Abstract
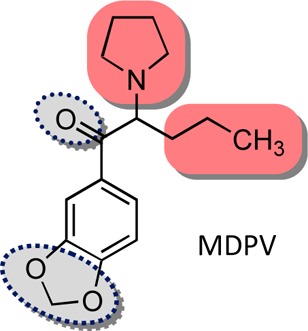
Synthetic cathinones, β-keto analogues of amphetamine (or, more correctly, of phenylalkylamines), represent a new and growing class of abused substances. Several such analogues have been demonstrated to act as dopamine (DA) releasing agents. Methylenedioxypyrovalerone (MDPV) was the first synthetic cathinone shown to act as a cocaine-like DA reuptake inhibitor. MDPV and seven deconstructed analogues were examined to determine which of MDPV’s structural features account(s) for uptake inhibition. In voltage-clamped (−60 mV) Xenopus oocytes transfected with the human DA transporter (hDAT), all analogues elicited inhibitor-like behavior shown as hDAT-mediated outward currents. Using hDAT-expressing mammalian cells we determined the affinities of MDPV and its analogues to inhibit uptake of [3H]DA by hDAT that varied over a broad range (IC50 values ca. 135 to >25 000 nM). The methylenedioxy group of MDPV made a minimal contribution to affinity, the carbonyl group and a tertiary amine are more important, and the extended α-alkyl group seems most important. Either a tertiary amine, or the extended α-alkyl group (but not both), are required for the potent nature of MDPV as an hDAT inhibitor.
Keywords: bk-Amphetamines, β-keto amphetamines, β-ketophenylalkylamines, hDAT, electrophysiology, “bath salts”, drug abuse
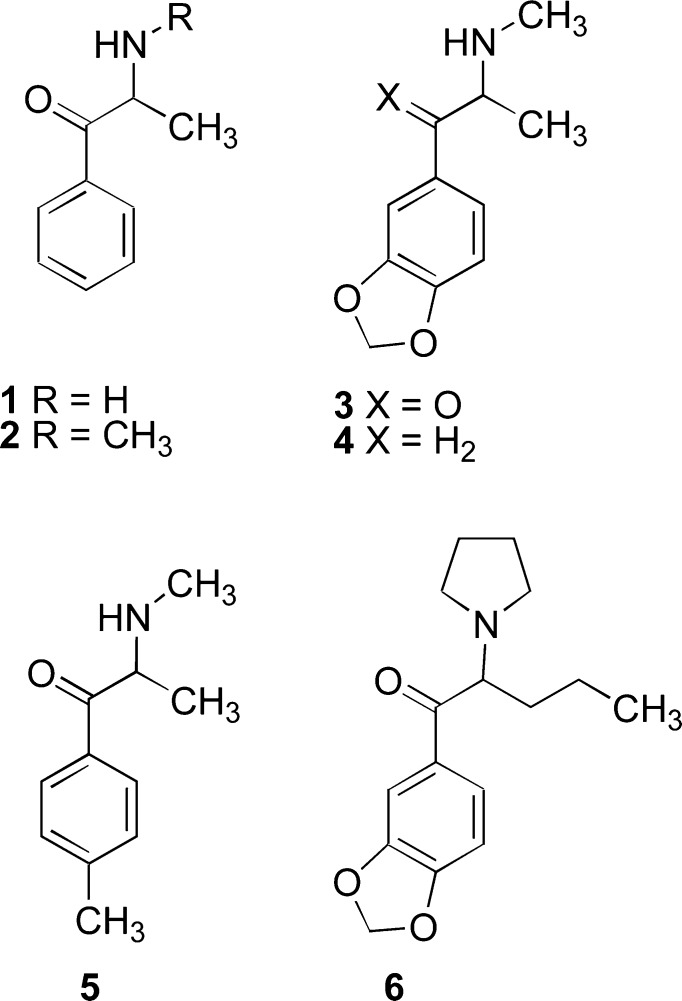
The naturally occurring β-keto analogue of the phenylalkylamine amphetamine, now termed cathinone (1; Figure 1), was first identified as the major central stimulant component of the shrub “khat” (Catha edulis) in 1975.1 The khat plant has been used for many hundreds of years in certain Middle Eastern countries for its stimulant action.2 Cathinone analogues (currently termed “synthetic cathinones”) seemingly erupted on the drug abuse scene just in the past few years. However, some analogues were known earlier. The first synthetic cathinone, termed “methcathinone” (2)3 (the N-methyl counterpart of cathinone or the β-keto analogue of methamphetamine), was actually a widely abused substance in the former Soviet Union since the early 1980s under the name of ephedrone; however, this information was not disseminated until 1989,4 and was not widely available for many years thereafter. Another synthetic cathinone is methylone or MDMC (3), the β-keto analogue of the empathogen MDMA (or N-methyl-(3,4-methylenedioxyphenyl)-2-aminopropane; 4). This synthetic cathinone analogue was independently reported by two groups of investigators in the mid-1990s.5,6
Figure 1.
Chemical structures of cathinone (1), methcathinone (2), methylone (MDMC; 3), MDMA (4), mephedrone (5), and MDPV (6).
In 2010, an alarm was sounded by the British Home Office that the abuse of synthetic cathinones in Europe was on the rise.7 One of the popular synthetic cathinones at the time was referred to as “bath salts” (although known by several other names) that contained either mephedrone (5), methylenedioxypyrovalerone (MDPV; 6), methylone (3), or a combination of two or more of these and/or other agents.8 MDPV was originally patented as a central stimulant in 1969,9 but its identification as a drug of potential abuse did not occur until nearly 40 years later.10 The three most common bath salts ingredients were emergency scheduled (U.S. Schedule I) in 2011;11 mephedrone and MDPV were permanently scheduled in 201212, and methylone was permanently scheduled in 2013.13 Recent reports confirm the central stimulant actions of MDPV.14,15 As of now, 44 synthetic cathinones have been encountered on the clandestine market.16
Cathinone and methcathinone, like amphetamine and methamphetamine, have been previously found to act, primarily, at the human dopamine (DA) membrane transporter (hDAT) by causing the release of DA.3,17 It was initially thought that mephedrone and MDPV, being cathinone analogues, might act in a similar fashion. However, we found that mephedrone induces hDAT-mediated inward currents characteristic of a substrate whereas MDPV induces hDAT-mediated outward currents characteristic of a cocaine-like inhibitor in cells voltage-clamped to −60 mV.18−20 Consistent with these findings, others have now found that mephedrone is (primarily) a DA releasing agent whereas MDPV is an hDAT reuptake inhibitor.21−24
MDPV is unique because, although it interacts with the hDAT, it appears to act in a mechanistically different manner than other synthetic cathinones already investigated. The purpose of the present study was to determine what it is about the structure of MDPV that makes it a mechanistically different type of synthetic-cathinone entity. We employed a deconstruction approach of the MDPV molecule to address the problem. We deconstructed the MDPV molecule, one structural change at a time (i.e., a chemical cladistics approach) to examine this; seven deconstructed analogues (7–13) of MDPV were synthesized and investigated.
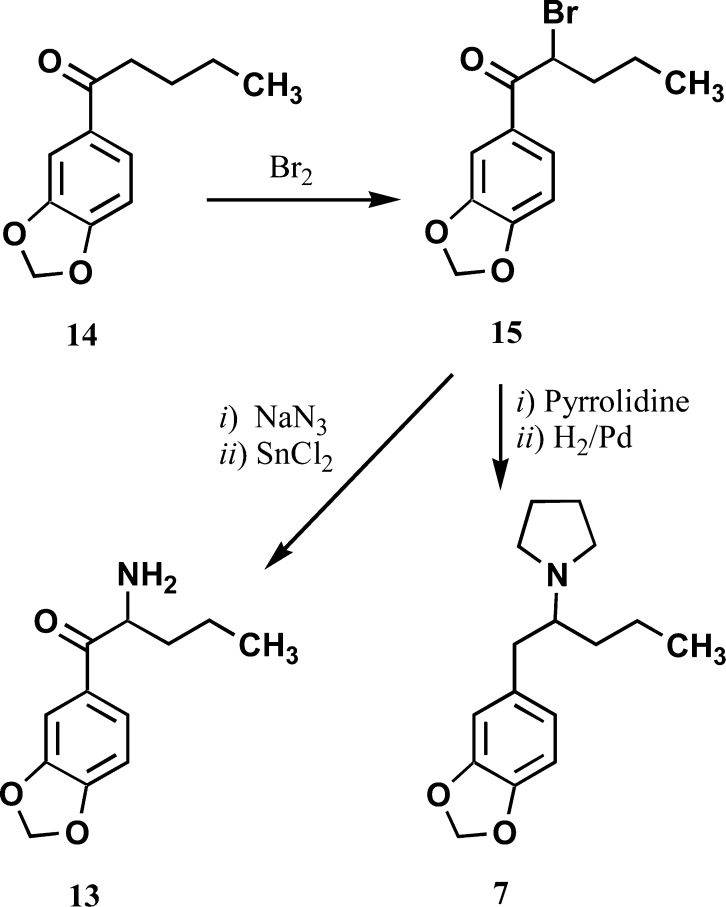
Some of the target compounds have been previously reported in the chemical literature; their synthesis was replicated here, and the targets were isolated as their hydrochloride salts: 6,268,259,2610,27,2811,29 and 12.27,28 1-(Benzo[d]-1,3-dioxol-5-yl)pentanone (14) was prepared as reported by Masazumi et al.30 and brominated with Br2 to afford 15 (Scheme 1). Compound 15 has been previously prepared by a different method;31 although its boiling point was not reported, the 1H NMR spectrum of 15 was identical with that reported in the literature. Reaction of 15 with pyrrolidine followed by catalytic reduction afforded 7 (Scheme 1).
Scheme 1.
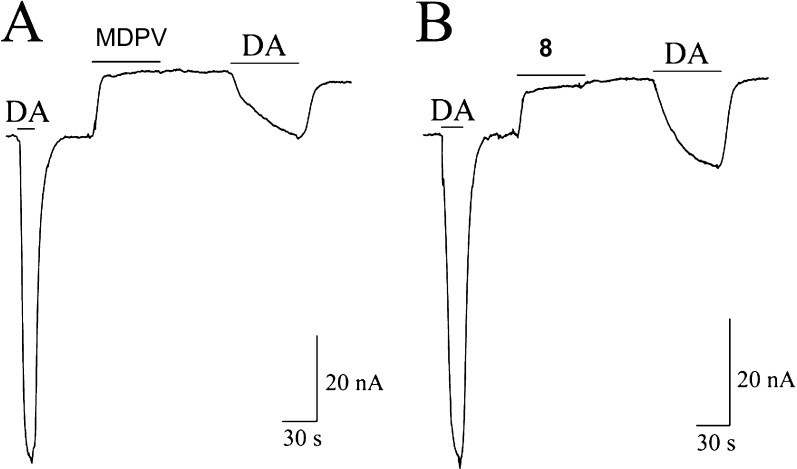
An early goal of these studies was to determine which molecular components of MDPV are responsible for its actions as a cocaine-like agent compared to, for example, methcathinone (which acts as a DA-like releasing agent at hDAT). To address this, we employed two-electrode voltage-clamp (TEVC) in hDAT-expressing Xenopus laevis oocytes (clamped to −60 mV) in response to MDPV and the MDPV analogs. In each recording, an oocyte was first exposed to a short application of 5 μM DA, followed by 10 μM application for 1 min of either MDPV (Figure 2A) or one of the analogues (see Figure 2B and Supporting Information). In contrast to the “synthetic cathinones” methcathinone and mephedrone, which produce hDAT-mediated inward currents in cells voltage-clamped to −60 mV, all of the deconstructed analogues, unexpectedly, behaved in the same manner as MDPV. At 10 μM, all the examined compounds produced qualitatively similar hDAT-mediated outward currents that were similar in amplitude (normalized to percent of the amplitude of the trace induced by the first DA application, Table 1). The outward current represents the block of an endogenous leak current and suggests that all the compounds behaved as hDAT inhibitors. Compound 8 has been previously found to act as a DA reuptake inhibitor.25
Figure 2.
MDPV (A) and compound 8 (B) confer long-lasting effects on hDAT. Currents were recorded in voltage-clamped (−60 mV) Xenopus laevis oocytes expressing hDAT (using a two-electrode voltage-clamp technique); results are representative. (A) Exposure to DA (5 μM) produced an hDAT-mediated inward current. MDPV application (10 μM, 1 min) induced an outward hDAT-mediated current that did not return to baseline when the drug was washed out. Following exposure to MDPV, a 5 μM DA-application induced a diminished hDAT-mediated inward current. (B) In a different oocyte, exposure to DA (5 μM) yielded an hDAT-mediated inward current. Application of compound 8 (10 μM, 1 min) induced an outward hDAT-mediated current that did not return to baseline when the drug was washed out. After exposure to compound 8, application of DA (5 μM) produced a diminished hDAT-mediated inward current (though slightly larger than the current elicited after MDPV application in A). (Representative tracings for the other six compounds not shown here are provided in the Supporting Information.)
Table 1. Magnitude of the Effect Produced by MDPV and Analogues 7–13 (10 μM), and Percent Recovery Following a Subsequent Application of 5 μM Dopamine.
| % response (±SEM)a | % recovery (±SEM)b | N | |
|---|---|---|---|
| MDPV | 16.4 (2.4) | 12.1 (1.1) | 7 |
| 7 | 15.3 (1.7) | 90.0 (1.6) | 5 |
| 8 | 14.8 (2.1) | 25.4 (1.7) | 5 |
| 9 | 18.0 (1.2) | 91.9 (1.9) | 6 |
| 10 | 14.1 (1.7) | 57.5 (2.4) | 3 |
| 11 | 18.7 (2.2) | 102.0 (0.8) | 5 |
| 12 | 16.9 (2.0) | 75.7 (4.5) | 5 |
| 13 | 19.2 (3.4) | 102.6 (1.5) | 5 |
Percent response (relative to the amplitude of the inward current produced by 5 μM DA.
Percent recovery upon application of 5 μM DA 1 min following the outward current produced by MDPV or 7–13.
In these same recordings, however, we sought to determine if exposure to MDPV and its analogues would affect hDAT activity. Following washout for 1 min, a subsequent application of 5 μM DA (after exposure to MDPV or its analogs) elicited hDAT-mediated inward currents with distinct amplitudes (Figure 2 and Supporting Information). For example, following MDPV exposure, DA elicited a diminished hDAT-mediated inward current (as compared to the first DA application) (Figure 2A). In contrast, after exposing hDAT to compound 8 (under the same conditions as Figure 2A), DA produced a somewhat larger hDAT-mediated inward current as compared to the current produced by the initial DA application (Figure 2B). Eventually, modifications to the structure of MDPV did not exhibit a diminished hDAT-mediated inward current and a full DA recovery was seen (as with compounds 11 and 13 (see Table 1 and Supporting Information).
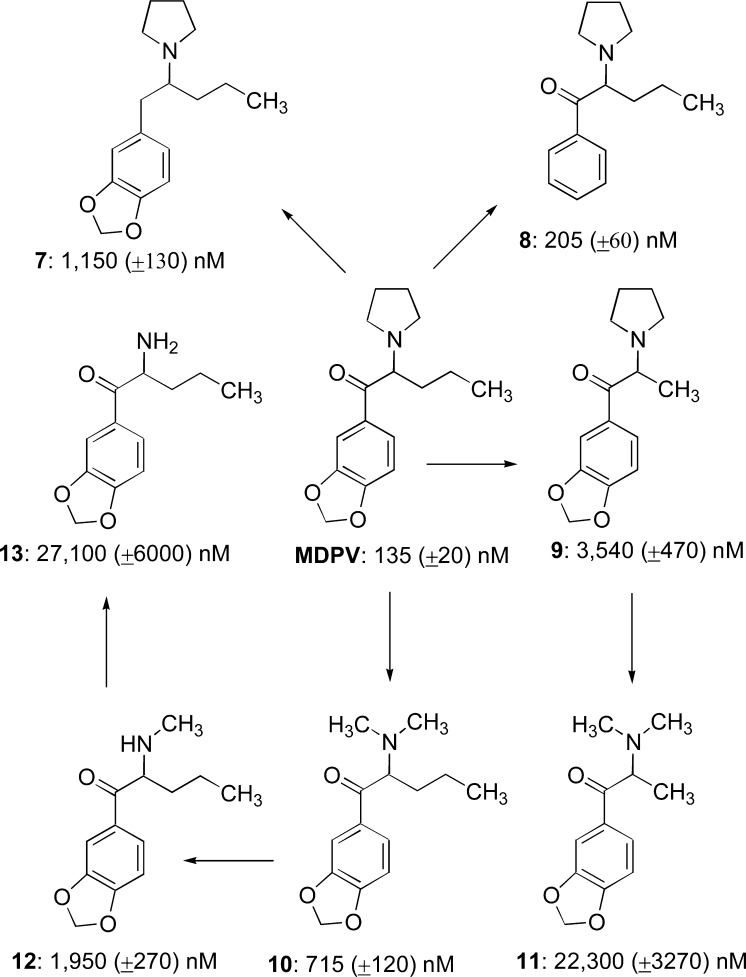
By employing hDAT-expressing HEK-293 cells, we determined the affinities of MDPV and 7–13 to inhibit uptake of [3H]DA by hDAT (Figure 3), which spanned a 200-fold range. None of the structural modifications resulted in a compound with a higher affinity than MDPV (IC50 = 135 nM). Removal of the carbonyl group of MDPV, essentially converting it from a “cathinone analogue” to an “amphetamine analogue”, reduced affinity by nearly 10-fold (7; IC50 = 1,150 nM). In contrast, compound 8 (IC50 = 205 nM), lacking the methylenedioxy group of MDPV, possessed an affinity similar to that of MDPV. The chief structural differences between MDPV and other cathinone analogues (including, for example, analogues 3, 5, and cathinone itself) is the presence of an α-n-propyl group and the pyrrolidine moiety. Shortening the α-n-propyl to an α-methyl group (i.e., 9; IC50 = 3540 nM) produced a 25-fold decrease in affinity. Replacement of the pyrrolidine ring of MDPV with an N,N-dimethylamine (i.e., 10; IC50 = 715 nM) decreased affinity by 5-fold. A similar decrease in affinity was observed when 9 was converted to its N,N-dimethylamine counterpart 11 (IC50 = 22 300 nM). Demethylation of 10 to secondary amine 12 (IC50 = 1,950 nM) reduced affinity by <3-fold, whereas primary amine 13 (IC50 = 27 100 nM) displayed another ∼15-fold decreased affinity.
Figure 3.
Affinity (IC50 ± SEM) of deconstructed analogues 7–13 for hDAT relative to that of MDPV itself. Each arrow represents a single molecular modification.
As described, several different structural features of MDPV are responsible for its potent actions as an hDAT inhibitor (as measured from the affinity values obtained in the uptake inhibition assay) and, accordingly, several different structural features of MDPV confer its long-lasting actions at hDAT (as seen in the recovery experiment). Following MDPV exposure, DA elicited an hDAT-mediated inward current 12.1% the size of the first DA application (Figure 2A, Table 1). The methylenedioxy group of MDPV, although making a small contribution, is not a major determinant for the long-lasting effect on hDAT, since DA recovery was 25.4% (viz. 8). The carbonyl group of MDPV, although a modest contributor to uptake inhibition affinity (10-fold lower than MDPV), seems to play a significant role to produce the long-lasting effect on hDAT (90% recovery) (viz. 7). Moreover, shortening the α-n-propyl chain to an α-methyl group (i.e., 9) resulted in a 25-fold decrease in affinity, which seems related to its pronounced role to elicit the long-lasting effect on hDAT (DA recovery = 91.9%). Comparing MDPV with 10, and 9 with 11, the presence of the cyclic ring system seems to contribute to affinity (∼5–6-fold drop in affinity for both comparisons), which parallels changes on DA recovery (MDPV (12%) vs 10 (57.5%) and 9 (91.9%) vs 11 (102%)). The secondary and primary amine counterparts of 10 (i.e., 12 and 13) elicited increased DA recovery (75.7% for 12 and 102.6% for 13) agreeing with 3-fold and 14-fold affinity reductions, respectively, as compared to 10. Conversion of the pyrrolidine moiety of MDPV to its simplest tertiary (i.e., N,N-dimethyl), secondary (i.e., N-monomethyl), and primary amine counterparts (i.e., 10, 12, and 13, respectively) resulted in relatively small decreases in affinity, but, taken together, conversion of the pyrrolidine moiety found in MDPV to its primary amine 13 resulted in a collective 200-fold decrease in affinity. Similarly, the conversion from MDPV to analogues with sequential pyrrolidine moiety modifications (10 to 12 to 13) shifted DA recovery from 12.1% to 102.6%, suggesting that a tertiary amine is a major contributor to the potent effect MDPV has at hDAT.
It would appear, then, that either (but not necessarily both) a tertiary amine (as found, for example, in MDPV, 9, and 10) or an extended side chain (as found, for example, in MDPV and 12) is required for high affinity, but that optimal affinity is associated with both substituents. The presence of either feature seems sufficient to convert the hDAT substrate-like nature of simple methcathinone (2) analogues (inferred by their ability to induce inward currents at −60 mV) to inhibitor-like agents (inferred by their ability to induce outward currents).
All compounds studied here acted similar to MDPV though their ability to inhibit hDAT uptake is weaker than MDPV. When comparing uptake assays with current measurements, note that, unlike current recordings, uptake inhibition is in the presence of DA and currents measured are under −60 mV voltage-clamp whereas in the uptake assay cells are unclamped.
One of the major differences among the compounds investigated herein is their effect on recovery (Table 1). This needs to be examined in more detail, and was examined here only with a single fixed (i.e., 5 μM) concentration of DA. Nevertheless, percent recovery is highly correlated with affinity (r = 0.928, n = 8, when pIC50 is plotted against % recovery) (Supporting Information Figure 2). This does not seem to be related to incomplete washout because the response to MDPV, the agent showing the least recovery following application of DA after 1 min of buffer washout, failed to return to baseline even following >30 min of washing.20 It would seem, then, that the SAR for recovery is similar to the SAR for affinity in that those analogues with the highest affinity resulted in the least recovery.
It can be concluded that (a) the methylenedioxy ring of MDPV is not a major contributor to its affinity for hDAT, (b) the carbonyl group of MDPV increases its affinity for hDAT, and (c) a tertiary amine or, more importantly, the extended side chain, are sufficient and critical contributors to affinity. From previous work, we have established that the direction of current elicited by a compound (inward vs outward) can be used to determine its action. Inward and outward currents would be expected to depolarize or hyperpolarize a cell, respectively. Mechanistically, MDPV acts in a different manner than, for example, methcathinone or mephedrone at hDAT. The purpose of this investigation was to specifically identify what structural features of MDPV account for these differences. The contribution of MDPV’s structural features to its action to induce hDAT-mediated outward currents has now been identified and quantified.
Once our studies had been completed, it came to light that several of the agents we examined here have now appeared on the clandestine market.16 For example, 8 (already known as α-PVP), 9 (now known as MDPPP), 11 (bk-MDDMA), and 12 (bk-MBDP) have been confiscated by law enforcement agents. So, in addition to an investigation of the SAR of MDPV as a cocaine-like agent, an unintended consequence of the present investigation is to provide some of the first information on the mechanism of action of several newly confiscated synthetic cathinones that have not been previously investigated mechanistically.
Methods
Chemistry
All commercially available reagents and solvents were purchased from Sigma-Aldrich Co. (St. Louis, MO) and used as delivered. Melting points were measured in glass capillary tubes (Thomas-Hoover melting point apparatus) and are uncorrected. 1H NMR spectra were recorded with a Bruker 400 MHz spectrometer. Chemical shifts (δ) are reported in parts per million (ppm) relative to tetramethylsilane as internal standard. Infrared (IR) spectra were recorded using a Thermo Scientific Nicolet iS10 FT-IR spectrometer. The purity of all compounds (>95%), established by elemental analysis, was performed by Atlantic Microlabs (Norcross, GA) and are within 0.4% of theory. Reactions and product mixtures were routinely monitored by thin-layer chromatography (TLC) on silica gel precoated F254 Merck plates.
1-(1-(Benzo[d]-1,3-dioxol-5-yl)pentan-2-yl)pyrrolidine Hydrochloride (7)
A mixture of MDPV (6) (0.20 g, 0.64 mmol), 10% Pd/C (0.05 g), and glacial AcOH (10 mL) was shaken in a Parr hydrogenator under H2 (ca. 55 psi) at room temperature for 16 h. During this time, the starting material was consumed and, presumably, 1-(benzo[d]-1,3-dioxol-5-yl)-2-(pyrrolidin-1-yl)pentan-1-ol was formed as determined by IR analysis. Additional amounts of 10% Pd/C (0.03 g) and perchloric acid (0.4 mL) were periodically added at 24 h intervals while the reaction mixture was further hydrogenated for a total 72 h until the intermediate alcohol was completely consumed.
The catalyst was removed by filtration through a Celite pad. The filtrate was basified with 15% NaOH to pH 9–10 and extracted with EtOAc (3 × 10 mL). The combined organic portion was washed with brine (10 mL) and dried (Na2SO4), and solvent was removed under reduced pressure. The oily residue was converted to its hydrochloride salt by dissolving it in anhydrous Et2O and adding a saturated solution of gaseous HCl in anhydrous Et2O. The salt separated as an oil. Solvent was removed under reduced pressure, and the oily residue solidified after being dried under high vacuum (0.15 g, 79%). The solid was recrystallized twice from a mixture of absolute EtOH/anhydrous Et2O to afford the product (0.06 g, 31%) as beige crystals: mp 135–137 °C; 1H NMR (DMSO-d6) δ 0.77 (t, J = 7.2 Hz, 3H, CH3), 1.16–1.34 (m, 2H, CH2), 1.52–1.57 (m, 2H, CH2), 1.89–1.97 (m, 4H, CH2), 2.75 (dd, J = 13.5, 9.7 Hz, 1H, CH2), 3.07–3.12 (m, 3H, CH2), 3.46–3.49 (m, 3H, CH2), 6.00 (s, 2H, CH2O), 6.75 (t, J = 7.8 Hz, 1H, ArH), 6.87 (d, J = 7.8 Hz, 1H, ArH), 6.92 (s, 1H, ArH), 10.41 (br s, 1H, NH+). Anal. Calcd for C16H23NO2·HCl·0.25H2O: C, 63.56; H, 8.17; N, 4.63. Found: C, 63.70; H, 7.97; N, 4.63.
2-Amino-1-(benzo[d]-1,3-dioxol-5-yl)pentan-1-one Hydrochloride (13)
Sodium azide (0.15 g, 2.31 mmol) was added to a solution of α-bromoketone 15 (0.66 g, 2.31 mmol) in anhydrous MeOH (10 mL), and the reaction mixture was allowed to stir at room temperature for 12 h. Solvent was removed under reduced pressure. The resulting solid residue was dissolved in H2O (20 mL) and extracted with EtOAc (3 × 6 mL). The combined organic portion was dried (Na2SO4), and the solvent was removed under reduced pressure to afford the α-azido ketone (0.52 g, 91%) as a dark-yellow oil that was used without further purification: 1H NMR (CDCl3) δ 0.98 (t, J = 7.4 Hz, 3H, CH3), 1.43–1.60 (m, 2H, CH2), 1.78–1.91 (m, 2H, CH2), 4.47 (dd, J = 8.4, 5.4 Hz, 1H, CH), 6.07 (s, 2H, CH2O), 6.88 (d, J = 8.2 Hz, 1H, ArH), 7.42 (d, J = 1.6 Hz, 1H, ArH), 7.52 (dd, J = 8.2, 1.6 Hz, 1H, ArH).
Tin(II) chloride dihydrate (0.99 g, 4.38 mmol) was added in one portion to a solution of the α-azido ketone (0.51 g, 2.19 mmol) in absolute EtOH (10 mL) at 0 °C (ice-bath), and the reaction mixture was allowed to stir at room temperature for 2 h. Solvent was removed under reduced pressure, and the oily residue was partitioned between a saturated solution of NaHCO3 and EtOAc (100 mL/25 mL). The organic layer was separated and the aqueous portion was extracted with EtOAc (2 × 25 mL). The combined organic portion was washed with brine (25 mL) and dried (Na2SO4), and solvent was removed under reduced pressure to give a yellow, oily residue. The oil was converted to its hydrochloride salt and recrystallized from absolute EtOH to afford 13 (0.26 g, 49%) as white crystals: mp 224–225 °C (dec); 1H NMR (DMSO-d6) δ 0.82 (t, J = 7.2 Hz, 3H, CH3), 1.16–1.29 (m, 1H, CH2), 1.32–1.45 (m, 1H, CH2), 1.66–1.83 (m, 2H, CH2) 5.02 (dd, J = 7.0, 4.7 Hz, 1H, CH), 6.18 (s, 2H, CH2O), 7.11 (d, J = 8.2 Hz, 1H, ArH), 7.54 (d, J = 1.6 Hz, 1H, ArH), 7.71 (dd, J = 8.2, 1.6 Hz, 1H, ArH), 8.44 (br s, 3H, NH3+). Anal. Calcd for C12H15NO3·HCl: C, 55.93; H, 6.26; N, 5.43. Found: C, 55.97; H, 6.11; N, 5.40.
1-(Benzo[d]-1,3-dioxol-5-yl)-2-bromopentan-1-one (15)
Bromine (0.35 mL, 1.09 g, 6.8 mmol) was added in one portion to a stirred mixture of 1-(benzo[d]-1,3-dioxol-5-yl)pentan-1-one (14)29 (9.45 g, 45.8 mmol) and freshly sublimed AlCl3 (0.31 g, 2.3 mmol) in anhydrous Et2O (150 mL) at 0 °C under an N2 atmosphere. After 10 min, the ice-bath was removed. The reaction mixture was allowed to warm to room temperature, and additional Br2 (2.00 mL, 6.23 g, 39.0 mmol) was added in a dropwise manner over a 5 min period. The reaction mixture was neutralized by adding a saturated solution of NaHCO3. The organic layer was separated and dried (Na2SO4), and solvent was removed under reduced pressure. The crude material was purified by Kugelrohr distillation to afford the product (11.24 g, 86%) as a yellow oil: bp 217 °C, 0.6 Torr; 1H NMR (CDCl3) δ 1.00 (t, J = 7.4 Hz, 3H, CH3), 1.40–1.62 (m, 2H, CH2), 2.07–2.22 (m, 2H, CH2), 5.09 (dd, J = 7.7, 6.6 Hz, 1H, CH), 6.09 (s, 2H, CH2O), 6.90 (d, J = 8.2 Hz, 1H, ArH), 7.51 (d, J = 1.7 Hz, 1H, ArH), 7.65 (dd, J = 8.2, 1.7 Hz, 1H, ArH).
Expression of hDAT in Xenopus laevis Oocytes
Oocytes were harvested and prepared from adult X. laevis females following standard procedures.32−34 Stage V–VI oocytes were selected for cRNA injection within 24 h of isolation. cRNA was transcribed from the pOTV vector using the mMessage Machine T7 kit (Ambion Inc., Austin, TX). Oocytes were injected with 50 ng of hDAT cRNA (Nanoject AutoOocyteInjector, Drummond Scientific Co., Broomall, PA) and incubated at 18 °C for 4–8 days in Ringers solution supplemented with sodium pyruvate (550 μg/mL), streptomycin (100 μg/mL), tetracycline (50 μg/mL), and 5% dialyzed horse serum.
Electrophysiology
Two-electrode voltage-clamp (TEVC) experiments were performed as previously described.35 Recordings were at room temperature (23–25 °C). Electrodes having a resistance of 1–5 MΩ were filled with 3 M KCl. X. laevis oocytes expressing hDAT were voltage-clamped to −60 mV with a GeneClamp 500 (Axon Instruments), and the holding current was recorded using a Clampex 10 (Axon Instruments). Extracellular buffer consisted of (in mM): 120 NaCl, 7.5 HEPES, 5.4 potassium gluconate, and 1.2 calcium gluconate, pH 7.4. In a typical recording, extracellular buffer was perfused until stable baseline currents were obtained, followed by experimental drugs (perfusion duration is indicated by a horizontal line on the trace).
Maintenance of Cells Stably Expressing hDAT (hDAT-HEK)
Cells were prepared in DMEM supplemented with 10% FBS, 2 mM l-glutamine, penicillin (100 units/mL), streptomycin (100 mg/mL), and G418.
Radiolabeled 3H-DA Uptake Competition Assay
hDAT-HEK cells in DMEM suspension were counted, and 200 000 cells were placed in individual Eppendorf tubes and incubated for 15 min at room temperature (23–25 °C) with mixtures of 1% [3H]DA. The compounds were tested (MDPV and analogs) at a broad range of concentrations. Ice-cold (4 °C) PBS was added to the samples, cells were centrifuged for 1 min at 2000 rpm, supernatant was removed, and cells were washed twice with PBS, centrifuged, and supernatant was removed. Cells were solubilized with Ecoscint H (National Diagnostics, Atlanta, GA), and the [3H]DA remaining was measured using a scintillation counter. To determine IC50 values, uptake values were plotted against concentration and data were fit using the Hill equation y = Vmax + (Vmin – Vmax) * xn/(kn + xn) using Origin 8 (OriginLab Corporation, Northampton, MA).
Supporting Information Available
Representative tracings for currents induced by analogues 7 and 9–13, and the correlation between % DA recovery versus hDAT affinity. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
R.K. and F.S. synthesized and characterized the target compounds, and E.S. performed the assays and analyzed the data; all provided manuscript input. R.A.G. conceived the initiation of the project; R.A.G. and J.L.D. oversaw the project, assisted with data analysis, and wrote the paper.
This work was supported in part by PHS Grant DA 033930.
The authors declare no competing financial interest.
Supplementary Material
References
- United Nations Narcotic Laboratory Document. (1975) Studies on the chemical composition of khat. III. Investigations on the phenylalkylamine fraction. MNAR/11/75, GE.75-12624. United Nations, Geneva. [Google Scholar]
- United Nations Narcotic Laboratory Document. (1974) Studies on the chemical composition of khat. I. Extraction, screening investigations and solvent separation of khat components. MNAR/12/74, GE.74-12580. United Nations, Geneva. [Google Scholar]
- Glennon R. A.; Yousif M.; Naiman N. A.; Kalix P. (1987) Methcathinone: A new and potent amphetamine-like agent. Pharmacol., Biochem. Behav. 26, 547–551. [DOI] [PubMed] [Google Scholar]
- Savenko V. G., Semkin E. P., Sorokin V. I., and Kazankov S. P. (1989) Expert examination of narcotic substances obtained from ephedrine. USSR Ministry of the Interior All-Union Scientific Research Institute Report, Moscow, pp 1–22.
- Jacob P., and Shulgin A. T. (1996) Novel N-substituted 2-amino-3′,4′-methylene-dioxypropiophenones. WO Patent 9639133, December 12, 1996.
- Dal Cason T. A.; Young R.; Glennon R. A. (1997) Cathinone: An investigation of several N-alkyl and methylenedioxy analogs. Pharmacol., Biochem. Behav. 58, 1109–1116. [DOI] [PubMed] [Google Scholar]
- Iversen L. E. (2010) Consideration of the cathinones. Advisory Council on the Misuse of Drugs. A report submitted to the Home Secretary of the UK (March 31, 2010).
- Spiller H. A.; Ryan M. L.; Weston R. G.; Jansen J. (2011) Clinical experience with analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin. Toxicol. 49, 499–505. [DOI] [PubMed] [Google Scholar]
- Boehringer Ingelheim. (1969) α-Substituted-ketones and processes for their preparation. British Patent 1,149,366, April 23, 1969.
- Fuwa T.; Fukumori N.; Tanaka T.; Kubo Y.; Ogata A.; Uehara S.; Honda Y.; Kodama T. (2007) Microdialysis study of drug effects on central nervous system: Changes of dopamine levels in mice striatum after oral administration of methylenedioxypyrovalerone. Ann. Rep. Tokyo Metrop. Inst. Public Health 58, 287–292. [Google Scholar]
- Federal Register. (2011) Schedules of Controlled Substances: Temporary placement of three synthetic cathinones into Schedule I. Federal Register (October 21, 2011), 4, 65371–65375. [PubMed]
- Synthetic Drug Abuse Prevention Act. (2012) Section 1152. Addition of synthetic drugs to Schedule I of the Controlled Substances Act, pp 138–140 (enacted January 3, 2012; signed July 9, 2012) [http://www.gpo.gov/fdsys/pkg/BILLS-112s3187enr/pdf/BILLS-112s3187enr.pdf].
- Federal Register. (2013) Schedules of Controlled Substances: Placement of methylone into Schedule I. Federal Register (April 12, 2013), 4, 21818–21825.
- Marusich J. A.; Grant K. R.; Blough B. E.; Wiley J. L. (2012) Effects of synthetic cathinones contained in “bath salts” on motor behavior and a functional observational battery in mice. Neurotoxicology 33, 1305–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi W. E.; Gannon B. M.; Zimmerman S. M.; Rice K. C. (2013) In vivo effects of abused “bath salt” constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: Drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology 38, 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The challenge of new psychoactive substances. (2013) United Nations Office of Drugs and Crime. A United Nations Publication, Vienna, Austria.
- Kalix P.; Glennon R. A. (1986) Further evidence for an amphetamine-like mechanism of action of the alkaloid cathinone. Biochem. Pharmacol. 35, 3015–3019. [DOI] [PubMed] [Google Scholar]
- Kolanos R., Cameron K. N., Vekariya R. H., De Felice L. J., and Glennon R. A. (2011) “Bath salts”: An imitation of methamphetamine plus cocaine? Southeast Regional Meeting of American Chemical Society (SERMACS), Richmond, VA, October 26–29. [Google Scholar]
- Cameron K.; Kolanos R.; Verkariya R.; De Felice L.; Glennon R. A. (2013a) Mephedrone and methylenedioxypyrovalerone (MDPV), major constituents of “bath salts,” produce opposite effects at the human dopamine transporter. Psychopharmacology 227, 493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron K. N.; Kolanos R.; Solis E.; Glennon R. A.; De Felice L. J. (2013b) Bath salts components mephedrone and methylenedioxypyrovalerone (MDPV) act synergistically at the human dopamine transporter. Br. J. Pharmacol. 68, 1750–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann M. H.; Ayestas M. A.; Partilla J. S.; Sink J. R.; Shulgin A. T.; Daley P. F.; Brandt S. D.; Rothman R. B.; Ruoho A. E.; Cozzi N. V. (2012) The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology 37, 1192–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann M. H.; Partilla J. S.; Lehner K. R.; Thorndike E. B.; Hoffman A. F.; Holy M.; Rothman R. B.; Goldberg S. R.; Lupica C. R.; Sitte H. H.; Brandt S. D.; Tella S. R.; Cozzi N. V.; Schindler C. W. (2013) Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive “bath salts” products. Neuropsychopharmacology 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler L. D.; Buser T. A.; Donzelli M.; Schramm Y.; Diue L. H.; Huwyler J.; Chaboz S.; Hoener M. C.; Liechti M. E. (2013) Pharmacological characterization of designer cathinones in vitro. Br. J. Pharmacol. 168, 458–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman A. J.; Wolfrum K. M.; Hatfield M. G.; Johnson R. A.; Murphy K. V.; Janowsky A. (2013) Substituted methcathinones differ in transporter and receptor interactions. Biochem. Pharmacol. 85, 1803–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer P. C.; Butler D.; Deschamps J. R.; Madras B. K. (2006) 1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (pyrovalerone) analogues: A promising class of monoamine uptake inhibitors. J. Med. Chem. 49, 1420–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köppe H., Ludwig G., and Zeile K. (1969) 1-(3′,4′-Methylenedioxy-phenyl)-2-pyrrolidino-alkanones-(1). US Patent 3478050, November 11, 1969.
- Koppë H., Ludwig G., and Zeile K. (1967) Verfahren zur Herstellung von substituierten Phenyl-alpha-aminoketonen und deren Saeureadditionssalzen bzw. deren optischen Antipoden. German Patent DE 1242241, June 15, 1967.
- Boehringer Ingelheim G.M.B.H. (1967) Aryl-α- aminoketone derivatives. British Patent GB1085135, September 27, 1967.
- Iwao J.; Samejima M. (1954) Alkanolamines. I. Syntheses of N-methyl-3,4-dihydroxyephedrine hydrochloride and its derivatives. Yakugaku Zasshi 74, 548–50. [Google Scholar]
- Masazumi T., Masaki S., and Setsuo Y. (1985) 1-Propanone Derivatives and Pharmaceutical Compositions Containing Same. Eur. Pat. Appl. 0163537, May 30, 1985.
- Yamauchi S.; Taniguchi E. (1992) Synthesis and insecticidal activity of sesquilignan analogues with 2-alkyl-6-methoxy-3-(3, 4-methylenedioxyphenyl)-1,4-benzodioxanyl group. Biosci. Biotech. Biochem. 56, 1751–1759. [Google Scholar]
- Iwamoto H.; Blakely L.; De Felice L. J. (2006) Na+, Cl–, and pH dependence of the human choline transporter (hCHT) in Xenopus oocytes: The proton inactivation hypothesis of hCHT in synaptic vesicles. J. Neurosci. 26, 9851–9859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey S.; De Felice L. J. (2002) Serotonin transporter function and pharmacology are sensitive to expression level: evidence for an endogenous regulatory factor. J. Biol. Chem. 277, 14475–14482. [DOI] [PubMed] [Google Scholar]
- Machaca K.; Hartzell H. (1998) Assymetric distribution of Ca-activated Cl channels in Xenopus oocytes. Biophys. J. 74, 1286–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. W.; Li C. Z.; Yang Z. F.; Zheng Y. Q.; Zhang Y.; Liu Y. M. (2006) Electrophysiological effect of fluoxetine on Xenopus oocytes heterologously expressing human serotonin transporter. Acta Pharmacol. Sin. 27, 289–293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.