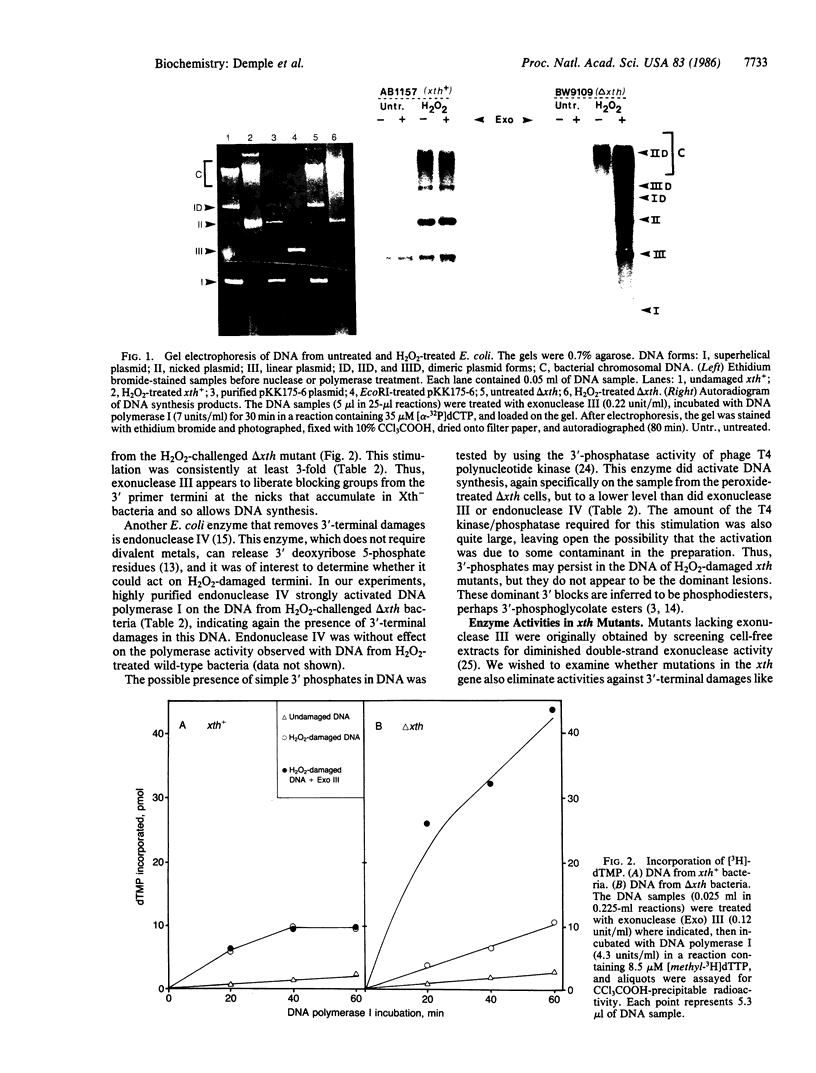
Abstract
Escherichia coli deficient in exonuclease III (xth gene mutants) are known to be hypersensitive to hydrogen peroxide. We now show that such mutants accumulate many more DNA single-strand breaks than do wild-type bacteria upon exposure to H2O2. DNA isolated from H2O2-treated xth- cells contains strand breaks that do not efficiently support synthesis by E. coli DNA polymerase I, indicating the presence of blocking groups at the DNA 3' termini. Purified E. coli exonuclease III activates this blocked DNA to allow substantial synthesis by polymerase I in vitro. Another E. coli enzyme, endonuclease IV, also activates primers for DNA polymerase. Exonuclease III accounts for greater than 95% of the total activity in E. coli crude extracts for removal of 3'-terminal phosphoglycolaldehyde esters from model DNA substrates. Purified exonuclease III and endonuclease IV can each efficiently remove 3'-terminal phosphoglycolaldehyde in vitro. An important physiological function for exonuclease III is thus the activation of blocked 3' ends for DNA repair synthesis. Endonuclease IV can also initiate the repair of ruptured 3'-deoxyribose in DNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Ananthaswamy H. N., Eisenstark A. Repair of hydrogen peroxide-induced single-strand breaks in Escherichia coli deoxyribonucleic acid. J Bacteriol. 1977 Apr;130(1):187–191. doi: 10.1128/jb.130.1.187-191.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J. Plasmid vectors for the selection of promoters. Gene. 1984 Feb;27(2):151–160. doi: 10.1016/0378-1119(84)90136-7. [DOI] [PubMed] [Google Scholar]
- Cameron V., Uhlenbeck O. C. 3'-Phosphatase activity in T4 polynucleotide kinase. Biochemistry. 1977 Nov 15;16(23):5120–5126. doi: 10.1021/bi00642a027. [DOI] [PubMed] [Google Scholar]
- Clements J. E., Rogers S. G., Weiss B. A DNase for apurinic/apyrimidinic sites associated with exonuclease III of Hemophilus influenzae. J Biol Chem. 1978 May 10;253(9):2990–2999. [PubMed] [Google Scholar]
- Demple B., Halbrook J. Inducible repair of oxidative DNA damage in Escherichia coli. Nature. 1983 Aug 4;304(5925):466–468. doi: 10.1038/304466a0. [DOI] [PubMed] [Google Scholar]
- Demple B., Halbrook J., Linn S. Escherichia coli xth mutants are hypersensitive to hydrogen peroxide. J Bacteriol. 1983 Feb;153(2):1079–1082. doi: 10.1128/jb.153.2.1079-1082.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Linn S. 5,6-Saturated thymine lesions in DNA: production by ultraviolet light or hydrogen peroxide. Nucleic Acids Res. 1982 Jun 25;10(12):3781–3789. doi: 10.1093/nar/10.12.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Linn S. DNA N-glycosylases and UV repair. Nature. 1980 Sep 18;287(5779):203–208. doi: 10.1038/287203a0. [DOI] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Giloni L., Takeshita M., Johnson F., Iden C., Grollman A. P. Bleomycin-induced strand-scission of DNA. Mechanism of deoxyribose cleavage. J Biol Chem. 1981 Aug 25;256(16):8608–8615. [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- Henner W. D., Grunberg S. M., Haseltine W. A. Enzyme action at 3' termini of ionizing radiation-induced DNA strand breaks. J Biol Chem. 1983 Dec 25;258(24):15198–15205. [PubMed] [Google Scholar]
- Hutchinson F. Chemical changes induced in DNA by ionizing radiation. Prog Nucleic Acid Res Mol Biol. 1985;32:115–154. doi: 10.1016/s0079-6603(08)60347-5. [DOI] [PubMed] [Google Scholar]
- Kow Y. W., Wallace S. S. Exonuclease III recognizes urea residues in oxidized DNA. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8354–8358. doi: 10.1073/pnas.82.24.8354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasin F., Hutchinson F. Repair of DNA double-strand breaks in Escherichia coli, which requires recA function and the presence of a duplicate genome. J Mol Biol. 1977 Oct 15;116(1):81–98. doi: 10.1016/0022-2836(77)90120-6. [DOI] [PubMed] [Google Scholar]
- Ljungquist S. A new endonuclease from Escherichia coli acting at apurinic sites in DNA. J Biol Chem. 1977 May 10;252(9):2808–2814. [PubMed] [Google Scholar]
- Milcarek C., Weiss B. Mutants of Escherichia coli with altered deoxyribonucleases. I. Isolation and characterization of mutants for exonuclease 3. J Mol Biol. 1972 Jul 21;68(2):303–318. doi: 10.1016/0022-2836(72)90215-x. [DOI] [PubMed] [Google Scholar]
- Mitzel-Landbeck L., Schutz G., Hagen U. In vitro repair of radiation-induced strand breaks in DNA. Biochim Biophys Acta. 1976 May 3;432(2):145–153. doi: 10.1016/0005-2787(76)90156-8. [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y., Yamashita K., Sekiguchi M. Purification and characterization of normal and mutant forms of T4 endonuclease V. J Biol Chem. 1982 Mar 10;257(5):2556–2562. [PubMed] [Google Scholar]
- Noguti T., Kada T. Studies on DNA repair in Bacillus subtilis. II. Partial purification and mode of action of an enzyme enhancing the priming activity of gamma-irradiated DNA. Biochim Biophys Acta. 1975 Jul 7;395(3):294–305. doi: 10.1016/0005-2787(75)90200-2. [DOI] [PubMed] [Google Scholar]
- Povirk L. F., Goldberg I. H. Endonuclease-resistant apyrimidinic sites formed by neocarzinostatin at cytosine residues in DNA: evidence for a possible role in mutagenesis. Proc Natl Acad Sci U S A. 1985 May;82(10):3182–3186. doi: 10.1073/pnas.82.10.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON C. C., KORNBERG A. A DEOXYRIBONUCLEIC ACID PHOSPHATASE-EXONUCLEASE FROM ESCHERICHIA COLI. I. PURIFICATION OF THE ENZYME AND CHARACTERIZATION OF THE PHOSPHATASE ACTIVITY. J Biol Chem. 1964 Jan;239:242–250. [PubMed] [Google Scholar]
- Repine J. E., Pfenninger O. W., Talmage D. W., Berger E. M., Pettijohn D. E. Dimethyl sulfoxide prevents DNA nicking mediated by ionizing radiation or iron/hydrogen peroxide-generated hydroxyl radical. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1001–1003. doi: 10.1073/pnas.78.2.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. G., Weiss B. Exonuclease III of Escherichia coli K-12, an AP endonuclease. Methods Enzymol. 1980;65(1):201–211. doi: 10.1016/s0076-6879(80)65028-9. [DOI] [PubMed] [Google Scholar]
- Seeberg E., Steinum A. L. Repair of x-ray-induced deoxyribonucleic acid single-strand breaks in xth mutants of Escherichia coli. J Bacteriol. 1980 Mar;141(3):1424–1427. doi: 10.1128/jb.141.3.1424-1427.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C., Becherer K. Cloning of the gene topA encoding for DNA topoisomerase I and the physical mapping of the cysB-topA-trp region of Escherichia coli. Nucleic Acids Res. 1983 Mar 25;11(6):1773–1790. doi: 10.1093/nar/11.6.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Demple B. F., Deutsch W. A., Kane C. M., Linn S. Apurinic/apyrimidinic endonucleases in repair of pyrimidine dimers and other lesions in DNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4602–4606. doi: 10.1073/pnas.77.8.4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajko D. M., Weiss B. Mutations simultaneously affecting endonuclease II and exonuclease III in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Feb;72(2):688–692. doi: 10.1073/pnas.72.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]