Abstract
Objectives
To describe the prevalence and longitudinal course of radiographic, erosive and symptomatic hand osteoarthritis (HOA) in the general population.
Methods
Framingham osteoarthritis (OA) study participants obtained bilateral hand radiographs at baseline and 9-year follow-up. The authors defined radiographic HOA at joint level as Kellgren–Lawrence grade (KLG)≥2, erosive HOA as KLG≥2 plus erosion and symptomatic HOA as KLG≥2 plus pain/aching/stiffness. Presence of HOA at individual level was defined as ≥1 affected joint. The prevalence was age-standardised (US 2000 Population 40–84 years).
Results
Mean (SD) baseline age was 58.9 (9.9) years (56.5% women). The age-standardised prevalence of HOA was only modestly higher in women (44.2%) than men (37.7%), whereas the age-standardised prevalence of erosive and symptomatic OA was much higher in women (9.9% vs 3.3%, and 15.9% vs 8.2%). The crude incidence of HOA over 9-year follow-up was similar in women (34.6%) and men (33.7%), whereas the majority of those women (96.4%) and men (91.4%) with HOA at baseline showed progression during follow-up. Incident metacarpophalangeal and wrist OA were rare, but occurred more frequently and from an earlier age in men than women. Development of erosive disease occurred mainly in those with non-erosive HOA at baseline (as opposed to those without HOA), and was more frequent in women (17.3%) than men (9.6%).
Conclusions
The usual female predominance of prevalent and incident HOA was less clear for radiographic HOA than for symptomatic and erosive HOA. With an ageing population, the impact of HOA will further increase.
INTRODUCTION
Hand osteoarthritis (HOA) is a heterogeneous disease with involvement of different joints, varying levels of symptoms and an erosive subset with radiographic central erosions.
The prevalence and pattern of radiographic HOA have previously been studied in population-based cohorts.1-16 The estimates show great variation, which may be due to differences in types of populations, disease definitions and/or risk factors such as genetic background or environmental exposures across cohorts. Most previous studies are limited by inclusion of small samples,1 only one sex,2-4 subjects within a limited range of age1,3-7 or only selected joints.8,9 The incidence and longitudinal course of HOA in the general population are less well described. Most studies on the natural history of HOA have been performed in cohorts with prevalent disease or at high risk of HOA,17-20 and the few longitudinal studies in the general population have either included small samples, older subjects or only men.21-24
Erosive HOA is frequently seen in postmenopausal women visiting rheumatology clinics.25 However, few studies have described the prevalence of erosive HOA in the general population,26,27 and no population-based studies have described its incidence.
Thus, prior studies have left an incomplete picture of the descriptive epidemiology of HOA. Among unresolved issues are the occurrence of osteoarthritis (OA) in the metacarpophalangeal (MCP) and wrist joints, the epidemiology of symptomatic and erosive disease, and the longitudinal course. Obtaining a comprehensive picture of the epidemiology of HOA would facilitate a better understanding of the public burden of disease and a better insight into whether and how much of HOA might be due to traumatic/mechanical aetiologies.
Using data from a large longitudinal population-based study, our first aim was to examine the prevalence, incidence and progression of radiographic HOA (including MCP and wrist OA) by age and sex. Second, we examined the prevalence and incidence of erosive and symptomatic HOA.
MATERIAL AND METHODS
Subjects
The Framingham OA Study consists of the Framingham Offspring and Community cohorts. Both cohorts were included in the analyses of HOA prevalence, whereas we used only the Offspring cohort for analyses of HOA incidence/progression. The Offspring cohort included children of the original Framingham Heart Study participants and the spouses of these children.28 Offspring (with parents previously studied for knee OA) and their spouses (if members of the Offspring cohort) were contacted by mail and a follow-up telephone call as part of a family study of OA, and 1800 (28–82 years) (representing 65% of those contacted) attended an examination in 1992–1995. Of those, 1293 (71.8%) returned in 2002–2005 after a median (interquartile range) follow-up time of 8.7 (7.9–9.5) years (n=12 with rheumatoid arthritis (RA) at baseline excluded, n=495 lost to follow-up). Those who were lost to follow-up (45.1% women) had a mean (standard deviation; SD) age of 57.6 (10.7) years and 36.0% had radiographic HOA. Of the 1293 who returned, 1275 eligible participants had baseline hand radiographs (n=10 with missing baseline radiographs, n=8 with RA in the interim), and their baseline and follow-up radiographs were read in pairs. Thus, only those at baseline who later met for follow-up were included in the analyses of HOA prevalence (online supplementary figure S1). One participant did not have hand radiographs at follow-up, and 1274 were included in the analyses of HOA incidence/progression.
We also used the Framingham Community cohort to describe HOA prevalence (online supplementary figure S1). Members were recruited through census-tract data and random-digit telephone dialling, and 1830 persons above 50 years expressed interest. Of those, 1039 were examined in 2002–2005 after application of exclusion criteria (including RA) as described elsewhere.29 Hand radiographs were available for 1028 participants, and 1026 were included in the analyses of HOA prevalence (n=2 with psoriatic arthritis excluded).
The Boston University Medical Center Institutional Review Board approved both studies, and written informed consents were obtained from all participants.
Hand radiographs
The Offspring members underwent bilateral posteroanterior hand radiographs at baseline (1992–1995) and follow-up (2002–2005), and one musculoskeletal radiologist (PA) read the paired radiographs with known time sequence. The Community members underwent bilateral posteroanterior hand radiographs at baseline (2002–2005), and one investigator (IKH) read the radiographs after a training session with PA and DTF.
The bilateral second to fifth distal interphalangeal (DIP), second to fifth proximal interphalangeal (PIP), first to fifth MCP, thumb interphalangeal (IP), thumb base (carpometacarpal/scaphotrapezial joint) and wrist joints were graded for HOA. We used a modified Kellgren–Lawrence (KL) Scale: KL grade (KLG) 0=no HOA; 1=minimal HOA, i.e. questionable osteophyte (OP) and/or joint space narrowing (JSN); 2=mild HOA, i.e. small OP(s) and/or mild JSN, sclerosis may be present; 3=moderate HOA, i.e. moderate OP(s) and/or moderate JSN, sclerosis and erosions may be present; 4=severe HOA, i.e. large OP(s) and/or severe JSN, sclerosis and erosions may be present.6 The joints were also scored for absence/presence of subchondral erosions.30
The same reader scored 42 randomly selected Offspring radiographs and 20 Community radiographs twice. Also, both readers scored 20 Community radiographs. Intra-reader and inter-reader reliability assessed by κ and intraclass correlation coefficients (two-way mixed effect model) were ‘good’ to ‘excellent’ (online supplementary table S1).31
Hand joint symptoms
An interview was conducted with 2212 and 1272 participants at the study visit at baseline and follow-up respectively. If they answered ‘Yes’ to the question, ‘On most days, do you have pain/aching/stiffness in any of your joints?’, they were shown a homunculus with the bilateral DIP, PIP, MCP, thumb IP and thumb base joints circled, and they were asked to indicate which hand joint(s) had complaints.
Statistics
At joint level, we defined radiographic HOA as KLG≥2, erosive OA as KLG≥2 plus central erosion and symptomatic HOA as KLG≥2 plus pain/aching/stiffness. At subject level, participants with ≥1 affected joint(s) according to the definitions above were classified as cases. We similarly classified a hand-joint group (eg, MCP) as having HOA if ≥1 joint(s) in the group was affected. We calculated the prevalence ratio (PR) of symptoms in HOA (KLG≥2) versus non-HOA joints (KLG≤1), in severe HOA (KLG≥3) versus no/mild HOA joints (KLG≤2) and in erosive versus non-erosive joints.
We examined the prevalence, incidence and progression of HOA at joint and subject level. Incidence was assessed in those who were free of HOA (KLG≤1) in all joints at baseline, whereas progression was assessed in those with ≥1 HOA joint(s) at baseline. Participants with maximum possible KL score (n=14 with KL score=8 for thumb base joints, n=1 with KL score=32 for DIP joints) at baseline were excluded from analyses of progression in the respective joint groups. Progression at joint level was defined as an increase in KL score (joints with KLG=4 at baseline excluded). We differed between changes in KL score in joints with HOA (KLG=2–3) versus those without HOA (KLG≤1) at baseline.
We divided the subjects by sex and into 10 age categories: <40 (n=38), 40–44 (n=109), 45–49 (n=220), 50–54 (n=458), 55–59 (n=436), 60–64 (n=394), 65–69 (n=307), 70–74 (n=170), 75–79 (n=110) and ≥80 years (n=59). The Community cohort recruited only subjects ≥50 years, leaving the combined study group under-represented for those below the age of 50. Therefore, we standardised our overall prevalence estimates to the distribution of the US 2000 Standard Population using data for those 40–84 years of age and 5-year strata. Few subjects were in the youngest (<40 years) and oldest (>84 years) age categories, and were excluded from the standardised estimates to increase the stability of the prevalence estimates. However, we present crude (non-standardised) estimates for other estimates of prevalence and incidence if not stated otherwise.
RESULTS
HOA prevalence
Men and women in the Framingham OA study had a mean (SD) baseline age of 59.2 (10.0) and 58.7 (9.9) years respectively. The majority, 2229 (96.9%), were Caucasians. The Community members (51–92 years) were older than the Offspring members (28–84 years) (online supplementary table S2). The crude HOA prevalence was slightly higher in women than men (table 1). The age-standardised prevalence was 44.2% in women and 37.7% in men. Figure 1 shows that the observed HOA prevalence was similar but slightly higher in men than women below 60 years, and higher in women than men 60 years of age or older. DIP, thumb base and PIP OA were more frequent in women than men, whereas MCP and wrist OA were more frequent in men (table 1). Similar sex-differences were found for age-standardised estimates (data not shown). The pattern of affected joints was different in the MCP joints compared with the DIP/PIP joints, with the fifth MCP infrequently affected (figure 2). Isolated MCP OA (i.e. MCP OA without DIP and/ or PIP OA in the same finger) was more common than isolated PIP OA (i.e. PIP OA without DIP OA in the same finger), and was more common in men than women (data not shown). DIP, PIP and MCP OA appeared symmetrical, whereas thumb base OA was more common in the left hand (figure 2). Seven of 40 wrists (six of 30 individuals) with OA showed signs of old wrist fractures. The crude prevalence of erosive HOA was higher in women than men (table 1), and similar to the age-standardised prevalence estimates (women 9.9%, men 3.3%). At least two erosive joints were present in 7.1% of women and 2.2% of men. Erosive HOA was especially frequent in women 60 years of age or older (figure 1). Of women and men >60 years with radiographic HOA, 24.3% and 9.5% showed erosive disease respectively. Erosive HOA was most frequent in the DIP joints, less in the PIP joints and was not seen in the MCP or thumb base joints (online supplementary figure S2).
Table 1.
Observed prevalence (%) of radiographic HOA in men and women in the Framingham Offspring and Community cohorts
| Men (n = 1001) | Women (n = 1300) | |
|---|---|---|
| Radiographic HOA (≥1 joint(s)) | 47.9 (44.8 to 50.9) | 50.5 (47.8 to 53.3) |
| DIP (2nd–5th) OA | 28.7 (25.9 to 31.5) | 35.1 (32.5 to 37.7) |
| PIP (2nd–5th) OA | 13.5 (11.4 to 15.6) | 16.5 (14.5 to 18.6) |
| Thumb IP OA | 13.0 (10.9 to 15.1) | 15.8 (13.9 to 17.8) |
| MCP (1st–5th) OA | 11.9 (9.9 to 13.9) | 6.8 (5.5 to 8.2) |
| Thumb base OA | 30.3 (27.4 to 33.1) | 32.9 (30.3 to 35.4) |
| Wrist OA | 1.7 (0.9 to 2.5) | 1.0 (0.5 to 1.5) |
| One joint group with HOA | 20.9 (18.4 to 23.4) | 21.5 (19.2 to 23.7) |
| Two joint groups with HOA | 12.6 (10.5 to 14.6) | 12.5 (10.7 to 14.3) |
| Three or more joint groups with HOA | 14.4 (12.2 to 16.6) | 16.5 (14.5 to 18.6) |
| Radiographic HOA (≥2 joints) | 35.7 (32.7 to 38.6) | 38.2 (35.6 to 40.9) |
| Symptomatic HOA (≥1 joint(s)) | 8.2 (6.5 to 10.0) | 15.9 (13.9 to 17.9) |
| Erosive HOA (≥1 joint(s)) | 3.6 (2.4 to 4.7) | 9.8 (8.2 to 11.4) |
| Total KL score (all hand joints) | 6.3 (2, 0–9) | 8.2 (3, 0–11) |
| HOA joints (KLG≥2) | 2.0 (0, 0–3) | 2.7 (1, 0–3) |
| Erosive joints* | 3.8 (2.5, 1–5) | 3.9 (3, 1–6) |
| Symptomatic HOA joints† | 3.1 (2, 1–3) | 3.4 (2, 1–4) |
Data presented as % (95% CI), except for total KL score and number of affected joints presented as mean (median, interquartile range).
In subjects with erosive HOA.
In subjects with symptomatic HOA.
DIP, distal interphalangeal; HOA, hand osteoarthritis; IP, interphalangeal; KL, Kellgren and Lawrence; KLG, Kellgren–Lawrence grade; MCP, metacarpophalangeal; OA, osteoarthritis; PIP, proximal interphalangeal.
Figure 1.
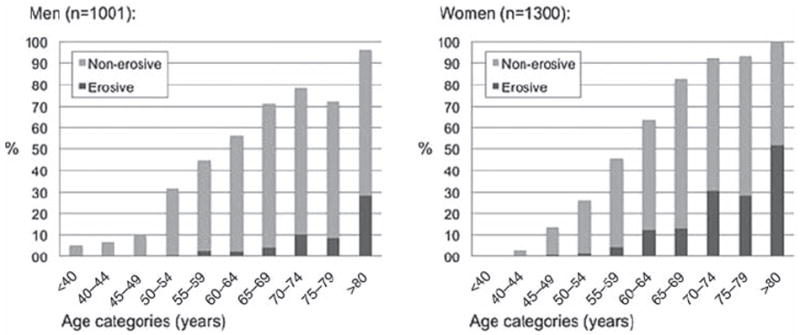
Prevalence (%) of radiographic hand osteoarthritis (HOA) in men and women across age categories at baseline. The proportions with non-erosive and erosive HOA are shown in bright and dark grey colours respectively.
Figure 2.
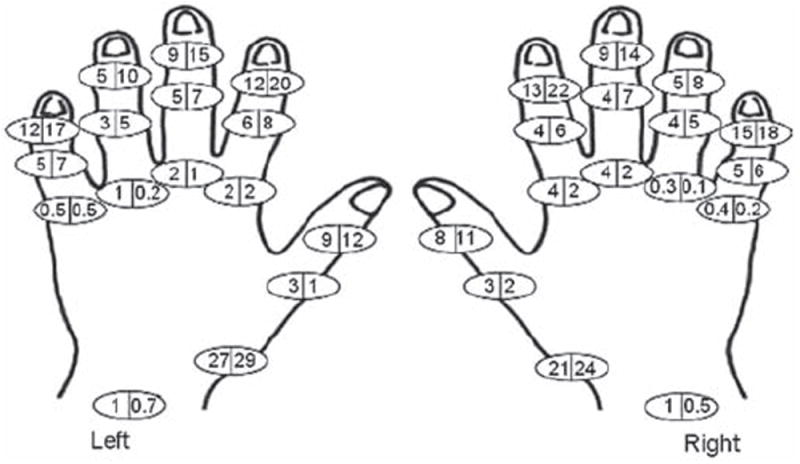
Prevalence (%) of radiographic hand osteoarthritis in the individual joints at baseline in men (left side of the circle) and women (right side of the circle).
The crude prevalence of symptomatic HOA was also higher in women than men (table 1), and similar to the age-standardised prevalence estimates (women 14.4%, men 6.9%). Symptomatic HOA was present in 59.0% of women and 55.9% of men with erosive HOA, and in 24.8% of women and 13.9% of men with non-erosive HOA. The thumb base and DIP joints were most frequently affected (online supplementary figure S2).
HOA joints had a higher prevalence of pain/aching/stiffness than non-HOA joints (17.5% vs 5.0%; PR 3.5), with the highest ratio for the thumb base (PR 5.2) and lowest for the MCP (PR 2.7) and DIP joints (PR 2.9). Erosive DIP/PIP joints were more likely to have pain/aching/stiffness than non-erosive joints (with/without HOA) (37.6% vs 6.3%; PR 5.9). Pain/aching/stiffness was similarly more frequent in DIP/PIP joints with severe OA (KLG≥3) than in no/mild OA (KLG≤2) (32.7% vs 6.1%; PR 5.3).
HOA incidence
Men and women without radiographic HOA at baseline (n=374, n=436) had mean (SD) baseline ages of 52.8 (8.5) and 50.8 (7.4) years respectively. The 9-year incidence was similar in men and women (table 2 and figure 3). MCP and wrist OA occurred more frequently and from an earlier age in men (table 2, online supplementary figure S3). Incident thumb base OA was more common in the left than the right hand (online supplementary figure S3). No men and 4.6% of those women with incident radiographic HOA had erosive disease (figure 3 and table 2). Of women and men with incident radiographic HOA, 27.8% and 11.9% had accompanying pain/aching/stiffness in >1 of the affected joint(s)respectively.
Table 2.
Cumulative 9-year incidence (%) of HOA in those with no HOA at baseline
| Men (n = 374) | Women (n = 436) | |
|---|---|---|
| Radiographic HOA (≥1 joint(s)) | 33.7 (28.9 to 38.5) | 34.6 (30.2, 39.1) |
| DIP (2nd–5th) OA | 16.0 (12.3 to 19.8) | 19.0 (15.4 to 22.7) |
| PIP (2nd–5th) OA | 5.1 (2.9 to 7.3) | 4.8 (2.8 to 6.8) |
| Thumb IP OA | 9.4 (6.4 to 12.3) | 8.7 (6.1 to 11.4) |
| MCP (1st–5th) OA | 6.1 (3.7 to 8.6) | 1.4 (0.3 to 2.5) |
| Thumb base OA | 17.4 (13.5 to 21.2) | 21.1 (17.3 to 24.9) |
| Wrist OA | 1.6 (0.3 to 2.9) | 0.2 (0.0 to 0.7) |
| Radiographic HOA (≥2 joints) | 25.1 (20.7 to 29.5) | 23.6 (19.6 to 27.6) |
| Symptomatic HOA (≥1 joint(s)) | 4.0 (2.0 to 6.0) | 9.7 (6.9 to 12.4) |
| Erosive HOA (≥1 joint(s)) | 0.0 (0.0 to 0.0) | 1.6 (0.4 to 2.8) |
| Total KL score (all hand joints) | 2.3 (0, 0–4) | 2.8 (0, 0–4) |
| HOA joints (KLG≥2) | 0.9 (0, 0–2) | 1.1 (0, 0–1) |
| Erosive joints* | 1.7 (1, 1–2) | 2.2 (2, 1–3) |
| Symptomatic HOA joints† | 2.1 (1, 1–2) | 2.7 (2, 1–3) |
Data presented as % (95% CI), except for total KL score and number of affected joints at follow-up presented as mean (median, interquartile range).
In subjects with incident erosive HOA.
In subjects with incident symptomatic HOA.
DIP, distal interphalangeal; HOA, hand osteoarthritis; IP, interphalangeal; KL, Kellgren and Lawrence; KLG, Kellgren–Lawrence grade; MCP, metacarpophalangeal; OA, osteoarthritis; PIP, proximal interphalangeal.
Figure 3.
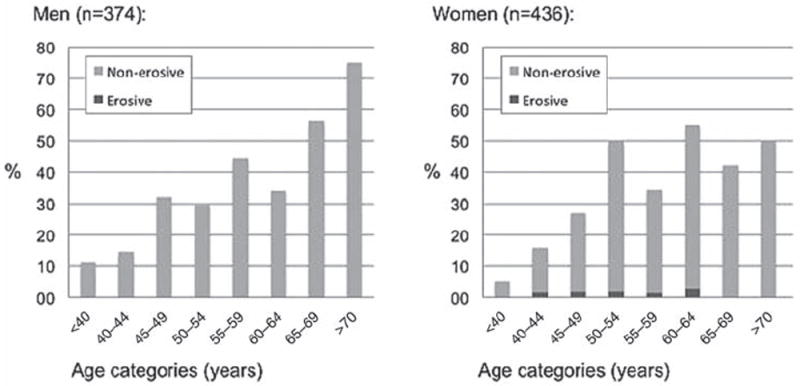
Frequency (%) of men and women with incident radiographic hand osteoarthritis (HOA) over a period of 9 years. The proportions with incident non-erosive and erosive HOA are shown in bright and dark grey colours respectively.
HOA progression
Men and women with radiographic HOA at baseline (n=187, n=277) had a mean (SD) baseline age of 60.6 (7.7) and 61.5 (7.4) years respectively. Almost all men and women showed progression during follow-up (table 3). The change in summed KL scores increased with higher age (data not shown).
Table 3.
Progression from baseline to 9-year follow-up (%) in individuals with radiographic HOA at baseline
| Men (n = 187) | Women (n = 277) | |
|---|---|---|
| Progression of HOA (change in summed KL score ≥1) | 91.4 (87.4 to 95.5) | 96.4 (94.2 to 98.6) |
| DIP (2nd–5th) OA | 59.4 (52.3 to 66.4) | 78.3 (73.4 to 83.1)* |
| PIP (2nd–5th) OA | 41.2 (34.1 to 48.2) | 57.0 (51.2 to 62.9) |
| Thumb IP OA | 31.6 (24.9 to 38.2) | 39.7 (33.9 to 45.5) |
| MCP (1st–5th) OA | 39.0 (32.0 to 46.0) | 24.5 (19.5 to 29.6) |
| Thumb base OA | 64.8 (57.9 to 71.8)* | 70.7 (65.2 to 76.1)* |
| Wrist OA | 3.2 (0.7 to 5.7) | 1.4 (0.0 to 2.8) |
| Change in total KL score (all hand joints) | 7.8 (6, 2–12) | 10.2 (9, 4–15) |
| Change in number of HOA joints (KLG≥2) | 5.0 (4, 2–8) | 6.4 (6, 3–9) |
| Number of joints with incident disease | 2.8 (2, 1–4) | 3.6 (3, 1–5) |
| Number or HOA joints with progression | 1.4 (1, 0–2) | 1.8 (1, 0–2) |
| Development of erosive HOA (≥1 joint(s)) † | 9.6 (5.2 to 13.9) | 17.3 (12.4 to 22.2) |
| Development of symptomatic HOA (≥1 joint(s)) ‡ | 25.9 (19.1 to 32.8) | 33.7 (26.8 to 40.6) |
Data presented as % (95% CI), except for changes in total KL score and number of affected joints presented as mean (median, interquartile range).
One woman with max KL score for the DIP joints, 4 men and 10 women with max KL scores for the thumb base joints were excluded from analyses.
In subjects with non-erosive HOA at baseline (178 men, 231 women).
In subjects with non-symptomatic HOA at baseline (158 men, 181 women).
DIP, distal interphalangeal; HOA, hand osteoarthritis; IP, interphalangeal; KL, Kellgren and Lawrence; KLG, Kellgren–Lawrence grade; MCP, metacarpophalangeal; OA, osteoarthritis; PIP, proximal interphalangeal.
Among the hands with some joints affected by OA at baseline, there was also incident disease in baseline unaffected joints (table 3). Incident HOA was present in 148 (79.1%) of the men and 242 (87.4%) of the women. Similarly, 114 (61.3%) of the men and 198 (71.5%) of the women showed progression of ≥1 joint(s) already affected by HOA at baseline.
Erosive and symptomatic development were more frequent in women than men (table 3). Among participants with non-symptomatic HOA at baseline, those with erosive HOA were more likely to develop symptoms than those with non-erosive HOA (54.2% vs 28.3%).
DISCUSSION
This study provides comprehensive information about prevalence, incidence and progression of radiographic HOA in the general population with a focus on subject level and individual joint level. Our results confirm that the prevalence rises with increasing age and is higher in women than men (figure 1),10-12 but not in all joint areas. Longitudinal data showed that men more frequently developed MCP and wrist OA, whereas erosive and symptomatic HOA were far more common in women (tables 1 and 2). Disease progression during 9-year follow-up was almost universal, but in most cases mild to moderate.
The epidemiology of MCP and wrist OA is less well described than the more common OA of the IP joints and the thumb base. In this large population-based cohort, we found a low prevalence of MCP10,13 and wrist OA (table 1 and figure 2).13 Knowledge about wrist OA prevalence is limited and conflicting.1,12,13 In accordance with previous studies, all carried out at least 25 years ago, we found a higher prevalence and incidence of wrist OA in men than women (tables 1 and 2).1,12,13 Signs of previous wrist fractures were present in 17.5% of wrists with OA, which could indicate that wrist fractures represent a risk factor for OA development.
A high prevalence of MCP OA has been found in rural communities from the former Soviet Union.14,15 The discrepancy between these and other populations (including Framingham) may be due to differences in disease-susceptibility genes or occupational/cultural lifestyles, as MCP OA has been associated with heavy labour.32 Biomechanical studies have proven that forces generated at the MCP joints during grip strength are higher than in more distal joints,33 and maximal grip strength has been more strongly associated with OA development in the MCP joints compared with more distal joints.34 We found a higher prevalence and incidence of MCP OA in men than women (tables 1 and 2), a different pattern of MCP joint involvement as opposed to the DIP and PIP joints (figure 2), and a higher frequency of isolated MCP OA. These results may suggest a different aetiology of MCP OA, such as mechanical stresses. However, we did not have data on markers of haemochromatosis, which is known to be associated with MCP OA.35
The longitudinal course of HOA has been less well described in previous studies. Our incidence estimates are lower than previously reported, which may be due to shorter period of follow-up, younger subjects,23 a more strict definition of incident HOA22 and the possibility that those at risk of HOA had already developed it at baseline. Older individuals were more likely to develop HOA during follow-up than younger ones, except the oldest women (figure 3). This decline among the oldest is probably due to fewer subjects at risk (only two women ≥70 years without HOA at baseline). Almost everyone with HOA at baseline showed disease progression during follow-up (table 3), and about two-thirds showed progression larger than the smallest detectable change (i.e. change≥4.8 in total KL score across hand joints). However, the changes were modest in most subjects, in support of previous studies.17-21,24
Consistent with previous studies,36 we found no clear evidence of higher HOA incidence in the right hand (usually dominant) (online supplementary figure S3). The symmetrical joint affection indicates that ‘wear and tear’ alone is not sufficient to explain the pattern of HOA, and neurogenic and hormonal influences have been suggested.37,38 As previously reported,9,10,39-41 thumb base OA occurred more frequently in the left hand. The articular configuration of the thumb base allows subluxation unless surrounding ligaments adequately stabilise the joint, and hypermobility/subluxation are proven OA risk factors.41-43 A left-side predominance of hypermobility has been reported,44 indicating that regular use of the dominant (presumably right) hand may protect against hypermobility and subsequent OA development through neuromuscular pathways and possibly strengthening of the thenar muscles, which dynamically stabilise this joint.
Symptomatic disease was twice as common in women than men (tables 1 and 2).6-7,45 Clinical and experimental studies show that women are at greater risk of several chronic pain conditions,46 suggesting differences in pain sensitivity, cognitive/affective mechanisms and pain reporting. We found that pain/aching/stiffness was substantially more frequent in HOA joints versus non-HOA joints, and especially in the thumb base joint, which may support that thumb joint OA has a greater impact on pain than DIP OA.47 Participants with erosive HOA reported symptoms more frequently than those with non-erosive HOA,48 which may contribute to the observed differences between women and men.
This study is the first to report the prevalence and incidence of erosive HOA in the general population. There exists no established definition of erosive HOA, and we required only one erosive joint (as in the Rotterdam study). However, the prevalence of erosive HOA was higher than in the Rotterdam Study (table 1).27 Incident erosive disease was much more frequent in those with radiographic HOA at baseline compared with those without HOA (tables 2 and 3). Further, we found a high prevalence of erosive disease in older men (figure 1), in contrast to the traditional view of erosive HOA as a disease that occurs in middle-aged women.
Some study limitations are noteworthy. Two different readers scored the radiographs in the two cohorts. However, the inter-reader reliability was very good. The amalgamation of participants from two different sampling frames in a limited geographic area may represent a potential limitation, and it is uncertain whether the results can be generalised to larger geographical areas or non-Caucasian groups. The Offspring cohort was not randomly selected from the population. However, the participants were not chosen based on joint symptoms, and previous studies have indicated that the cohort is reasonably representative of the US population.49 The mean time of follow-up was 9 years, and almost all participants showed progression, making discrimination between groups difficult. It is possible but unproven that reading of radiographs in known time sequence may lead to overestimation of progression.
In conclusion, the usual female predominance of prevalent and incident HOA was less clear for radiographic HOA than for symptomatic and erosive HOA. Incident radiographic HOA in the younger age groups and in specific joint groups such as wrist and MCP were more frequent in men. Among those with radiographic HOA at baseline, the majority showed mild to moderate progression during follow-up. With an ageing population, the impact of HOA will further increase.
Supplementary Material
Acknowledgments
The authors thank the participants of the Framingham Offspring and Community Cohorts for helping them to perform the study.
Funding The Framingham Osteoarthritis Study is supported by the National Institutes of Health (NIH) AR47785. IKH is funded by grants from the South-Eastern Norway Regional Health Authority and a scholarship from OARSI. ME is funded by the Swedish Research Council and the Faculty of Medicine, Lund University, Sweden.
Footnotes
Additional data (supplementary tables and figures) are published online only. To view these files please visit the journal online (http://ard.bmj.com).
Competing interests None.
Patient consent Obtained.
Ethics approval This study was conducted with the approval of the Boston University Medical Center Institutional Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Kellgren JH, Lawrence JS. Osteo-arthrosis and disk degeneration in an urban population. Ann Rheum Dis. 1958;17:388–97. doi: 10.1136/ard.17.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plato CC, Norris AH. Osteoarthritis of the hand: age-specific joint-digit prevalence rates. Am J Epidemiol. 1979;109:169–80. doi: 10.1093/oxfordjournals.aje.a112672. [DOI] [PubMed] [Google Scholar]
- 3.Egger P, Cooper C, Hart DJ, et al. Patterns of joint involvement in osteoarthritis of the hand: the Chingford Study. J Rheumatol. 1995;22:1509–13. [PubMed] [Google Scholar]
- 4.Sowers M, Lachance L, Hochberg M, et al. Radiographically defined osteoarthritis of the hand and knee in young and middle-aged African American and Caucasian women. Osteoarthr Cartil. 2000;8:69–77. doi: 10.1053/joca.1999.0273. [DOI] [PubMed] [Google Scholar]
- 5.Bagge E, Bjelle A, Edén S, et al. Osteoarthritis in the elderly: clinical and radiological findings in 79 and 85 year olds. Ann Rheum Dis. 1991;50:535–9. doi: 10.1136/ard.50.8.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Niu J, Kelly-Hayes M, et al. Prevalence of symptomatic hand osteoarthritis and its impact on functional status among the elderly: the Framingham Study. Am J Epidemiol. 2002;156:1021–7. doi: 10.1093/aje/kwf141. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Xu L, Nevitt MC, et al. Lower prevalence of hand osteoarthritis among Chinese subjects in Beijing compared with white subjects in the United States: the Beijing Osteoarthritis Study. Arthritis Rheum. 2003;48:1034–40. doi: 10.1002/art.10928. [DOI] [PubMed] [Google Scholar]
- 8.Acheson RM, Collart AB. New Haven survey of joint diseases. XVII. Relationship between some systemic characteristics and osteoarthrosis in a general population. Ann Rheum Dis. 1975;34:379–87. doi: 10.1136/ard.34.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilder FV, Barrett JP, Farina EJ. Joint-specific prevalence of osteoarthritis of the hand. Osteoarthr Cartil. 2006;14:953–7. doi: 10.1016/j.joca.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Dahaghin S, Bierma-Zeinstra SM, Ginai AZ, et al. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study) Ann Rheum Dis. 2005;64:682–7. doi: 10.1136/ard.2004.023564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haara MM, Manninen P, Kröger H, et al. Osteoarthritis of finger joints in Finns aged 30 or over: prevalence, determinants, and association with mortality. Ann Rheum Dis. 2003;62:151–8. doi: 10.1136/ard.62.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Saase JL, van Romunde LK, Cats A, et al. Epidemiology of osteoarthritis: Zoetermeer survey. Comparison of radiological osteoarthritis in a Dutch population with that in 10 other populations. Ann Rheum Dis. 1989;48:271–80. doi: 10.1136/ard.48.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler WJ, Hawthorne VM, Mikkelsen WM, et al. Prevalence of radiologically defined osteoarthritis in the finger and wrist joints of adult residents of Tecumseh, Michigan, 1962-65. J Clin Epidemiol. 1988;41:467–73. doi: 10.1016/0895-4356(88)90048-0. [DOI] [PubMed] [Google Scholar]
- 14.Kalichman L, Li L, Kobyliansky E. Prevalence, pattern and determinants of radiographic hand osteoarthritis in Turkmen community-based sample. Rheumatol Int. 2009;29:1143–9. doi: 10.1007/s00296-008-0815-1. [DOI] [PubMed] [Google Scholar]
- 15.Kalichman L, Li L, Batsevich V, et al. Prevalence, pattern and determinants of radiographic hand osteoarthritis in five Russian community-based samples. Osteoarthr Cartil. 2010;18:803–9. doi: 10.1016/j.joca.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Kalichman L, Li L, Batsevich V, et al. Hand osteoarthritis in the Abkhazian population. Homo. 2009;60:429–39. doi: 10.1016/j.jchb.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Bijsterbosch J, Watt I, Meulenbelt I, et al. Clinical and radiographic disease course of hand osteoarthritis and determinants of outcome after 6 years. Ann Rheum Dis. 2011;70:68–73. doi: 10.1136/ard.2010.133017. [DOI] [PubMed] [Google Scholar]
- 18.Botha-Scheepers S, Riyazi N, Watt I, et al. Progression of hand osteoarthritis over 2 years: a clinical and radiological follow-up study. Ann Rheum Dis. 2009;68:1260–4. doi: 10.1136/ard.2008.087981. [DOI] [PubMed] [Google Scholar]
- 19.Paradowski PT, Lohmander LS, Englund M. Natural history of radiographic features of hand osteoarthritis over 10 years. Osteoarthr Cartil. 2010;18:917–22. doi: 10.1016/j.joca.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Harris PA, Hart DJ, Dacre JE, et al. The progression of radiological hand osteoarthritis over ten years: a clinical follow-up study. Osteoarthr Cartil. 1994;2:247–52. doi: 10.1016/s1063-4584(05)80076-7. [DOI] [PubMed] [Google Scholar]
- 21.Plato CC, Norris AH. Osteoarthritis of the hand: longitudinal studies. Am J Epidemiol. 1979;110:740–6. doi: 10.1093/oxfordjournals.aje.a112855. [DOI] [PubMed] [Google Scholar]
- 22.Kallman DA, Wigley FM, Scott WW, Jr, et al. The longitudinal course of hand osteoarthritis in a male population. Arthritis Rheum. 1990;33:1323–32. doi: 10.1002/art.1780330904. [DOI] [PubMed] [Google Scholar]
- 23.Chaisson CE, Zhang Y, McAlindon TE, et al. Radiographic hand osteoarthritis: incidence, patterns, and influence of pre-existing disease in a population based sample. J Rheumatol. 1997;24:1337–43. [PubMed] [Google Scholar]
- 24.Busby J, Tobin J, Ettinger W, et al. A longitudinal study of osteoarthritis of the hand: the effect of age. Ann Hum Biol. 1991;18:417–24. doi: 10.1080/03014469100001712. [DOI] [PubMed] [Google Scholar]
- 25.Maheu E, Michon M, Carrat F, et al. Erosive versus non-erosive hand OA: Prospective cross-sectional comparison of clinical data. Osteoarthritis Cartilage. 2008;16:S130. abstract. [Google Scholar]
- 26.Cavasin F, Punzi L, Ramonda R, et al. Prevalence of erosive osteoarthritis of the hand in a population from Venetian area. Reumatismo. 2004;56:46–50. doi: 10.4081/reumatismo.2004.46. [DOI] [PubMed] [Google Scholar]
- 27.Kwok WY, Kloppenburg M, Rosendaal FR, et al. Erosive hand osteoarthritis: its prevalence and clinical impact in the general population and symptomatic hand osteoarthritis. Ann Rheum Dis. 2011 doi: 10.1136/ard.2010.143016. Published Online First: 6 April 2011. [DOI] [PubMed] [Google Scholar]
- 28.Feinleib M, Kannel WB, Garrison RJ, et al. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–25. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 29.Englund M, Guermazi A, Gale D, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008;359:1108–15. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthr Cartil. 2007;15(Suppl A):A1–56. doi: 10.1016/j.joca.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Altman DG. Inter-rater agreement. In: Altman DG, editor. Practical Statistics for Medical Research. London, UK: Chapman and Hall/CRC; 1991. p. 404. [Google Scholar]
- 32.Williams WV, Cope R, Gaunt WD, et al. Metacarpophalangeal arthropathy associated with manual labor (Missouri metacarpal syndrome). Clinical radiographic, and pathologic characteristics of an unusual degeneration process. Arthritis Rheum. 1987;30:1362–71. doi: 10.1002/art.1780301207. [DOI] [PubMed] [Google Scholar]
- 33.Cooney WP, 3rd, Chao EY. Biomechanical analysis of static forces in the thumb during hand function. J Bone Joint Surg Am. 1977;59:27–36. [PubMed] [Google Scholar]
- 34.Chaisson CE, Zhang Y, Sharma L, et al. Higher grip strength increases the risk of incident radiographic osteoarthritis in proximal hand joints. Osteoarthr Cartil. 2000;8(Suppl A):S29–32. doi: 10.1053/joca.2000.0333. [DOI] [PubMed] [Google Scholar]
- 35.Schumacher HR., Jr Haemochromatosis. Baillieres Best Pract Res Clin Rheumatol. 2000;14:277–84. doi: 10.1053/berh.2000.0065. [DOI] [PubMed] [Google Scholar]
- 36.Lane NE, Bloch DA, Jones HH, et al. Osteoarthritis in the hand: a comparison of handedness and hand use. J Rheumatol. 1989;16:637–42. [PubMed] [Google Scholar]
- 37.Kidd BL, Mapp PI, Gibson SJ, et al. A neurogenic mechanism for symmetrical arthritis. Lancet. 1989;2:1128–30. doi: 10.1016/s0140-6736(89)91491-8. [DOI] [PubMed] [Google Scholar]
- 38.Spector TD, Campion GD. Generalised osteoarthritis: a hormonally mediated disease. Ann Rheum Dis. 1989;48:523–7. doi: 10.1136/ard.48.6.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haara MM, Heliövaara M, Kröger H, et al. Osteoarthritis in the carpometacarpal joint of the thumb. Prevalence and associations with disability and mortality. J Bone Joint Surg Am. 2004;86-A:1452–7. doi: 10.2106/00004623-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Acheson RM, Chan YK, Clemett AR. New Haven survey of joint diseases. XII. Distribution and symptoms of osteoarthrosis in the hands with reference to handedness. Ann Rheum Dis. 1970;29:275–86. doi: 10.1136/ard.29.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jónsson H, Elíasson GJ, Jónsson A, et al. High hand joint mobility is associated with radiological CMC1 osteoarthritis: the AGES-Reykjavik study. Osteoarthr Cartil. 2009;17:592–5. doi: 10.1016/j.joca.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jónsson H, Valtýsdóttir ST, Kjartansson O, et al. Hypermobility associated with osteoarthritis of the thumb base: a clinical and radiological subset of hand osteoarthritis. Ann Rheum Dis. 1996;55:540–3. doi: 10.1136/ard.55.8.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hunter DJ, Zhang Y, Sokolove J, et al. Trapeziometacarpal subluxation predisposes to incident trapeziometacarpal osteoarthritis (OA): the Framingham Study. Osteoarthr Cartil. 2005;13:953–7. doi: 10.1016/j.joca.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Al-Rawi ZS, Al-Aszawi AJ, Al-Chalabi T. Joint mobility among university students in Iraq. Br J Rheumatol. 1985;24:326–31. doi: 10.1093/rheumatology/24.4.326. [DOI] [PubMed] [Google Scholar]
- 45.Niu J, Zhang Y, LaValley M, et al. Symmetry and clustering of symptomatic hand osteoarthritis in elderly men and women: the Framingham Study. Rheumatology (Oxford) 2003;42:343–8. doi: 10.1093/rheumatology/keg110. [DOI] [PubMed] [Google Scholar]
- 46.Fillingim RB, King CD, Ribeiro-Dasilva MC, et al. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–85. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bijsterbosch J, Visser W, Kroon HM, et al. Thumb base involvement in symptomatic hand osteoarthritis is associated with more pain and functional disability. Ann Rheum Dis. 2010;69:585–7. doi: 10.1136/ard.2009.104562. [DOI] [PubMed] [Google Scholar]
- 48.Bijsterbosch J, Watt I, Meulenbelt I, et al. Clinical burden of erosive hand osteoarthritis and its relationship to nodes. Ann Rheum Dis. 2010;69:1784–8. doi: 10.1136/ard.2009.125435. [DOI] [PubMed] [Google Scholar]
- 49.Vasan RS, Sullivan LM, Wilson PW, et al. Relative importance of borderline and elevated levels of coronary heart disease risk factors. Ann Intern Med. 2005;142:393–402. doi: 10.7326/0003-4819-142-6-200503150-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


