center, Shreveport, LA; SI: Kimberly L. Jones, M.D.; RC: Antoinette Gardner.
Loyola University Medical Center, Maywood, IL; SI: Sean M.Forsythe, M.D.; RC: Cathy Kalnicky and Theresa Krause.
Massachusetts General Hospital, Boston, MA; SI: Henry L. Dorkin, M.D.; RC: Lauren Kelly and Jane Solomon and Monica Ulles.
Medical College of Georgia / MCG Healthcare, Inc., Augusta, GA; SI: Margaret F. Guill, M.D.; RC: Kathy Dyer and Julie C. Hall.
Medical University of South Carolina, Charleston, SC; SI: C. Michael Bowman, M.D., Ph.D.; RC: Terry Byars.
Morristown Memorial Hospital, Morristown, NJ; SI: Stanley B. Fiel, M.D.; RC: Paula Lomas.
Naval Medical Center - Portsmouth, VA; SI: Rees Lee, M.D.; RC: Loretta Daniels.
Nemours Children’s Clinic Jacksonville, Jacksonville, FL; SI: David A. Schaeffer, M.D. Kathryn V. Blake, Ph.D. (Previous PI); RC: Betty DeLuca.
Nemours Children’s Clinic Orlando, Orlando, FL; SI: David E. Geller, M.D.; RC: Sondra Sadler.
New York Medical College/Westchester Medical Center, Valhalla, NY; SI: Allen J. Dozor, M.D. Nikhil S. Amin, M.D. (Previous PI); RC: Ingrid Gherson.
North Suburban Pulmonary and Critical Care Consultants, Niles, IL; SI: Arvey M. Stone, M.D.; RC: Suellen Moen.
Pediatric Breathing Disorders Clinic, Anchorage, Anchorage, AK; SI: Dion Roberts, M.D.; RC: Vicki Roberts.
Pediatric Pulmonary Associates, LLC, Columbia, SC; SI: Daniel C. Brown, M.D. Roxanne Marcille, M.D. (Previous PI); RC: Carolyn Turner.
Pediatric Pulmonary Center / Virginia Commonwealth University, Richmond, VA; SI: Gregory R. Elliott, M.D.; RC: Juellisa Gadd.
Penn-Presbyterian Medical Center, Philadelphia, PA; SI: Denis Hadjiliadis, M.D.; RC: Marianne Ferrin.
Pennsylvania State Milton S. Hershey Medical Center, Hershey, PA; SI: Gavin R. Graff, M.D.; RC: Diane Kitch.
Phoenix Children’s Hospital, Phoenix, AZ; SI: Peggy J. F. Radford, M.D.; RC: Natalia Argel.
Riley Hospital for Children, Indianapolis, IN; SI: Michelle S. Howenstine, M.D.; RC: Mary Blagburn.
St. Barnabas Ambulatory Care Center, Livingston, NJ; SI: Dorothy S. Bisberg, M.D.; RC: Pamela Pock.
St. Christopher’s Hospital for Children, Philadelphia, PA; SI: Laurie Varlotta, M.D.; RC: Joanne Gambo.
St. Louis University, St. Louis, MO; SI: Ravi Nayak, M.D.; RC: Patricia Dettenmeier and Jennifer Dizes.
State University of NewYork (SUNY) Upstate Medical University Hospital, Syracuse, NY; SI: Ran Anbar, M.D.; RC: Donna Lindner.
Texas Children’s Hospital / Baylor College of Medicine, Houston, TX; SI: Christopher M. Oermann, M.D.; RC: Charles Sellers.
Tufts New England Medical Center, Floating Hospital for Infants and Children, Boston, MA; SI: William F.H. Yee, M.D.; RC: Karen Murray and Corri Nelson.
University of Alabama at Birmingham, Birmingham, AL; SI: J.P. Clancy, M.D.; RC: Ginger Reeves.
University of Arkansas for Medical Sciences, Little Rock, AR; SI: Paula J. Anderson, M.D.; RC: Adam Taggart.
University of California, San Diego, San Diego, CA; SI: Douglas J. Conrad, M.D.; RC: Bobbie Munden.
University of Michigan, Ann Arbor, MI; SI: Samya Z. Nasr, M.D.; RC: Ermee Sakmar.
University of Minnesota, Minneapolis, MN; SI: Joanne L. Billings, M.D. Carlos E. Milla, M.D. (Previous SI); RC: Brooke Noren and Jacquelyn Zirbes.
University of Missouri, Columbia, MO; SI: Peter Konig, M.D.; RC: Donna Smith.
University of North Carolina Hospital at Chapel Hill, Chapel Hill, NC; SI: George Z. Retsch-Bogart, M.D.; RC: Diane Towle.
University of Utah, Salt Lake City, UT; SI: Theodore G. Liou, M.D.; RC: Kristyn Packer.
Via Christi Specialty Clinics / CF Center, Wichita, KS; SI: C. Maria Riva, M.D.; RC: Janet Messamore and Karen Wiant.
West Virginia Hospital, Pediatric Infectious Diseases, Morgantown, WV; SI: Kathryn S. Moffett, M.D.; RC: Ryan Adamec.
Women & Children’s Hospital of Buffalo, Buffalo, NY; SI: Drucy S.Borowitz, M.D.; RC: Nadine Caci and Jeanne Smith.
Yale University/New Haven Hospital, New Haven, CT; SI: John R. McArdle, M.D.; RC: Kathryn Engle.
Footnotes
Conflict of Interest Statement: Dr. Oermann received clinical research support as a site investigator conducting clinical trials for Gilead Sciences and Inspire Pharmaceuticals. Dr. Retsch-Bogart received clinical research support as a site investigator conducting clinical trial for Gilead Sciences, Inspire Pharmaceuticals, Genentech, Pathogenesis Corp., Boehringer-Ingelheim, and Cystic Fibrosis Foundation Therapeutics, Inc. Dr. Quittner was a consultant, served on an advisory board for Gilead Sciences, and has an investigator-initiated grant in another population. Dr. Gibson received clinical research support as a site investigator conducting clinical trials for Gilead Sciences, Inspire Pharmaceuticals, and Cystic Fibrosis Foundation Therapeutics. Dr. McCoy received clinical research support as a site investigator conducting clinical trials for Gilead Sciences, Inspire Pharmaceuticals, and Genentech. Dr. Montgomery is employed by Gilead Sciences. He is patent author on aztreonam for inhalation solution and Gilead Sciences is patent holder. He holds equity interest in Gilead Sciences. Dr. Cooper received clinical research support as a site investigator for clinical trials sponsored by Gilead Sciences.
All authors contributed to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article and/or revising it critically for important intellectual content; and final approval of the version to be published. Dr. Oermann and Dr. Montgomery (Gilead Sciences, Inc.) assure that this manuscript is free of bias.
REFERENCES
- 1.Flume PA, O’Sullivan BP, Robinson KA, Goss CH, Mogayzel PJ, Jr, Willey-Courand DB, Bujan J, Finder J, Lester M, Quittell L, Rosenblatt R, Vender RL, Hazle L, Sabadosa K, Marshall B. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176:957–969. doi: 10.1164/rccm.200705-664OC. [DOI] [PubMed] [Google Scholar]
- 2.Gibson RL, Retsch-Bogart GZ, Oermann C, Milla C, Pilewski J, Daines C, Ahrens R, Leon K, Cohen M, McNamara S, Callahan TL, Markus R, Burns JL. Microbiology, safety, and pharmacokinetics of aztreonam lysinate for inhalation in patients with cystic fibrosis. Pediatr Pulmonol. 2006;41:656–665. doi: 10.1002/ppul.20429. [DOI] [PubMed] [Google Scholar]
- 3.Bristol-Myers Squibb, Bristol-Myers Squibb. AZACTAM® (aztreonam for injection) [package insert] 2007 [Google Scholar]
- 4.Dietzsch HJ, Gottschalk B, Heyne K, Leupoid W, Wunderlich P. Cystic fibrosis: comparison of two mucolytic drugs for inhalation treatment (acetylcysteine and arginine hydrochloride) Pediatrics. 1975;55:96–100. [PubMed] [Google Scholar]
- 5.McCoy KS, Quittner AL, Oermann CM, Gibson RL, Retsch-Bogart GZ, Montgomery AB. Inhaled aztreonam lysine for chronic airway Pseudomonas aeruginosa in cystic fibrosis. Am J Respir Crit Care Med. 2008;178:921–928. doi: 10.1164/rccm.200712-1804OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Retsch-Bogart GZ, Quittner AL, Gibson RL, Oermann CM, McCoy KS, Montgomery AB, Cooper PJ. Efficacy and safety of inhaled aztreonam lysine for airway Pseudomonas in cystic fibrosis. Chest. 2009;135:1223–1232. doi: 10.1378/chest.08-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, Vasiljev-K M, Borowitz D, Bowman CM, Marshall BC, Marshall S, Smith AL. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med. 1999;340:23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 8.Bucholski A, Keller M, Balcke A, Lintz FC, Seemann S, Flitter WD, Hofmann TIn. vitro Performance of eFlowTM, an electronic inhaler for administration of a novel aztreonam formulation to CF patients [abstract 396] Pediatr Pulmonol Suppl. 2003;25:321. [Google Scholar]
- 9.American Thoracic Society Committee on Diagnostic Standards for Non-Tuberculous Respiratory Diseases. Standardization of spirometry 1994 update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 10.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127:725–734. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 11.Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire-Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest. 2009;135:1610–1618. doi: 10.1378/chest.08-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, Durie PR, Legrys VA, Massie J, Parad RB, Rock MJ, Campbell PW, 3rd, Cystic Fibrosis Foundation. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153:S4–S14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guyatt GH. Making sense of quality-of-life data. Med Care. 2000:II175–II179. doi: 10.1097/00005650-200009002-00027. [DOI] [PubMed] [Google Scholar]
- 14.Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10:407–415. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 15.Burns JL, Emerson J, McNamara S, Worrell K, Buccat A. Antibiotic resistance in cystic fibrosis sputum isolates [abstract 373] Pediatr Pulmonol. 2008;43:334. doi: 10.1002/ppul.21198. [DOI] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; eighteenth informational supplement. Wayne (PA): CLSI (formerly NCCLS); 2008. pp. M100–S18. [Google Scholar]
- 17.Quittner A, Modi A, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference (MCID) scores for the Cystic Fibrosis Questionnaire-Revised (CFQ-R) Respiratory Symptom scale in two populations of patients with CF and chronic Pseudomonas aeruginosa airway infection. Chest. 2009;135:1610–1618. doi: 10.1378/chest.08-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pathogenesis Corporation. Technical Monograph TOBI Tobramycin Solution for Inhalation, International Edition. Seattle: The Corporation; 2000. [Google Scholar]
- 19.Konstan MW, Schluchter MD, Xue W, Davis PB. Clinical use of ibuprofen is associated with slower FEV1 decline in children with cystic fibrosis. Am J Respir Crit Care Med. 2007;176:1084–1089. doi: 10.1164/rccm.200702-181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyle MP. Adult cystic fibrosis. JAMA. 2007;298:1787–1793. doi: 10.1001/jama.298.15.1787. [DOI] [PubMed] [Google Scholar]
- 21.Gozdzik J, Cofta S, Piorunek T, Batura-Gabryel H, Kosicki J. Relationship between nutritional status and pulmonary function in adult cystic fibrosis patients. J Physiol Pharmacol. 2008;59:253–260. [PubMed] [Google Scholar]
- 22.Montgomery AB, S. Lewis M, K. Higuchi M, Marshall B, Oermann C. Hospitalization risk of current standard of care (SOC) vs aztreonam for inhalation solution (AZLI) in patients with cystic fibrosis (CF) Am J Respir Crit Care Med. 2009;179:A1188. [Google Scholar]
- 23.Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry Annual Data Report To The Center Directors 2006. Bethesda, Maryland: 2007. [Google Scholar]
- 24.Retsch-Bogart GZ, Burns JL, Otto KL, Liou TG, McCoy K, Oermann C, Gibson RL. A phase 2 study of aztreonam lysine for inhalation to treat patients with cystic fibrosis and Pseudomonas aeruginosa infection. Pediatr Pulmonol. 2008;43:47–58. doi: 10.1002/ppul.20736. [DOI] [PubMed] [Google Scholar]
- 25.Oie S, Fukui Y, Yamamoto M, Masuda Y, Kamiya A. In vitro antimicrobial effects of aztreonam, colistin, and the 3-drug combination of aztreonam, ceftazidime and amikacin on metallobeta-lactamase-producing Pseudomonas aeruginosa. BMC Infect Dis. 2009;9:123–127. doi: 10.1186/1471-2334-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong Y, Zhao X, Kreiswirth BN, Drlica K. Mutant prevention concentration as a measure of antibiotic potency: studies with clinical isolates of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2000;44:2581–2584. doi: 10.1128/aac.44.9.2581-2584.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward H, Perron GG, Maclean RC. The cost of multiple drug resistance in Pseudomonas aeruginosa. J Evol Biol. 2009;22:997–1003. doi: 10.1111/j.1420-9101.2009.01712.x. [DOI] [PubMed] [Google Scholar]
- 28.Bakare N, Rickerts V, Bargon J, Just-Nübling G. Prevalence of Aspergillus fumigatus and other fungal species in the sputum of adult patients with cystic fibrosis. Mycoses. 2003;46:19–23. doi: 10.1046/j.1439-0507.2003.00830.x. [DOI] [PubMed] [Google Scholar]
- 29.Appel GB. Aminoglycoside nephrotoxicity. Am J Med. 1990:38S–42S. doi: 10.1016/0002-9343(90)90082-o. 16S-20S; discussion. [DOI] [PubMed] [Google Scholar]
- 30.Myhre JL, DePaoli A, Keim GR. Ototoxicity of subcutaneously administered aztreonam in neonatal rats. Toxicol Appl Pharmacol. 1985;77:108–115. doi: 10.1016/0041-008x(85)90272-8. [DOI] [PubMed] [Google Scholar]