Abstract
Objective. Artemisia ciniformis (Asteraceae) and A. biennis are two of 34 Artemisia species growing naturally in Iran. In this study we investigated whether different extracts of A. ciniformis and A. biennis have protective effect against hydrogen peroxide-induced cytotoxicity in rat cardiomyoblast cells (H9c2). Method. The dried and ground aerial parts of these two species were extracted successively using petroleum ether (40–60), dichloromethane, ethyl acetate (EA), ethanol (EtOH) and ethanol : water (1 : 1) by maceration method. To evaluate whether different extracts of A. ciniformis and A. biennis protect cardiomyoblast H9c2 cells from H2O2 cytotoxicity, we examined the direct cytotoxic effect of H2O2 on H9c2 cells in the presence and absence of different extracts. After then, cell viability was measured by MTT assay. Results. H2O2 induced cytotoxicity in a concentration dependent manner. The IC50 value was 62.5 μM for 24 h exposure. However, pretreatment of cells with various concentrations of EA, EtOH, and EtOH/wt extract of A. ciniformis protected cells from H2O2-induced cytotoxicity. Moreover, pretreatment with EA, EtOH and EtOH/wt extracts of A. ciniformis lead to a decrease in the reactive oxygen species (ROS) generation. Taken together our observation indicated that nontoxic concentration of different extracts of A. ciniformis has protective effect on H2O2-induced cytotoxicity in H9c2 cells.
1. Introduction
Artemisia biennis Willd. and A. ciniformis Krasch. & Popov ex Poljakov. (Compositae) grow wildly in Iran [1]. Analysis of the essential oils from the aerial parts of A. biennis growing in Iran and western Canada revealed the presence of camphor and [E] beta-farnesene as the major constituents, respectively [2, 3]. Myrcene [4] and davanone [5] have been reported as the main constituent in the aerial parts oils of A. ciniformis
Cytotoxicity of some fractions of A. biennis and A. ciniformis as well as significant effects of ethanolic extracts of the species on in vitro leishmanicidal activity have been proved [6–8]. Iranshahi et al. [9] reported the presence of high amounts of sesquiterpene lactonesin A. ciniformis. Another study showed that antioxidant activity and total phenolic content of hydroethanolic extract of A. biennis were higher than those of other extracts [10].
Oxidative stress corresponds to an imbalance between the rate of oxidant production and degradation. It causes numerous biological effects ranging from alternation in signal transduction and gene expression to mutagenesis and finally cell death. It is well known that oxidative stress plays a significant role in the pathogenesis of heart dysfunctions [11]. In our previous study we evaluated the antioxidant activity and total phenolic content of different extracts of A. biennis using cell free systems [10]. In the current, study we aimed to examine the effects of A. biennis and A. ciniformis extracts on hydrogen peroxide (H2O2)-induced cytotoxicity and oxidative stress in H9c2 cardiomyoblast cells.
2. Material and Methods
2.1. Reagents and Chemicals
Hydrogen peroxide H2O2, 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT), 2,5 dichlorofluorescin diacetate (DCF-DA) were bought from Sigma Aldrich (St Louis, MO, USA). Cell culture medium, penicillin-streptomycin, and fetal bovine serum (FBS) were purchased from Gibco (Gibco, Grand Island, NY, USA). All the solvents used for extraction were purchased from Caledon (Ontaria, Canada) and Scharlau (Sentmenate, Spain).
2.2. Plant Material
Aerial parts of A. ciniformis Krasch. & Popov ex Poljakov. and A. biennis Willd. were collected from Tandoureh national park and Zoshk, respectively (Razavi Khorasan province, Iran), in September 2010. Samples were identified by Dr Valiollah Mozaffarian (Research Institute of Forest and Rangelands, Tehran, Iran). The voucher specimen (Nos. 12569 and 12570) have been deposited in the herbarium, Department of Pharmacognosy, Faculty of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran.
2.3. Preparation of Extracts and Fractions
The dried powdered aerial parts (80 g) of A. biennis and A. ciniformis were extracted with petroleum ether (40–60) (PE), dichloromethane (DCM), ethyl acetate (EA), ethanol (EtOH) and ethanol-water (1 : 1 v/v) (EtOH/wt), respectively (Sequential maceration with ca. 3 × 0.8 L of each solvent). The extracts were filtrated with filter paper and dried using rotary evaporator at a reduced pressure at a temperature below 45°C to yield 4.30, 5.60, 0.39, 1.28, and 8.10 g of each extract for A. biennis and 4.13, 9.66, 0.29, 2.54, and 16.08 g for A. ciniformis, respectively.
2.4. Cell Culture Conditions
Cardiac H9c2 cells are a clonal heart muscle cell line originated from embryonic rat hearts that presents many cardiomyocyte phenotypes [12]. The H9c2 cells maintained in Dubblico modified Eagle's medium (DMEM ATCC) with 10% (V/V) heat inactivated FBS, penicillin G (100 U/mL) and streptomycin (100 mg/mL) at 37°C in 95% CO2 humified incubator. The medium was changed 2-3 days and subcultured when the cell population density reached to 70–80% confluence. Cells were seeded at an appropriate density according to each experimental design.
2.5. Cell Viability Assay
Cellular toxicities of hydrogen peroxide and different extracts of A. biennis and A. ciniformis were analysed in H9c2 cells using MTT methods. Four sets of experiments were performed at standard culture conditions: (1) untreated control cells, (2) cells were treated with different concentrations of A. biennis and A. ciniformis (10–50 μg/mL), (3) cells were treated with different concentrationsof hydrogen peroxide (25–250 μM), and (4) cells were pretreated with different concentrations of extracts for 24 h, then medium was changed and cells were treated with IC50 concentration of hydrogen peroxide for another 24 h. Viability of cells were analyzed using MTT methods. Briefly, after treatment, 20 μL of a 5 mg/mL MTT solution was added to each well. After 2 h incubation, the medium was carefully aspirated and the purple formazan crystals were solubilized with 100 μL DMSO. Optical density was measured at 570 nm (reference wavelength 630 nm) in a microplate reader (Bio-Tek, ELX 800, USA). The absorbance of the untreated culture was set at 100%.
2.6. Determination of Intracellular ROS
Intracellular ROS levels were examined using DCF-DA. DCF-DA is a nonfluorescent lipophilic ester that easily crosses the plasma membrane. Into the cytosol the acetate group is rapidly removed by unspecific esterases. The oxidation of this molecule to the fluorochrome DCF results in green fluorescence. The intensity of this fluorescence is generally considered to reflect the level to which ROS are present [12].
After seeding for 24 h, H9c2 cells were washed with PBS buffer (pH 7.4). The cells pretreated with test samples for 24 h were then treated with H2O2 for an additional 24 h. After washing with PBS, the cells were incubated with 20 μL DCF-DA at 37°C for 30 min. The percentage of DMSO insolution did not exceed from 0.5%. After incubation, cells were lysed with Triton X-100. The fluorescence was measured at an excitation wavelength of 488 nm and an emission wavelength of 528 nm using a fluorescence microplate reader (BioTek, H1M, USA).
2.7. Statistical Analysis
Each experiment was performed at least three times and the results were presented as mean ± S.E.M. One-way analysis of variance (ANOVA) followed by Turkey's test was used to compare the differences between means. A probability value of P < 0.05 was considered to be statistically significant.
3. Results
3.1. Cell Viability after Exposure to H2O2, A. biennis, and A. ciniformis Extracts Alone
The viability of H9c2 cardiomyoblast cells was evaluated after 24 h exposure to different concentrations of H2O2. Cell viability was evaluated by the MTT method. As shown in Figure 1, H2O2-induced cytotoxicity was dose dependent. The mean ± SEM IC50 value was 62.5 ± 0.034 μM for 24 h exposure to H2O2. In order to set extracts at concentrations which are nontoxic to cells but could prevent H2O2-induced cytotoxicity, we also examined the effects of different concentrations of A. biennis and A. ciniformis extracts on cell viability in H9c2 cells.
Figure 1.

The effect of H2O2 on H9c2 cell viability. The cell viability was determined by MTT assay as described in material and methods. Data are expressed as the mean ± SEM of three separate experiments (n = 6).
Figure 2 clearly revealed that 24 h treatment with PE, DCM, EA, EtOH, and EtOH/wt extracts of A. biennis had no cytotoxic effect at the concentrations up to 50 μg/mL, while 24 h exposure to DCM and PE extracts of A. ciniformis induced dose response cytotoxicity.
Figure 2.
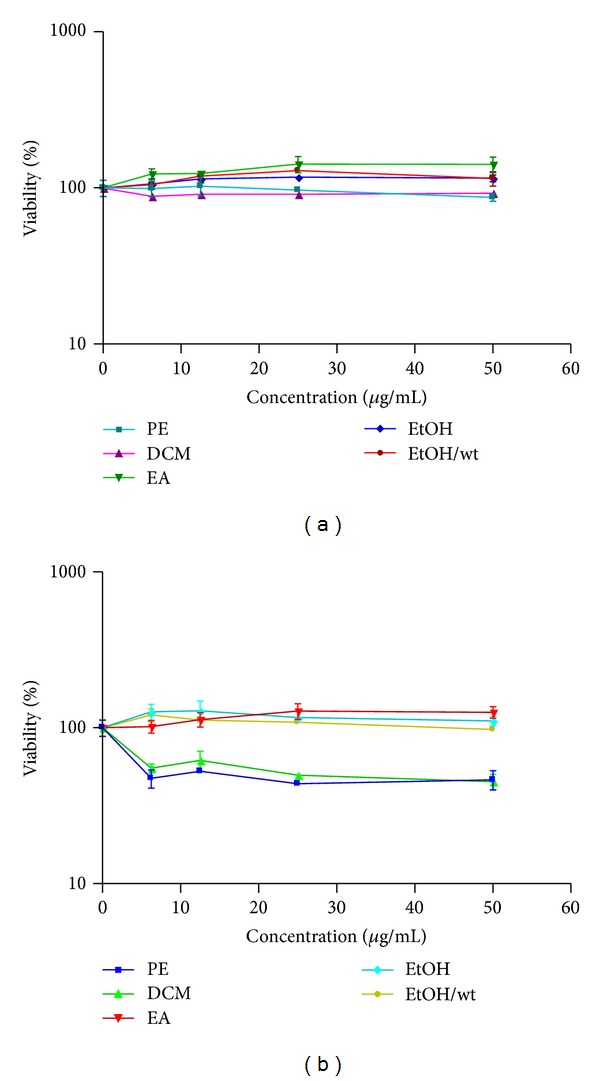
Cell viability of H9c2 cells after exposure to (a) A. biennis and (b) A. ciniformis Cells were treated with different concentration of extracts for 24 h. The cell viability was determined by MTT assay. Data are expressed as the mean ± SEM of three separate experiments (n = 6).
3.2. Effect of Pretreatment with Different Extracts of A. biennis and A. ciniformis on H2O2 Induced Cell Death
For evaluation of effect of pretreatment with different extracts on H2O2 induced cytotoxicity, H9c2 cells were pretreated for 24 h with nontoxic concentrations of extracts, then the medium was changed and cells treated with IC50 concentration (62.5 mM) of H2O2 for another 24 h. As shown in Figure 1, H2O2 treatment significantly decreased cell viability to 50 ± 2.2% of control. Adding EA, EtOH, and EtOH/wt extracts of A. ciniformis (25 μg/mL) before H2O2 treatment increased the cell viability to 76 ± 4.53, 72 ± 1.25 and 82 ± 3.21% of control, respectively (Figure 3). Other extracts were not able to protect H9c2 cells against H2O2-induced cytotoxicity.
Figure 3.

The effect of different extracts of A. biennis and A. ciniformis on H2O2-induced cytotoxicity in H9c2 cells. Cell pretreated with different extracts of A. biennis and A. ciniformis for 24 h before exposure to 62.5 μM of H2O2. Data are expressed as the mean ± SEM of three separate experiments (n = 6). ## P < 0.01 versus control, *P < 0.05, versus H2O2 treated cells.
3.3. Effect of EA, EtOH, and EtOH/wt Extracts of A. ciniformis on ROS Induced by H2O2 in H9c2 Cardiac Muscle Cells
In order to measure oxidative stress induced by H2O2, fluorescent dye DCF-DA was used to measure ROS generation. As anticipated adding H2O2 to H9c2 cells caused a significant increase in ROS level. Therefore, cardiomyoblast cells are probably killed due to oxidative stress, since H2O2 increases intracellular ROS levels. We investigated the inhibitory effect of different extracts on ROS production in the presence of H2O2. Pretreatment with EA, EtOH, and EtOH/wt extracts of A. ciniformis decreased intra cellular ROS levels in H9c2 cells, significantly. These results indicate that the aforementioned extracts have potential for prevention of ROS mediated events (Figure 4).
Figure 4.

The effect of different extracts of A. ciniformis pretreatment on H2O2-induced ROS generation. Data are expressed as the mean ± SEM of three separate experiments (n = 4). ### P < 0.001 versus Control, and ***P < 0.001 versus H2O2-treated cells.
4. Discussion
Oxidative stress is considered to be an important condition to promote cell death in response to a variety of signals and pathophysiological condition [13]. It results from increased formation of ROS and/or decreased antioxidant store. Oxidative stress can be identified in most of the key stages in the pathophysiology of atherosclerosis and the main clinical manifestations of cardiovascular disease [14, 15]. Previous reports demonstrated thatanti-oxidant natural substances including herbal medicines could inhibit ROS generation [16].
In the current study we examined the protective effect of different extracts of A. biennis and A. ciniformis on the cytotoxicity induced by H2O2. The obtained results showed that only EA, EtOH, and EtOH/wt extracts of A. ciniformis are able to protect H9c2 cardiomyoblast cells against H2O2 cytotoxicity.
Next, it was investigated whether pretreatment with above mentioned extracts had an effect on ROS generation by H2O2. The obtained results showed that pretreatment with EA, EtOH, and EtOH/wt extracts of A. ciniformis leads to a decrease in the ROS generation. One possible explanation for the effect of EA, EtOH, and EtOH/wt extracts of A. ciniformis on the oxidative stress induced by H2O2 concerns its polyphenolic content, because it is known that plant-derived polyphenolics are potent antioxidants and free radical scavengers [17].
Despite the fact that hydro ethanolic extract of A. biennis showed potent antioxidant effects using free radical scavenging methods it was not able to protect H9c2 cells from cytotoxicity induced by H2O2 in the current study [10]. This is due to the actual antioxidant activity in physiological conditions such as specific target radicals, localization in different phases and their possible interaction. Therefore, cell free methods may not be sufficient to assessment of antioxidant properties of phytochemicals. Taken together, our data suggested that EA, EtOH, and EtOH/wt extracts of A. ciniformis, protected cardiomyoblasts against H2O2-induced cell death by a mechanism believed to be free radical scavenging and/or the inhibition of reactive oxygen species. Thus, EA, EtOH, and EtOH/wt extracts of A. ciniformis contains principals that may be useful for the prevention and treatment of cardiovascular diseases associated with ROS. Polyphenolics [18], nitrogen containing compounds [19], Polysaccharide fractions [20] and terpenoids [21] are examples of different classes of plant-derived antioxidants. Isolation and characterization of the active and/or major components as well as further studies to determine the molecular mechanisms by which the extracts exert their cardioprotective role are needed.
Conflict of Interests
The authors declares there is no conflict of interests.
Acknowledgment
This work was performed in partial fulfillment of the requirements for Pharm. D. of Maryam Jamshidi, Kermanshah University of Medical Sciences, Kermanshah, Iran.
References
- 1.Mozaffarian V. A Dictionary of Iranian Plant Names. Tehran, Iran: Farhang Moaser; 1998. [Google Scholar]
- 2.Nematollahi F, Rustaiyan A, Larijani K, Nadimi M, Masoudi S. Essential oil composition of Artemisiabiennis willd. and Pulicariaundulata (L.) C.A. Mey., two compositae herbs growing wild in Iran. Journal of Essential Oil Research. 2006;18(3):339–341. [Google Scholar]
- 3.Lopes-Lutz D, Alviano DS, Alviano CS, Kolodziejczyk PP. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry. 2008;69(8):1732–1738. doi: 10.1016/j.phytochem.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Rustaivan A, Masoudi S, Kazemi M. Volatile oils constituents from different parts of Artemisia ciniformis Krasch. et M. Pop. ex Poljak and Artemisia incana (L.) Druce. from Iran. Journal of Essential Oil Research. 2007;19(6):548–551. [Google Scholar]
- 5.Firouzni A, Vahedi H, Sabbaghi F, Bigdeli M. Composition of the essential oil of Artemisia ciniformis, A. kopetdaghensis, and A. khorasanica in Iran. Chemistry of Natural Compounds. 2008;44(6):804–806. [Google Scholar]
- 6.Emami A, Zamani Taghizadeh Rabe SH, Ahi A, Mahmoudi M. Study on toxic effects of Artemisisa spp. fractions from Iran on human cancer cell lines. Journal of Zanjan University of Medical Sciences and Health Services. 2010;18(70):58–67. [Google Scholar]
- 7.Taghizadeh Rabe SZ, Mahmoudi M, Ahi A, Emami SA. Antiproliferative effects of extracts from Iranian Artemisia species on cancer cell lines. Pharmaceutical Biology. 2011;49(9):962–969. doi: 10.3109/13880209.2011.559251. [DOI] [PubMed] [Google Scholar]
- 8.Emami SA, Rabe SZT, Ahi A, Mahmoudi M. Inhibitory activity of eleven Artemisia species from Iran against Leishmania major parasites. Iranian Journal of Basic Medical Sciences. 2012;15(2):807–811. [PMC free article] [PubMed] [Google Scholar]
- 9.Iranshahi M, Emami SA, Mahmoud-Soltani M. Detection of sesquiterpene lactones in ten Artemisia species population of Khorasan provinces. Iranian Journal of Basic Medical Sciences. 2007;10:183–188. [Google Scholar]
- 10.Hatami T, Emami SA, Miraghaee SS, Mojarrab M. Total phenolic contents and antioxidant activities of different extracts and fractions from the aerial parts of Artemisia biennis Willd. Iranian Journal of Basic Medical Sciences. In press. [PMC free article] [PubMed] [Google Scholar]
- 11.Herrera B, Murillo MM, Álvarez-Barrientos A, Beltrán J, Fernández M, Fabregat I. Source of early reactive oxygen species in the apoptosis induced by transforming growth factor-β in fetal rat hepatocytes. Free Radical Biology and Medicine. 2004;36(1):16–26. doi: 10.1016/j.freeradbiomed.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Karlsson M, Kurz T, Brunk UT, Nilsson SE, Frennesson CI. What does the commonly used DCF test for oxidative stress really show? Biochemical Journal. 2010;428(2):183–190. doi: 10.1042/BJ20100208. [DOI] [PubMed] [Google Scholar]
- 13.Matés JM, Snchez-Jiménez FM. Role of reactive oxygen species in apoptosis: implications for cancer therapy. The International Journal of Biochemistry & Cell Biology. 2000;32(2):157–170. doi: 10.1016/s1357-2725(99)00088-6. [DOI] [PubMed] [Google Scholar]
- 14.Sorg O. Oxidative stress: a theoretical model or a biological reality? Comptes Rendus. 2004;327(7):649–662. doi: 10.1016/j.crvi.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Schnabel R, Blankenberg S. Oxidative stress in cardiovascular disease: successful translation from bench to bedside? Circulation. 2007;116(12):1338–1340. doi: 10.1161/CIRCULATIONAHA.107.728394. [DOI] [PubMed] [Google Scholar]
- 16.Brookins Danz ED, Skramsted J, Henry N, Bennett JA, Keller RS. Resveratrol prevents doxorubicin cardiotoxicity through mitochondrial stabilization and the Sirt1 pathway. Free Radical Biology and Medicine. 2009;46(12):1589–1597. doi: 10.1016/j.freeradbiomed.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Ishige K, Schubert D, Sagara Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radical Biology and Medicine. 2001;30(4):433–446. doi: 10.1016/s0891-5849(00)00498-6. [DOI] [PubMed] [Google Scholar]
- 18.Miller AL. Antioxidant flavonoids: structure, function and clinical usage. Alternative Medicine Review. 1996;1(2):103–111. [Google Scholar]
- 19.Drolet G, Dumbroff EB, Legge RL, Thompson JE. Radical scavenging properties of polyamines. Phytochemistry. 1986;25(2):367–371. [Google Scholar]
- 20.Wang J, Zhang Q, Zhang Z, Li Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica . International Journal of Biological Macromolecules. 2008;42(2):127–132. doi: 10.1016/j.ijbiomac.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Das J, Mao AA, Handique PJ. Terpenoid compositions and antioxidant activities of two Indian valerian oils from the Khasi Hills of North-east India. Natural Product Communications. 2011;6(1):129–132. [PubMed] [Google Scholar]


