Abstract
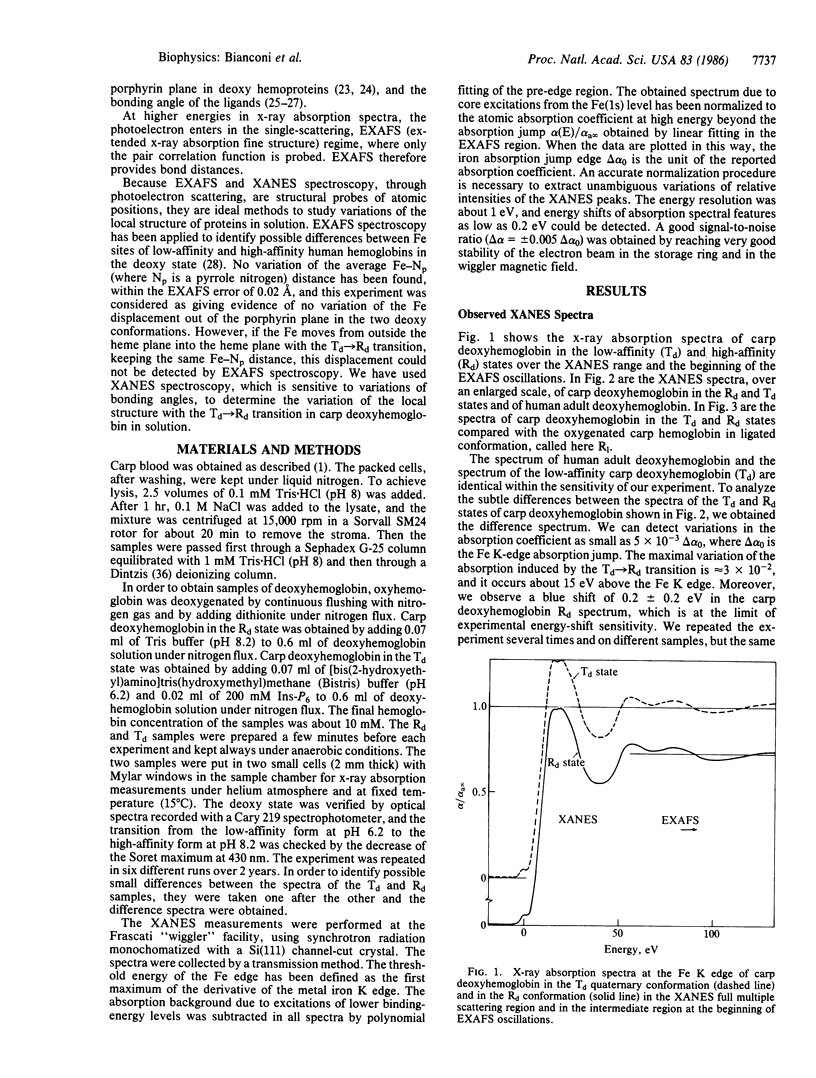
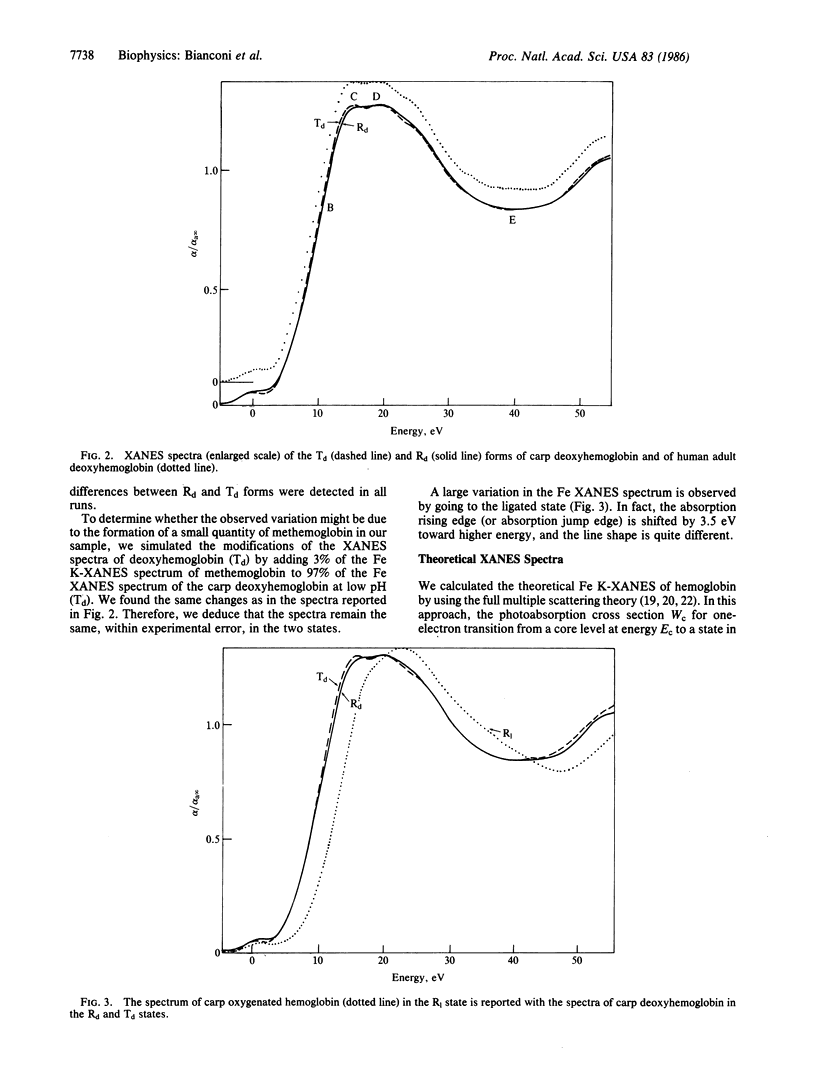
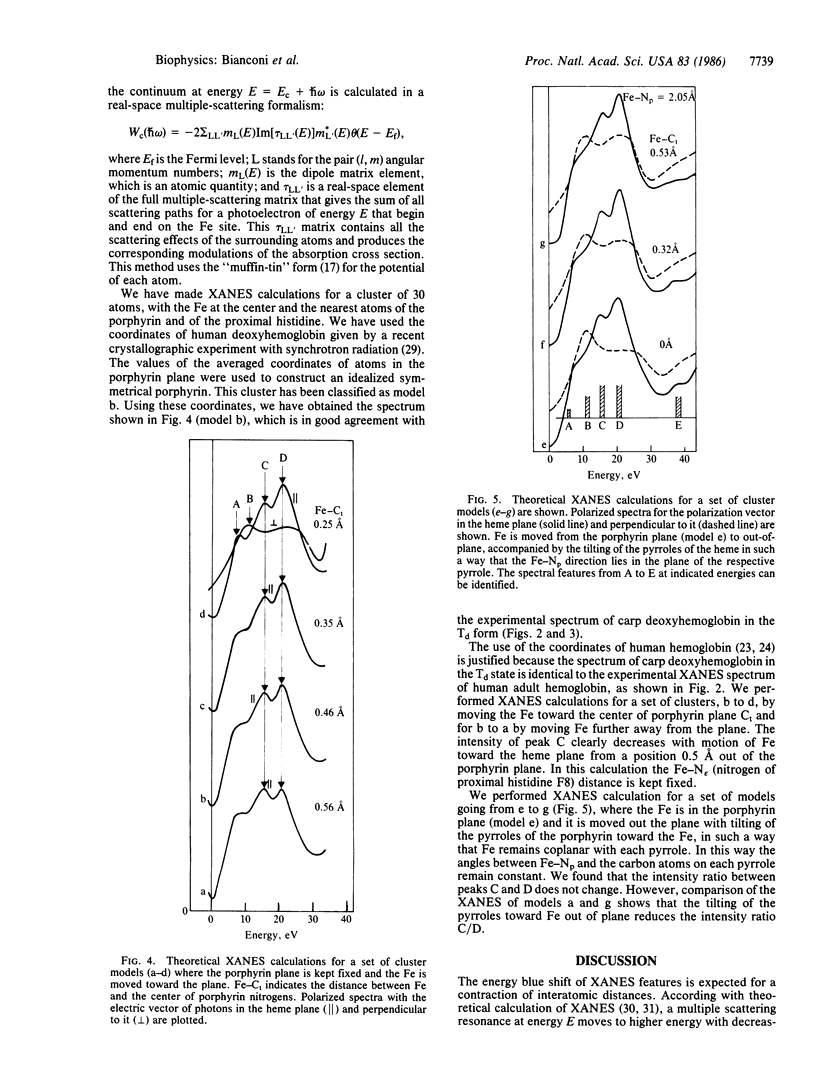
The Fe-site structure variation in the transition from the low-affinity tense (T) quaternary structure to the high-affinity relaxed (R) structure in carp deoxyhemoglobin was studied by analysis of multiple scattering resonances in the XANES (x-ray absorption near edge structure) spectra. High signal-to-noise XANES spectra were measured at the Frascati "wiggler" synchrotron radiation facility. We find that the forces on the Fe active site due to the change of quaternary protein conformation do not induce variations greater than 0.01 A in interatomic Fe-N distances, variations greater than 0.1 A in the Fe displacement toward the heme plane, or the "doming" of the heme. The relevance of these results to the mechanism of protein control of ligand binding is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianconi A., Congiu-Castellano A., Dell'Ariccia M., Giovannelli A., Burattini E., Castagnola M., Durham P. J. Changes in Fe site structure from fetal to adult hemoglobin probed by XANES. Biochim Biophys Acta. 1985 Sep 20;831(1):120–124. doi: 10.1016/0167-4838(85)90158-x. [DOI] [PubMed] [Google Scholar]
- Bianconi A., Congiu-Castellano A., Dell'Ariccia M., Giovannelli A., Burattini E., Durham P. J. Increase of the Fe effective charge in hemoproteins during oxygenation process. Biochem Biophys Res Commun. 1985 Aug 30;131(1):98–102. doi: 10.1016/0006-291x(85)91775-9. [DOI] [PubMed] [Google Scholar]
- Bianconi A., Congiu-Castellano A., Dell'Ariccia M., Giovannelli A., Durham P. J., Burattini E., Barteri M. XANES study of iron displacement in the haem of myoglobin. FEBS Lett. 1984 Dec 3;178(1):165–170. doi: 10.1016/0014-5793(84)81263-6. [DOI] [PubMed] [Google Scholar]
- Bianconi A., Congiu-Castellano A., Durham P. J., Hasnain S. S., Phillips S. The CO bond angle of carboxymyoglobin determined by angular-resolved XANES spectroscopy. Nature. 1985 Dec 19;318(6047):685–687. doi: 10.1038/318685a0. [DOI] [PubMed] [Google Scholar]
- Bianconi A., Giovannelli A., Castellani L., Alema S., Fasella P., Oesch B., Mobilio S. X-ray absorption near edge structure (XANES) determination of calcium sites of troponin C and parvalbumin. J Mol Biol. 1983 Mar 25;165(1):125–138. doi: 10.1016/s0022-2836(83)80246-0. [DOI] [PubMed] [Google Scholar]
- Bianconi A, Fritsch E, Calas G, Petiau J. X-ray-absorption near-edge structure of 3d transition elements in tetrahedral coordination: The effect of bond-length variation. Phys Rev B Condens Matter. 1985 Sep 15;32(6):4292–4295. doi: 10.1103/physrevb.32.4292. [DOI] [PubMed] [Google Scholar]
- Dalvit C., Cerdonio M., Fontana A., Mariotto G., Vitale S., De Young A., Noble R. W. Resonance Raman studies of the quaternary structural change in carp deoxy hemoglobin. FEBS Lett. 1982 Apr 19;140(2):303–306. doi: 10.1016/0014-5793(82)80919-8. [DOI] [PubMed] [Google Scholar]
- Dalvit C., Miura S., de Young A., Noble R. W., Cerdonio M., Ho C. A high-resolution proton nuclear-magnetic-resonance investigation of carp hemoglobin. Conformational differences between carp and human normal adult hemoglobins in solution. Eur J Biochem. 1984 Jun 1;141(2):255–259. doi: 10.1111/j.1432-1033.1984.tb08185.x. [DOI] [PubMed] [Google Scholar]
- Desbois A., Lutz M., Banerjee R. Resonance Raman spectra of deoxyhemoproteins. Heme structure in relation to dioxygen binding. Biochim Biophys Acta. 1981 Dec 29;671(2):177–183. doi: 10.1016/0005-2795(81)90132-x. [DOI] [PubMed] [Google Scholar]
- Durham P., Bianconi A., Congiu-Castellano A., Giovannelli A., Hasnain S. S., Incoccia L., Morante S., Pendry J. B. X-ray absorption near edge structure (XANES) for CO, CN and deoxyhaemoglobin: geometrical information. EMBO J. 1983;2(9):1441–1443. doi: 10.1002/j.1460-2075.1983.tb01605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fermi G., Perutz M. F., Shaanan B., Fourme R. The crystal structure of human deoxyhaemoglobin at 1.74 A resolution. J Mol Biol. 1984 May 15;175(2):159–174. doi: 10.1016/0022-2836(84)90472-8. [DOI] [PubMed] [Google Scholar]
- Friedman J. M., Rousseau D. L., Ondrias M. R., Stepnoski R. A. Transient Raman study of hemoglobin: structural dependence of the iron-histidine linkage. Science. 1982 Dec 17;218(4578):1244–1246. doi: 10.1126/science.7146910. [DOI] [PubMed] [Google Scholar]
- Friedman J. M. Structure, dynamics, and reactivity in hemoglobin. Science. 1985 Jun 14;228(4705):1273–1280. doi: 10.1126/science.4001941. [DOI] [PubMed] [Google Scholar]
- La Mar G. N., Jue T., Hoffman B. M., Nagai K. Proton nuclear magnetic resonance investigation of the allosteric transition in ligated and unligated carp hemoglobin. Evidence for structural heterogeneity in the heme pocket. J Mol Biol. 1984 Oct 5;178(4):929–939. doi: 10.1016/0022-2836(84)90320-6. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Nagai K., Kitagawa T. Differences in Fe(II)-N epsilon(His-F8) stretching frequencies between deoxyhemoglobins in the two alternative quaternary structures. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2033–2037. doi: 10.1073/pnas.77.4.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K., Kitagawa T., Morimoto H. Quaternary structures and low frequency molecular vibrations of haems of deoxy and oxyhaemoglobin studied by resonance raman scattering. J Mol Biol. 1980 Jan 25;136(3):271–289. doi: 10.1016/0022-2836(80)90374-5. [DOI] [PubMed] [Google Scholar]
- Noble R. W., Parkhurst L. J., Gibson Q. H. The effect of pH on the reactions of oxygen and carbon monoxide with the hemoglobin of the carp, Cyprinus carpio. J Biol Chem. 1970 Dec 25;245(24):6628–6633. [PubMed] [Google Scholar]
- Ondrias M. R., Rousseau D. L., Simon S. R. Resonance Raman detection of structural dynamics at the active site in hemoglobin. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1511–1514. doi: 10.1073/pnas.79.5.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F., Brunori M. Stereochemistry of cooperative effects in fish an amphibian haemoglobins. Nature. 1982 Sep 30;299(5882):421–426. doi: 10.1038/299421a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Species adaptation in a protein molecule. Mol Biol Evol. 1983 Dec;1(1):1–28. doi: 10.1093/oxfordjournals.molbev.a040299. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Rousseau D. L., Ondrias M. R. Resonance Raman spectra of photodissociated hemoglobins: implications on cooperative mechanisms. Biophys J. 1985 Apr;47(4):537–545. doi: 10.1016/S0006-3495(85)83948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaanan B. Structure of human oxyhaemoglobin at 2.1 A resolution. J Mol Biol. 1983 Nov 25;171(1):31–59. doi: 10.1016/s0022-2836(83)80313-1. [DOI] [PubMed] [Google Scholar]
- Tan A. L., De Young A., Noble R. W. The pH dependence of the affinity, kinetics, and cooperativity of ligand binding to carp hemoglobin, Cyprinus carpio. J Biol Chem. 1972 Apr 25;247(8):2493–2498. [PubMed] [Google Scholar]


